4.1. The Net N Balance
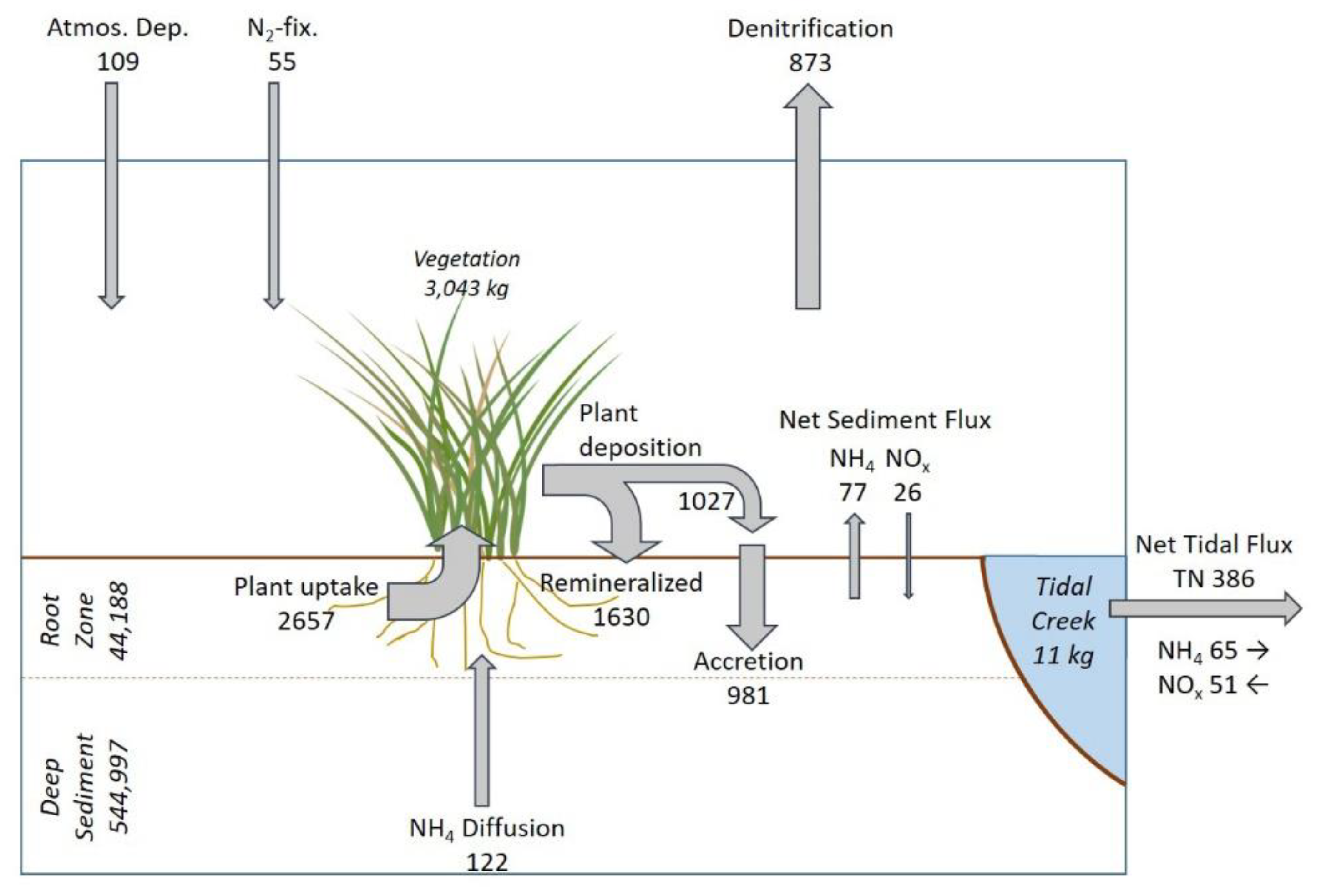
The dredged material used to construct the Cell 1B marsh contains a very large reservoir of N (
Figure 7). Most of this reservoir exists in the deeper soil below the root zone, where it is largely unavailable for direct plant uptake but provides a long-term source via diffusion of NH
4 into the root zone. Compared with the size of the sediment reservoir, only small amounts of N are being exported annually from the marsh. The largest internal N transfer is short-term uptake of NH
4 by macrophytic vegetation, leading to long-term accumulation of carbon-rich marsh soil and associated N. Denitrification is a major sink for N in this marsh, and while NH
4 fluxes from the sediment may be high, internal recycling appears to reduce losses to the estuary.
