Noble Pen Shell (Pinna nobilis) Mortalities along the Eastern Adriatic Coast with a Study of the Spreading Velocity
Abstract
:1. Introduction
2. Materials and Methods
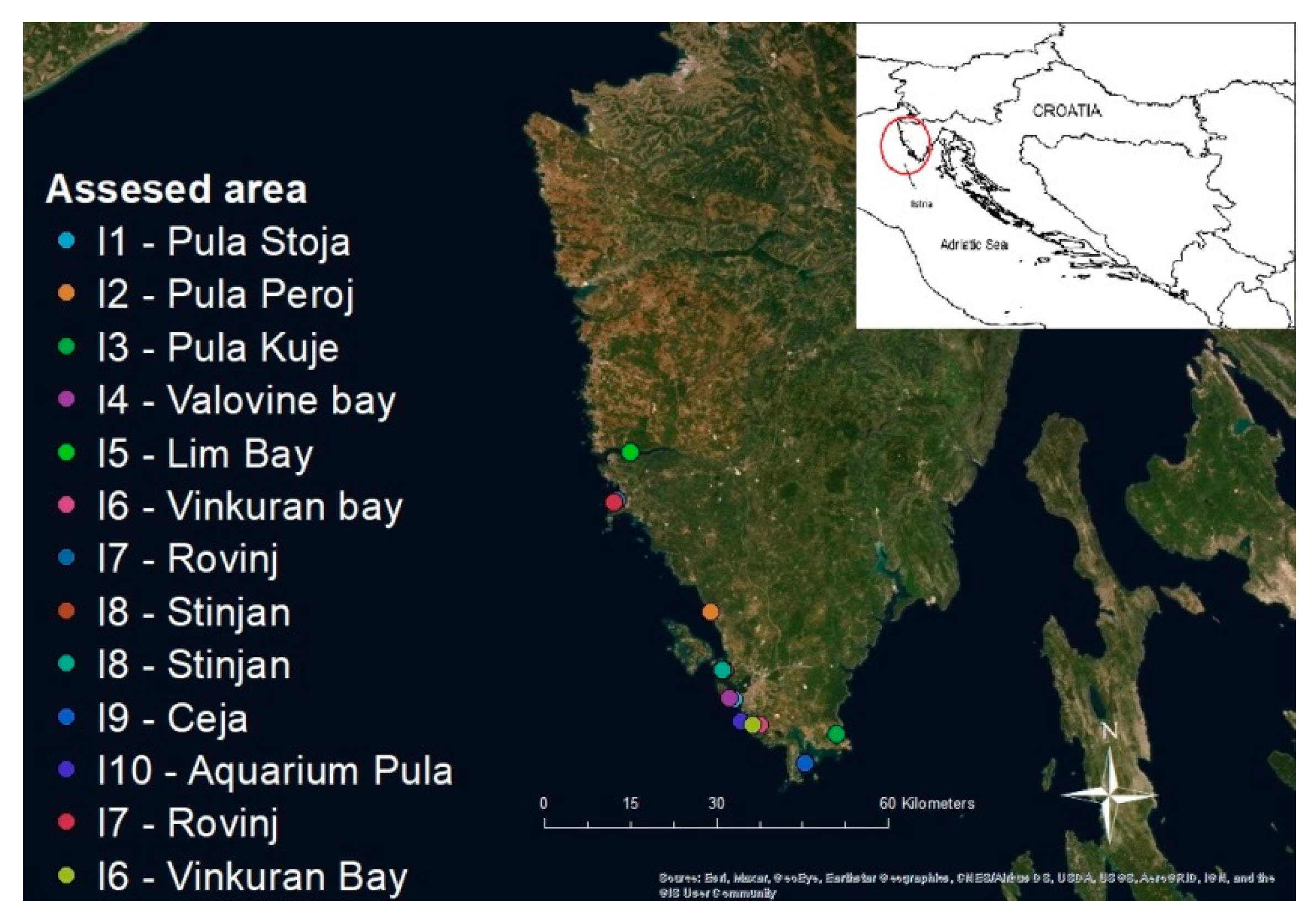
2.1. Sampling Sites and Sample Collection
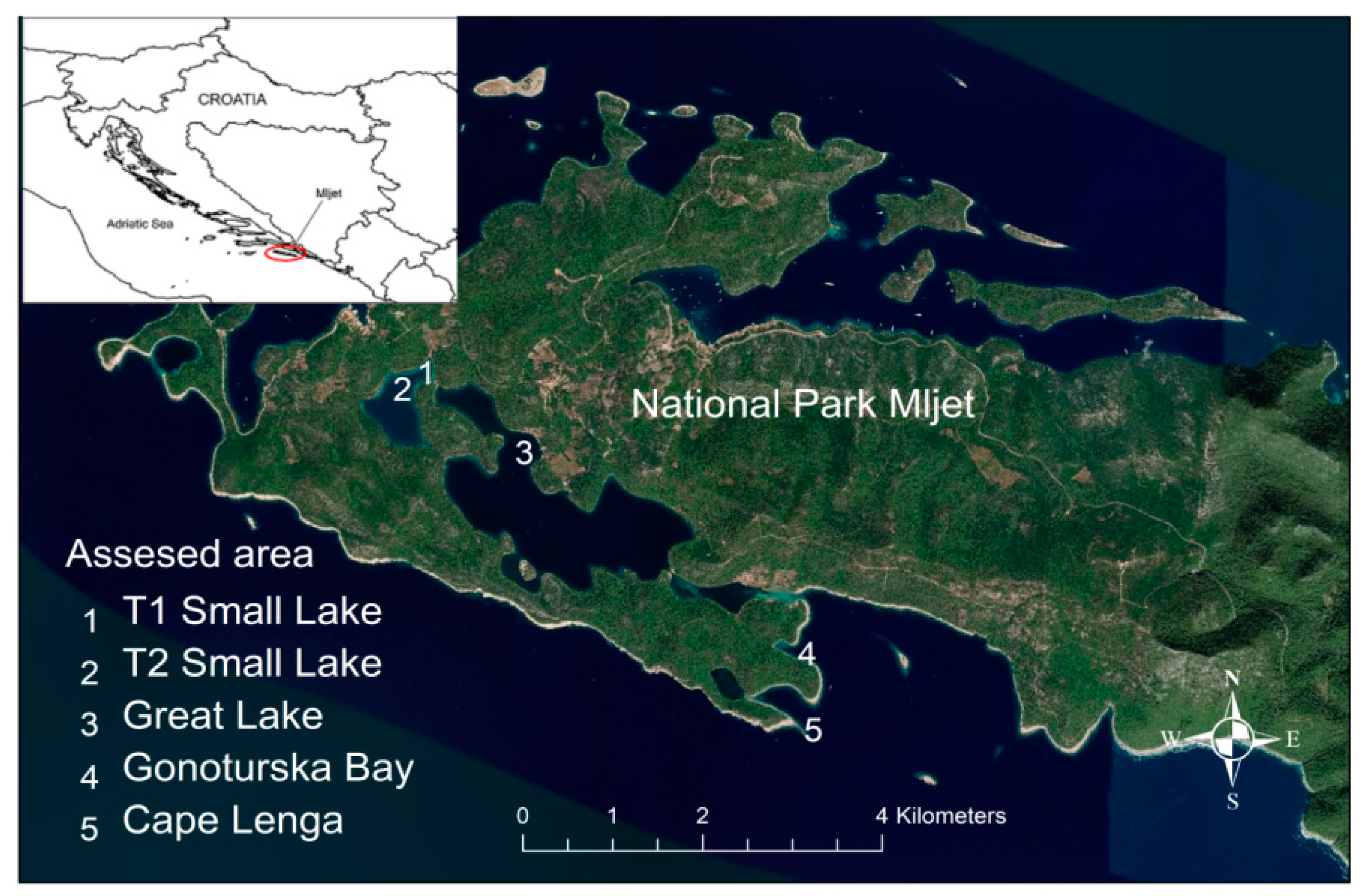
2.2. Site and Design of the Small-Scale Study within the MNP
2.3. Non-Lethal Sampling
2.4. Statistical Analysis
2.5. Laboratory Procedures
2.6. Histological Analysis
2.7. DNA Extraction
2.8. PCR for Detection of Haplosporidium spp.
2.9. PCR for Detection of Mycobacterium spp.
2.10. Sequencing and Phylogeny
3. Results
3.1. Description of the Pen Shells Collected in Situ
3.2. Results of the Pilot Study (Morphometrics, Environmental Conditions, and Mortality Patterns)
3.3. Necropsy and Histological Findings
3.4. Molecular Analysis for the Presence of Haplosporidium spp. and Mycobacterium spp.
3.4.1. Sequencing of Haplosporidium spp. Isolates
3.4.2. Sequencing of Mycobacterium spp. Isolates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zavodnik, D.; Hrs-Brenko, M.; Legas, M. Synopsis on the fan shell Pinna nobilis L. in the eastern Adriatic Sea. Les Espèces Marines à protegér en Méditerranée. Gis Posidonie. Publ. Marseille 1991, 169–178. [Google Scholar]
- Cabanellas-Reboredo, M.; Vazquez-Luis, M.; Mourre, B.; Álvarez, E.; Deudero, S.; Amores, Á.; Addis, P.; Bal-lesteros, E.; Barrajon, A.; Coppa, S.; et al. Tracking a mass mortality outbreak of pen shell Pinna nobilis populations: A collaborative effort of scientists and citizens. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tsatiris, A.; Papadopoulos, V.; Makri, D.; Topouzelis, K.; Manoutsoglou, E.; Hasiotis, T.; Katsanevakis, S. Spatial distribution, abundance and habitat use of the endemic Mediterranean fan mussel Pinna nobilis in Gera Gulf, Lesvos (Greece): Comparison of design-based and model-based approaches. Mediterr. Mar. Sci. 2018, 19, 642–655. [Google Scholar] [CrossRef] [Green Version]
- Katsanevakis, S. Growth and mortality rates of the fan mussel Pinna nobilis in Lake Vouliagmeni (Korin-thiakos Gulf, Greece): A generalized additive modelling approach. Mar. Biol. 2007, 152, 1319–1331. [Google Scholar] [CrossRef]
- Richardson, C.A.; Peharda, M.; Kennedy, H.; Kennedy, P.; Onofri, V. Age, growth rate and season of recruitment of Pinna nobilis (L) in the Croatian Adriatic determined from Mg:Ca and Sr:Ca shell profiles. J. Exp. Mar. Biol. Ecol. 2004, 299, 1–16. [Google Scholar] [CrossRef]