Our estimates show that the annual N export via denitrification, soil accumulation, and tidal flux were about 39%, 44%, and 17% of TN export, respectively. The rate of tidal TN export varied seasonally and, importantly for SAV, the highest export occurred during the colder months, following senescence of the marsh vegetation. Dissolved inorganic N (DIN) released from the marshes at this time of year is less likely to have a negative impact on SAV, which is largely dormant in the cold winter months in upper Chesapeake Bay [
41]. The seasonal variability in TN exchange between the marsh and estuary is driven by the influence of temperature on biogeochemical processes as well as the seasonality of the primary producers (macrophyte and edaphic algae). Uptake of dissolved N during the growing season is followed by export of dissolved and particulate N during the dormant season, in agreement with other studies [
40,
42,
43].
An examination of the balance of diffusive and new N inputs to the marsh (286 kg y
−1) and N exports as gaseous exchange and in tidal waters (1259 kg y
−1, not including soil accumulation) indicates that an important source term has not been directly quantified. This missing “source” of N, which would help balance inputs with export, is likely to result from remineralization of N associated with labile organic matter in the dredged material, primarily in the root zone where microbial activity is enhanced by rhizosphere oxygenation by
S. alterniflora [
44].
4.2. Internal N Cycling
Temporal variation in denitrification may have been influenced by variation in estuarine water column NO
3 concentrations, with the lowest rate occurring in September 2013 when NO
3 concentrations in the flood water were lowest, and higher rates occurring on other sampling dates when estuarine NO
3 concentrations were higher. However, the import of NO
3 represents only about 5% of N denitrified, suggesting that the source of most denitrified N is within the marsh. Coupled nitrification and denitrification may occur in the near surface sediment or in the macrophyte rhizosphere, where NH
4 and organic carbon are abundant and oxygen is present, and is likely enhanced due to rapid remineralization when temperatures rise in the spring. Limitation of denitrification due to inhibition by sulfide is unlikely to occur in these young marshes, where sulfide concentrations tend to be below detection in the first few years of development due to the presence of free iron resulting from pyrite oxidation during dewatering of the dredged material prior to marsh establishment [
16].
The root zone TN pool would meet the annual macrophyte N requirement for more than a decade, suggesting that the vegetation will not be N limited for many years under present conditions. However, porewater concentrations of dissolved NH
4, the preferred form of N for uptake by
S. alterniflora [
45], show depletion after only 2 years, suggesting that other sources of N are more available and energetically favorable. We estimate that diffusive transport of NH
4 from the deeper sediment into the root zone would supply only about 3% of annual macrophyte demand, while atmospheric deposition and N
2-fixation, would supply about 4% and 2%, respectively. Translocation at the end of the growing season would conserve a small percentage for the next season’s growth [
46]. However, remineralization resulting from plant decomposition, which returned about 61% of assimilated N over two years in this study, is likely to be an important source of N for macrophyte and algal growth, emphasizing the importance of internal N cycling in the overall N budget for the marsh. The estimate of plant N deposition (29.84 mg N m
−2 d
−1) based on plant production and decomposition data compares well with the estimate obtained from the measured rate of vertical accretion (28.50 mg N m
−2 d
−1), emphasizing the importance of internal cycling and the utility of measurements of vertical accretion for estimating soil N accumulation. Future studies of soil N trajectories will help characterize the rate of N depletion in the original substrate, N accumulation in accreting soil and organic matter, and how long N enriched conditions may continue to enhance macrophyte growth.
4.3. Sources of Error
Scaling up discreet measurements to derive annual estimates of nutrient fluxes is subject to several sources of error. Seasonal (temporal) variability in N fluxes may occur due to temperature regulation of biogeochemical processes as well as changes in plant uptake driven by phenology. Spatial variability can be high, and may be difficult to assess with limited sampling and the use of ex situ methods (e.g., core incubations). Furthermore, the use of monitoring data which were not designed for a mass balance study sometimes means that the most appropriate data for the analysis are not obtainable. In the study presented here we combined several sources of data to develop the mass balance, and discuss potential sources of error below.
Direct atmospheric N deposition is recognized as a significant source in some locations, including coastal systems [
47,
48], and direct and indirect deposition combined are estimated to contribute 8% of TN inputs in Chesapeake Bay [
49]. The estimate used here included only direct deposition on the marsh, because there is, currently, minimal upland drainage into the marshes. The actual catchment used to calculate deposition may be slightly larger due to runoff from the surrounding dikes and access roads. Additionally, the use of data from NADP/NTN site BWR139 rather than site MD13, which is closer to Poplar Island and has a higher average wet DIN deposition for the 2012–2014 period (3.66 kg N ha
−1 y
−1 versus 2.75 kg N ha
−1 y
−1, respectively), but lacks TN deposition data, may have led to an underestimate of the TN deposition rate. These two small sources of error would have opposing effects, and would not change the conclusion that atmospheric deposition is a small term in our N mass balance.