- Deudero, S.; Grau, A.; Vázquez-Luis, M.; Álvarez, E.; AlOmar, C.; Hendriks, I.E. Reproductive investment of the pen shell Pinna nobilis Linnaeus, 1758 in Cabrera National Park (Spain). Mediterr. Mar. Sci. 2017, 18, 271. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Luis, M.; Álvarez, E.; Barrajón, A.; García-March, J.R.; Grau, A.; Hendriks, I.E.; Jiménez, S.; Kersting, D.; Moreno, D.; Pérez, M.; et al. S.O.S. Pinna nobilis: A Mass Mortality Event in Western Mediterranean Sea. Front. Mar. Sci. 2017, 4, 220. [Google Scholar] [CrossRef] [Green Version]
- Catanese, G.; Grau, A.; Valencia, J.M.; March, J.R.G.; Vázquez-Luis, M.; Alvarez, E.; Deudero, S.; Darriba, S.; Carballal, M.J.; Villalba, A. Haplosporidium pinnae sp. Nov., a haplosporidan parasite associated with mass mortalities of the fan mussel, Pinna nobilis, in the Western Mediterranean Sea. J. Invertebr. Pathol. 2018, 157, 9–24. [Google Scholar] [CrossRef]
- Panarese, R.; Tedesco, P.; Chimienti, G.; Latrofa, M.S.; Quaglio, F.; Passantino, G.; Buonavoglia, C.; Gustinelli, A.; Tursi, A.; Otranto, D. Haplosporidium pinnae associated with mass mortality in endangered Pinna nobilis (Linnaeus 1758) fan mussels. J. Invertebr. Pathol. 2019, 164, 32–37. [Google Scholar] [CrossRef]
- Carella, F.; Aceto, S.; Pollaro, F.; Miccio, A.; Iaria, C.; Carrasco, N.; Prado, P.; De Vico, G. A mycobacterial disease is associated with the silent mass mortality of the pen shell Pinna nobilis along the Tyrrhenian coast-line of Italy. Sci. Rep. 2019, 9, 2725. [Google Scholar] [CrossRef]
- Katsanevakis, S. The cryptogenic parasite Haplosporidium pinnae invades the Aegean Sea and causes the collapse of Pinna nobilis populations. Aquat. Invasions 2019, 14, 150–164. [Google Scholar] [CrossRef]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Michaelidis, B. First detection of the invasive Haplosporidian and Mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020, 155, 104889. [Google Scholar] [CrossRef] [PubMed]
- Tiscar, P.G.; Rubino, F.; Fanelli, G.; Paoletti, B.; Della Salda, L. Mass Mortality of the Fan Mussel Pinna nobilis in Apulia (Ionian Sea) Caused by Haplosporidium pinnae. In Proceedings of the 42nd CIESM Congress, Cascais, Portugal, 7–11 October 2019. [Google Scholar]
- Čižmek, H.; Čolić, B.; Gračan, R.; Grau, A.; Catanese, G. An emergency situation for pen shells in the Mediterranean: The Adriatic Sea, one of the last Pinna nobilis shelters, is now affected by a mass mortality event. J. Invertebr. Pathol. 2020, 173, 107388. [Google Scholar] [CrossRef] [PubMed]
- Šarić, T.; Župan, I.; Aceto, S.; Villari, G.; Palić, D.; De Vico, G.; Carella, F. Epidemiology of Noble Pen Shell (Pinna nobilis L. 1758) Mass Mortality Events in Adriatic Sea Is Characterised with Rapid Spreading and Acute Disease Progression. Pathogens 2020, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Künili, I.E.; Gürkan, S.E.; Aksu, A.; Turgay, E.; Çakir, F.; Gürkan, M.; Altinağaç, U. Mass mortality in endangered fan mussels Pinna nobilis (Linnaeus 1758) caused by co-infection of Haplosporidium pinnae and multiple Vibrio infection in Çanakkale Strait, Turkey. Biomarkers 2021, 26, 450–461. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature (IUCN). Mediterranean Noble Pen Shell Crisis (Pinna nobilis)-January 2020 Update. Available online: https://www.iucn.org/news/mediterranean/202001/mediterranean-noble-pen-shell-crisis-pinna-nobilis-january-2020-update (accessed on 10 March 2021).
- Kersting, D.; Benabdi, M.; Čižmek, H.; Grau, A.; Jimenez, C.; Katsanevakis, S.; Öztürk, B.; Tuncer, S.; Tunesi, L.; Vázquez-Luis, M.; et al. Pinna Nobilis. The IUCN Red List of Threatened Species 2019; IUCN Red List: London, UK, 2019. [Google Scholar] [CrossRef]
- Pavlinec, Ž.; Zupičić, I.G.; Oraić, D.; Petani, B.; Mustać, B.; Mihaljević, Ž.; Beck, R.; Zrnčić, S. Assessment of predominant bacteria in noble pen shell (Pinna nobilis) collected in the Eastern Adriatic Sea. Environ. Monit. Assess. 2020, 192, 1–10. [Google Scholar] [CrossRef]
- Carella, F.; Antuofermo, E.; Farina, S.; Salati, F.; Mandas, D.; Prado, P.; Panarese, R.; Marino, F.; Fiocchi, E.; Pretto, T.; et al. In the wake of the ongoing mass mortality events: Co-occurrence of Mycobacterium, Haplosporidium and other pathogens in Pinna nobilis collected in Italy and Spain (Mediterranean Sea). Front. Mar. Sci. 2020, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Prado, P.; Carrasco, N.; Catanese, G.; Grau, A.; Cabanes, P. Presence of Vibrio mediterranei associated to major mortality in stabled individuals of Pinna nobilis L. Aquaculture 2020, 519, 734899. [Google Scholar] [CrossRef]