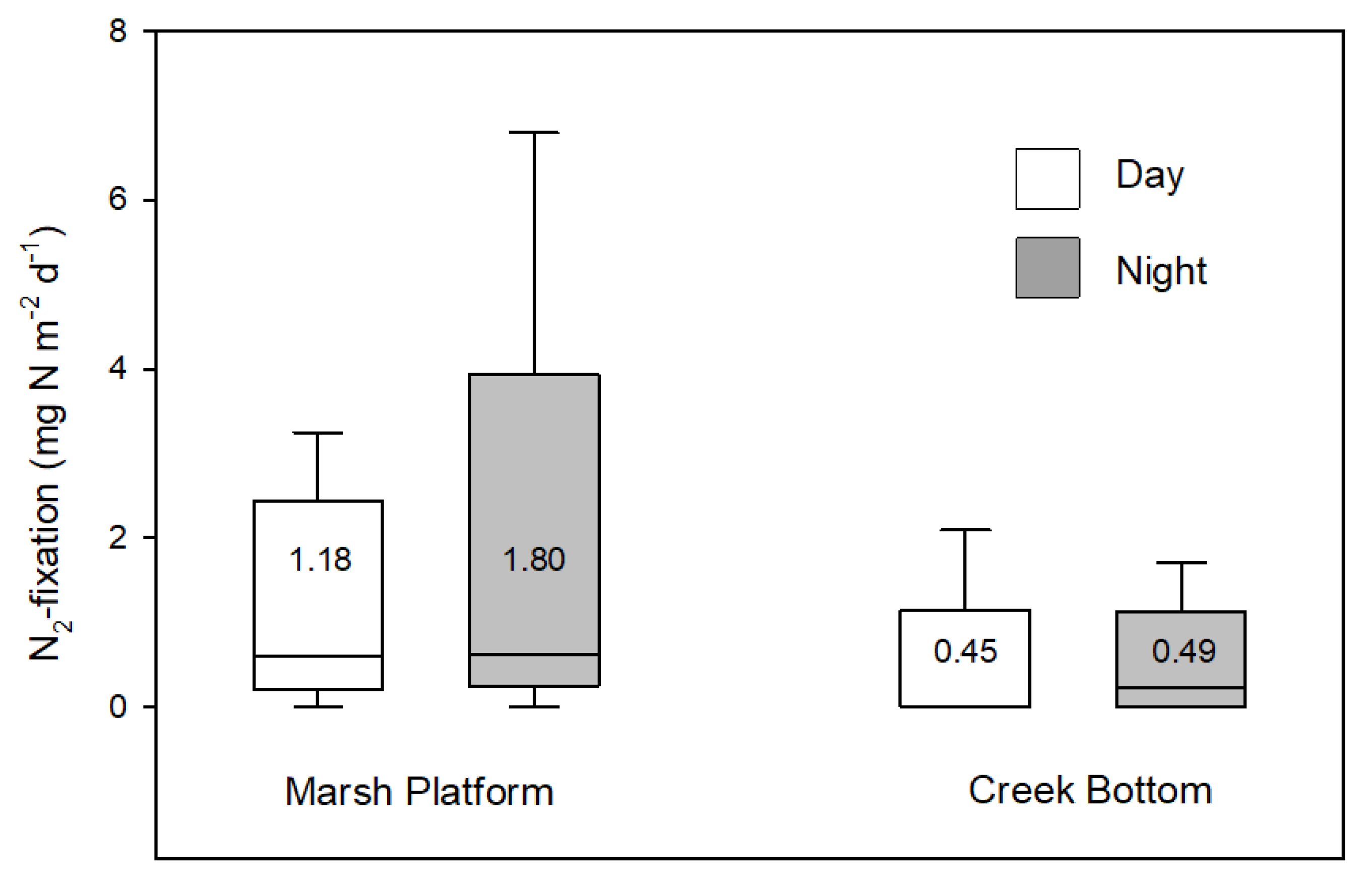
Nitrogen fixation rates were spatially variable but very low for both marsh platform and creek sediments, as expected in this N rich system. The estimate used here was based on measurements made during the summer, near the peak in seasonal variation [
18] and, therefore, likely overestimates the annual flux. The contribution to the N mass balance was minimal, however, and a more accurate annual rate would likely further reduce the estimate of N contributed by N
2-fixation.
Estimates of sediment N fluxes on the marsh platform may be biased upward due to underrepresentation of the warmest and coldest months of the year. On an annual basis, denitrification is still likely to represent the dominant sink in this two-year-old marsh, but estimates of annual fluxes of dissolved N could also be more accurately represented by evenly distributed seasonal measurements. Further improvements may be made by accounting for the effects of unflooded periods on the marsh platform. During periods in which the soil is unflooded, pore water constituents can accumulate near the soil surface; continuous flooding during incubations may result in measured fluxes that overestimate the actual fluxes because of depuration of the stored nutrient concentrations during flooding. This may result in the daily assignment of rates that reflect gradients that are too large over too long a period of time. In addition, estimates of NH4+ and NOx for the creeks may be a more accurate reflection of actual rates than estimates for the marsh surface, which is not continuously flooded.