- Lattos, A.; Bitchava, K.; Giantsis, I.; Theodorou, J.; Batargias, C.; Michaelidis, B. The Implication of Vibrio Bacteria in the Winter Mortalities of the Critically Endangered Pinna nobilis. Microorganisms 2021, 9, 922. [Google Scholar] [CrossRef]
- Scarpa, F.; Sanna, D.; Azzena, I.; Mugetti, D.; Cerruti, F.; Hosseini, S.; Cossu, P.; Pinna, S.; Grech, D.; Cabana, D.; et al. Multiple Non-Species-Specific Pathogens Possibly Triggered the Mass Mortality in Pinna nobilis. Life 2020, 10, 238. [Google Scholar] [CrossRef]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Theodorou, J.A.; Michaelidis, B. Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS. Pathogens 2020, 9, 1002. [Google Scholar] [CrossRef]
- Orepić, N.; Vidmar, J.; Zahtila, E.; Zavodnik, D. A marine benthos survey in the lakes of the National park Mljet (Adriatic Sea). Period. Biol. 1997, 99, 229–245. [Google Scholar]
- Šiletić, T.; Peharda, M. Population study of the fan shell Pinna nobilis L. in Malo and Veliko Jezero of the Mljet National Park (Adriatic Sea). Sci. Mar. 2003, 67, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Peharda, M.; Hrs-Brenko, M.; Bogner, D.; Onofri, V.; Benović, A. Spatial distribution of live and dead bivalves in saltwater lake Malo Jezero (Mljet National Park). Period. Biol. 2002, 104, 115–122. [Google Scholar]
- Howard, D.; Lewis, E.; Keller, J.; Smith, C. Histological Techniques for Marine Bivalve Mollusks and Crustaceans, 2nd ed.; NOAA (National Ocean Service): Oxford, MD, USA, 2004; 60p. [Google Scholar]
- Garcia-March, J.R.; Vicente, N. Protocol to Study and Monitor Pinna nobilis Populations within Marine Protected Areas. Report of the Malta Environment and Planning Authority (MEPA) and MedPAN-Interreg IIIC-Project. 2006. Available online: https://drive.google.com/file/d/131bRQrcQQ62NUep1V1J3f_W33JAmANbO/view (accessed on 5 September 2019).
- Sanna, D.; Cossu, P.; Dedola, G.L.; Scarpa, F.; Maltagliati, F.; Castelli, A.; Franzoi, P.; Lai, T.; Cristo, B.; Curini-Galletti, M.; et al. Mitochondrial DNA Reveals Genetic Structuring of Pinna nobilis across the Mediterranean Sea. PLoS ONE 2013, 8, e67372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, W.N.; Wickham, R.; Coombs, M.N. An Introduction to Survival Statistics: Kaplan-Meier Analysis. J. Adv. Pr. Oncol. 2016, 7, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Morton, B.; Puljas, S. An improbable opportunistic predator: The functional morphology of Pinna nobilis (Bivalvia: Pterioida: Pinnidae). J. Mar. Biol. Assoc. UK 2019, 99, 359–373. [Google Scholar] [CrossRef]
- Renault, T.; Stokes, N.; Chollet, B.; Cochennec, N.; Berthe, F.; Gerard, A.; Burreson, E. Haplosporidiosis in the Pacific oyster Crassostrea gigas from the French Atlantic coast. Dis. Aquat. Org. 2000, 42, 207–214. [Google Scholar] [CrossRef]
- Telenti, A.; Marchesi, F.; Balz, M.; Bally, F.; Böttger, E.C.; Bodmer, T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 1993, 31, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böddinghaus, B.; Rogall, T.; Flohr, T.; Blöcker, H.; Böttger, E.C. Detection and identification of mycobacteria by amplification of rRNA. J. Clin. Microbiol. 1990, 28, 1751–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Mizrachi, I.K.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2012, 41, D36–D42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashish, E.; Merwad, A.-R.; Elgaml, S.; Amer, A.; Kamal, H.; Elsadek, A.; Marei, A.; Sitohy, M. Mycobacterium marinum infection in fish and man: Epidemiology, pathophysiology and management; a review. VetIQ Petcare 2018, 38, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Box, A.; Capó, X.; Tejada, S.; Catanese, G.; Grau, A.; Deudero, S.; Sureda, A.; Valencia, J.M. Reduced Antioxidant Response of the Fan Mussel Pinna nobilis Related to the Presence of Haplosporidium pinnae. Pathogens 2020, 9, 932. [Google Scholar] [CrossRef]
- Arzul, I.; Carnegie, R.B. New perspective on the haplosporidian parasites of molluscs. J. Invertebr. Pathol. 2015, 131, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Mihanović, H.; Cosoli, S.; Vilibić, I.; Ivanković, D.; Dadić, V.; Gačić, M. Surface current patterns in the northern Adriatic extracted from high-frequency radar data using self-organizing map analysis. J. Geophys. Res. Space Phys. 2011, 116, 08033. [Google Scholar] [CrossRef] [Green Version]
- Haskin, H.H.; Andrews, J.D. Uncertainties and Speculations about the Life Cycle of the Eastern Oyster Pathogen Haplosporidium nelsoni (MSX). In Disease Processes in Marine Bivalve Molluscs; American Fisheries Society: Bethesda, MD, USA, 1988; Available online: https://scholarworks.wm.edu/cgi/viewcontent.cgi?article=1117&context=vimsbooks (accessed on 5 March 2021).