The calculation of the upward diffusive flux of pore water NH
4+ utilizes average pore water values from a single date; these gradients change over time due to pore water depletion of NH
4+ in both the upper (0–10 cm) and the deep (20–47 cm) soil layers [
16].
While the N standing stock and fluxes associated with edaphic algae were not quantified in this study, this community can play an important role in internal biogeochemical cycles and food webs [
50,
51]. A thick layer of edaphic algae has been observed on the sediment surface in the Poplar Island marshes and may create a boundary which limits oxygen penetration into the sediment. As a result, anoxic conditions appear to occur on the sediment surface immediately beneath the algal layer during the warmer months of the year. Decomposition of algal and macrophyte tissue in this layer likely leads to consumption of oxygen and remineralization of organic N, and may enhance denitrification. Further study of the role of edaphic algae in nutrient cycling in these nutrient rich marshes would likely enhance our understanding of internal nutrient processing.
Variations in tidal prism caused by subtidal (time scales longer than a tidal cycle) water level changes can be a large source of error in tidal flux estimates [
52], as these changes impact the degree to which the marsh plain is flooded (or not). In this study, water level measured seasonally over two tidal cycles was considered representative of each of the full seasons. The tide ranges recorded during each deployment were within the great diurnal tide range for Poplar Island [
53], but a more careful analysis of seasonal water levels would indicate how typically the tidal prisms used here compared with seasonal averages.
Nitrogen fluxes associated with transient nekton species have received less attention than carbon fluxes, but can be significant in some systems [
54]. Large nekton migrations (e.g., penaid shrimp) do not appear to occur in the Poplar Island marshes [
55], but foraging by small fish in the tidal creeks and on the marsh platform, which may be consumed by predatory fish or birds, has been observed. Although trophic relay is not expected to be a major N flux in this young marsh, quantification of TN fluxes associated with the fauna would help refine the mass balance.
The scale of the N flux estimates presented here suggests that modest errors would not change the conclusions that, in this young marsh, denitrification was a large, permanent N sink, internal N cycling was intense, and there was a modest TN but minimal DIN export to the estuary.
4.4. Comparison with Previous Studies
Atmospheric exchange of gaseous N
2 with the sediment is attributed to microbially mediated denitrification and, possibly, anaerobic ammonium oxidation (ANAMMOX), as well as N
2 fixation. High rates of ANAMMOX occur in some coastal environments, but are low where NO
3 is low and organic carbon is abundant [
56,
57], including tidal marshes, and is therefore unlikely to represent a significant fraction of the N
2 fluxes measured here. Thus, the annual net N
2 (21.31 mg N m
−2 d
−1), assumed to represent denitrification, is on the higher end of the range reported for denitrification in most natural marshes in a recent review of saltmarsh biogeochemistry (0–27 mg N m
−2 d
−1), although rates up to 279 mg N m
−2 d
−1 have been reported [
39,
58]. Nitrogen input via N
2-fixation (1.33 mg N m
−2 d
−1) is a small fraction of denitrification, and on the low end of the range reported for mature marshes, <1.4–18.6 mg N m
−2 d
−1 [
58]. N
2-fixation in the Cell 1B marsh is very low compared with rates reported for restored and young marshes, which can range up to 95.9 mg N m
−2 d
−1 [
18]. The low rates in this young marsh likely result from high N availability in the fine-grained dredged material, and emphasize that chronological age is not always a good indicator of marsh function [
59].
The estimate of soil N accumulation (28.5 mg N m
−2 y
−1) is within the range of values reported for natural marshes using a variety of techniques [
42,
60,
61]. It is notable that this rate, derived from the rate of vertical accretion measured with SETs and soil N density, is similar to the estimate that can be derived from the difference between plant uptake and remineralization based on annual biomass and decomposition measurements (27.9 mg N m
−2 d
−1). Due to the high rate of macrophyte biomass production and the observed retention of biomass on the marsh platform, and low inorganic sediment inputs at this site, organic matter is the primary contributor to vertical accretion and N accumulation is largely associated with organic matter accumulation, similar to many natural marshes [
62,
63].
The mean annual TN flux via tidal exchange (9.42 mg N m
−2 d
−1) is of the same order of magnitude reported for several U. S.
S. alterniflora marshes [
64]. Although the timing of sediment and tidal flux measurements were not synchronized, the direction of tidal exchange of dissolved N estimated in the tidal flux study is largely supported by the sediment flux measurements, which are of similar magnitude and direction.
Finally, our estimate of atmospheric TN deposition is similar to other estimates reported for Chesapeake Bay [
47,
65,
66,
67], and is consistent with the dominant agricultural land use in the region [
67].