| Date of Sampling (D/M/Y) | Name and Number of the Site | Sea Temp. °C | Sample | Results of Laboratory Analysis | ||
|---|---|---|---|---|---|---|
| PCR Haplo | PCR Myco | Histology | ||||
| 19/04/19 | MNP1—Small Lake | 17.2 | Whole animal | 0/3 | 2/3 | neg |
| 19/04/19 | MNP5—Cape Lenga | 15.9 | Whole animal | 2/2 | 0/2 | Haplo + |
| 19/04/19 | MNP4—Gonoturska | 15.9 | Whole animal | 0/1 | 0/1 | neg |
| 04/09/19 | I1—Pula-Stoja | 24.5 | Organs in EtOH * | 0/3 | 0/3 | n/d |
| 04/09/19 | I2—Pula-Peroj | 24.8 | Organs in EtOH | 0/1 | 0/1 | n/d |
| 04/09/19 | I3—Pula-Kuje | 25.2 | Organs in EtOH | 0/1 | 0/1 | n/d |
| 24/09/19 | MNP1 Mljet-Small Lake | 28 | Mantles in EtOH | 2/4 | 1/4 | n/d |
| 30/09/19 | MNP1 Mljet-Small Lake | 26 | Mantles in EtOH | 2/16 | 1/16 | n/d |
| 13/01/20 | I4—Uvala Valovine | 12.3 | Organs in EtOH | 0/1 | 0/1 | n/d |
| 13/01/20 | I5—Limski Bay | 12.8 | Organs in RNAlater | 0/1 | 0/1 | n/d |
| 18/02/20 | I6—Vinkuran Bay | 12.2 | Organs in EtOH | 0/1 | 0/1 | n/d |
| 19/02/20 | I7—Rovinj | 12.2 | Organs in EtOH | 0/2 | 0/2 | n/d |
| 20/02/20 | I8—Štinjan | 11.9 | Organs in EtOH | 0/1 | 0/1 | n/d |
| 19/05/20 | I8—Štinjan | 21.5 | Organs in EtOH | 2/2 | 2/2 | n/d |
| 19/05/20 | I9—Ceja | 22.7 | Organs in EtOH | 1/1 | 0/1 | n/d |
| 28/05/20 | I10—Aquarium Pula | 18.0 | Organs in EtOH and HCHO ** | 0/1 | 1/1 | n/d |
| 28/05/20 | I7—Rovinj | 18.5 | Organs in EtOH and HCHO | 1/1 | 1/1 | Haplo + |
| 28/05/20 | I6—Vinkuranska vala | 17.8 | Organs in EtOH and HCHO | 1/1 | 0/1 | Haplo + |
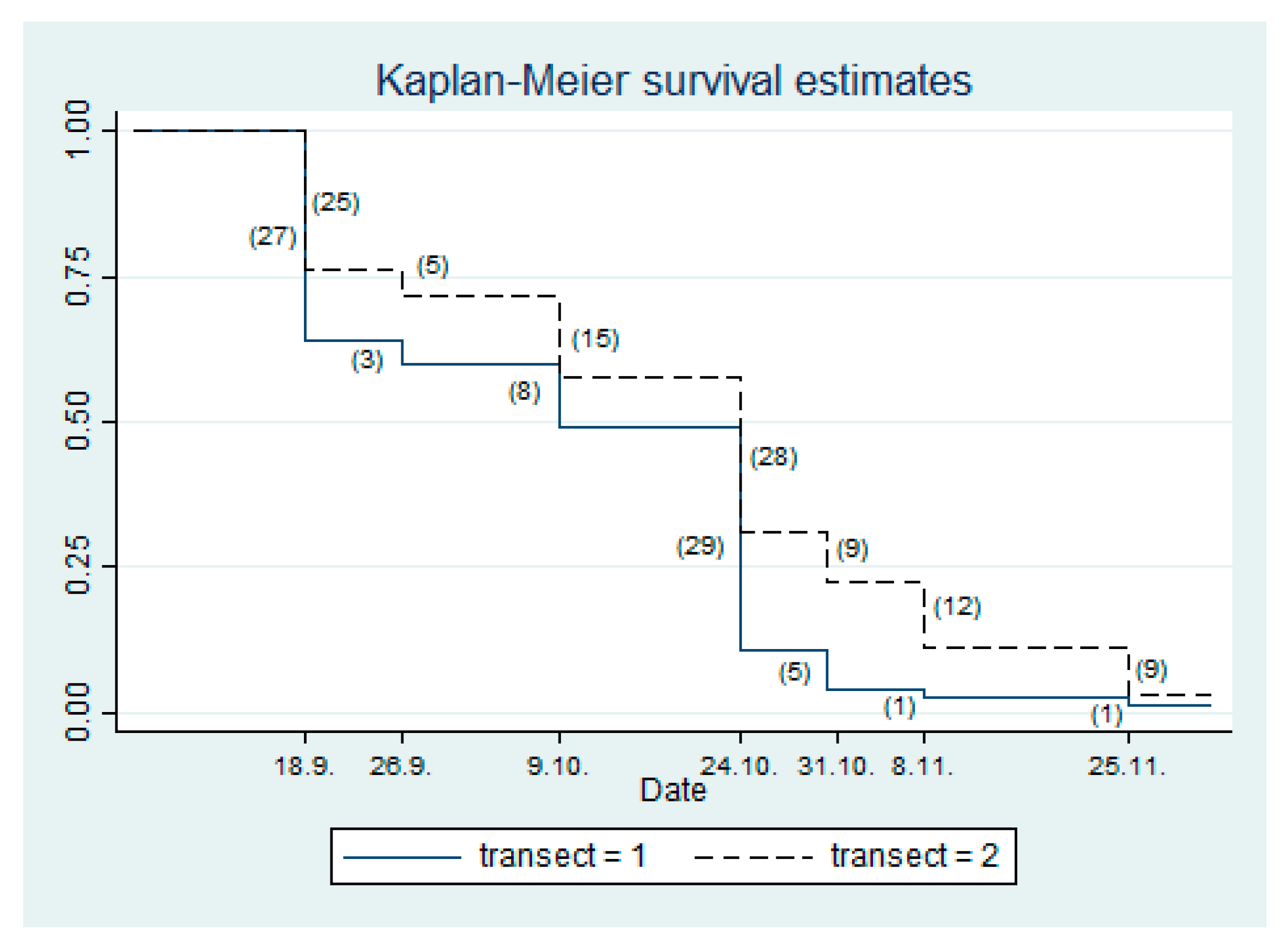
| Date | Number of Dead Noble Pen Shells found in the Small Lake | |
|---|---|---|
| Transect 1 (T1) | Transect 2 (T2) | |
| 4 September | 62 | 28 |
| 18 September | 27 | 25 |
| 26 September | 3 | 5 |
| 9 October | 8 | 15 |
| 24 October | 29 | 28 |
| 31 October | 5 | 9 |
| 8 November | 1 | 12 |
| 25 November | 1 | 9 |
| Total | 74/75 | 103/106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaljević, Ž.; Pavlinec, Ž.; Zupičić, I.G.; Oraić, D.; Popijač, A.; Pećar, O.; Sršen, I.; Benić, M.; Habrun, B.; Zrnčić, S. Noble Pen Shell (Pinna nobilis) Mortalities along the Eastern Adriatic Coast with a Study of the Spreading Velocity. J. Mar. Sci. Eng. 2021, 9, 764. https://doi.org/10.3390/jmse9070764
Mihaljević Ž, Pavlinec Ž, Zupičić IG, Oraić D, Popijač A, Pećar O, Sršen I, Benić M, Habrun B, Zrnčić S. Noble Pen Shell (Pinna nobilis) Mortalities along the Eastern Adriatic Coast with a Study of the Spreading Velocity. Journal of Marine Science and Engineering. 2021; 9(7):764. https://doi.org/10.3390/jmse9070764
Chicago/Turabian StyleMihaljević, Željko, Željko Pavlinec, Ivana Giovanna Zupičić, Dražen Oraić, Aleksandar Popijač, Osvin Pećar, Ivan Sršen, Miroslav Benić, Boris Habrun, and Snježana Zrnčić. 2021. "Noble Pen Shell (Pinna nobilis) Mortalities along the Eastern Adriatic Coast with a Study of the Spreading Velocity" Journal of Marine Science and Engineering 9, no. 7: 764. https://doi.org/10.3390/jmse9070764






