Benthic Diatoms on Sheltered Coastal Soft Bottoms (Baltic Sea)—Seasonal Community Production and Respiration
Abstract
:1. Introduction
2. Materials and Methods
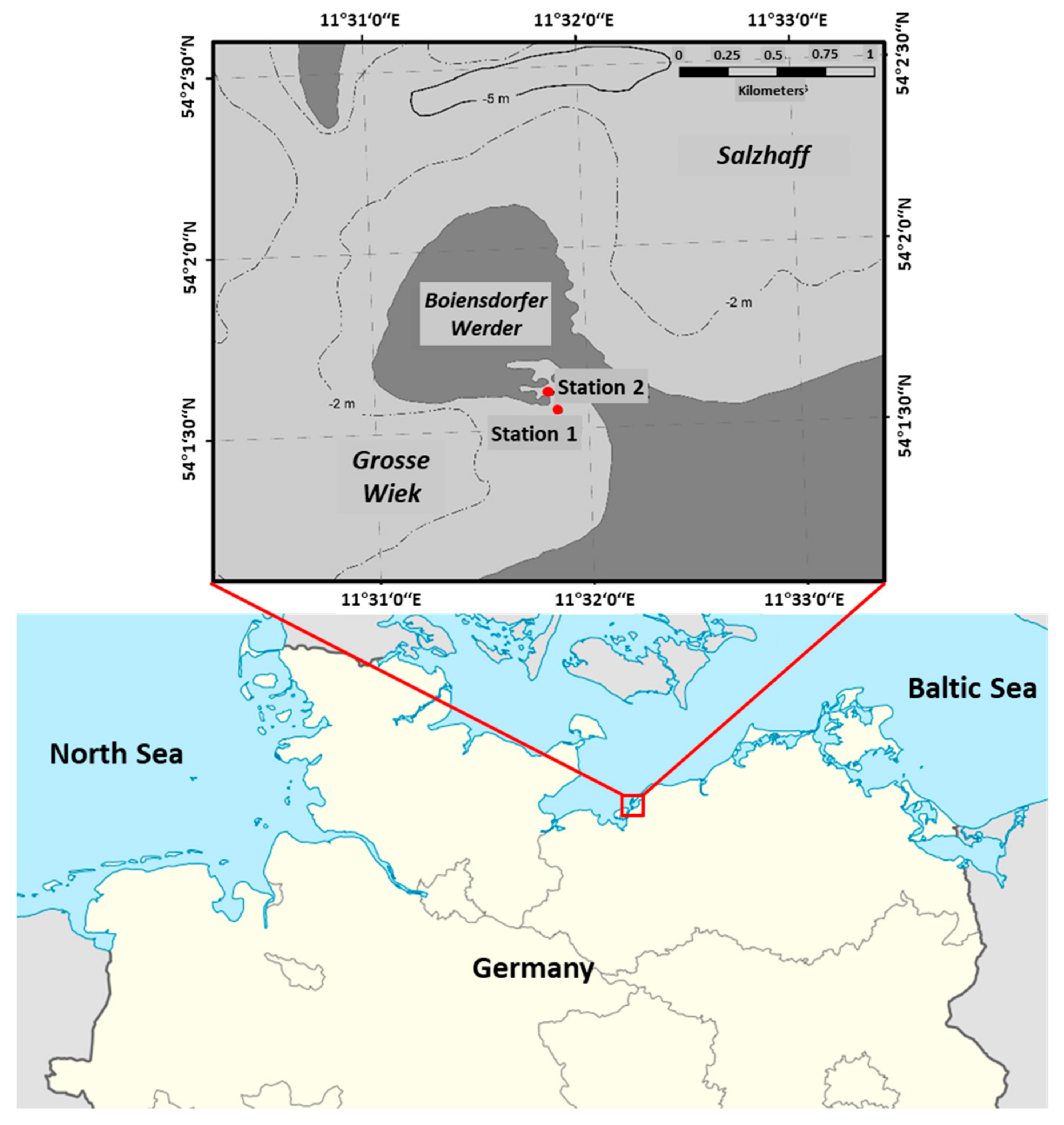
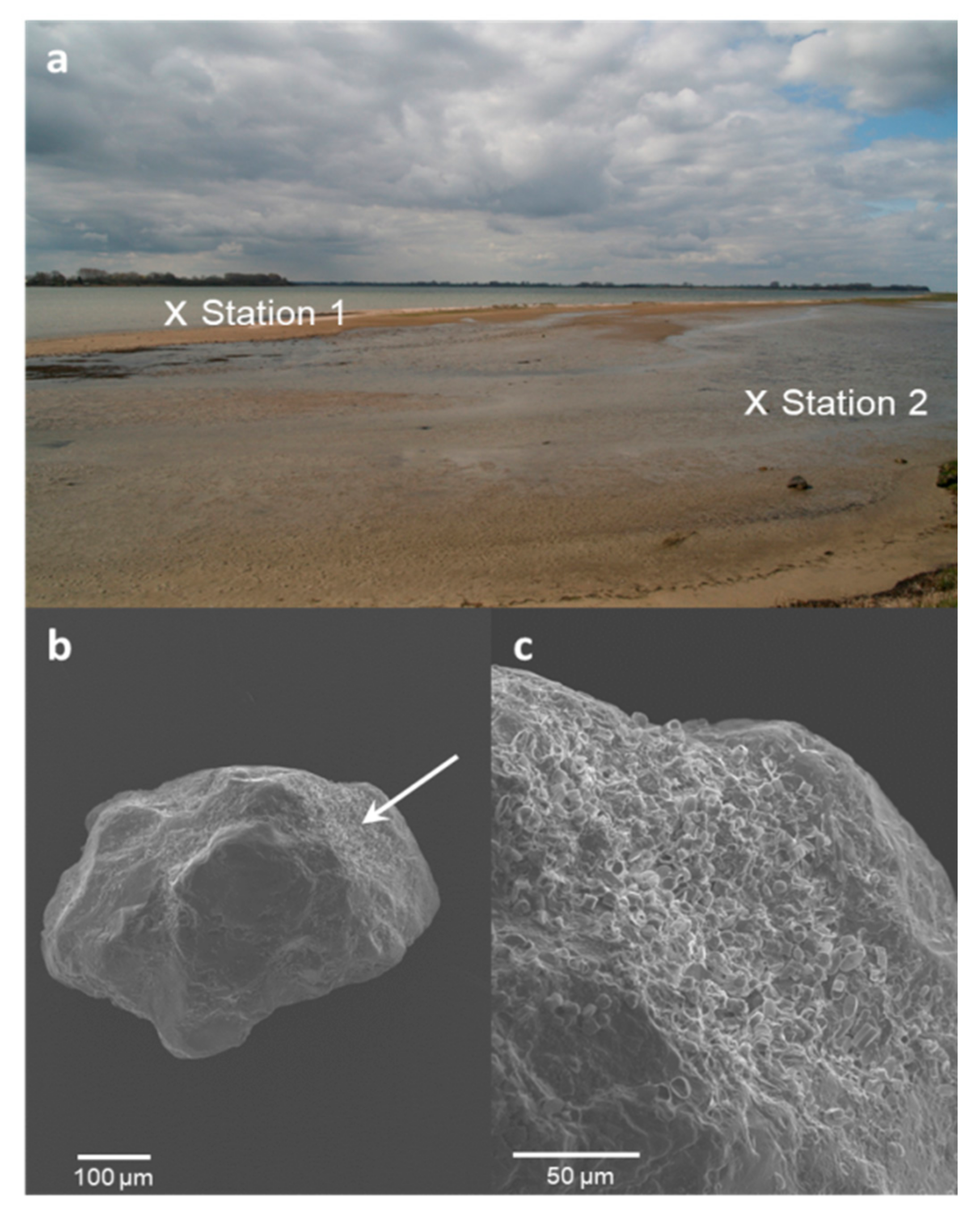
2.1. Site Characteristics and Sampling
2.2. Biomass and Sediment Properties
2.3. Primary Production and Respiration
2.4. Diatom Visualization Using Scanning Electron Microscopy
2.5. Statistical Data Treatment
3. Results
3.1. Abiotic Conditions and Sediment Properties
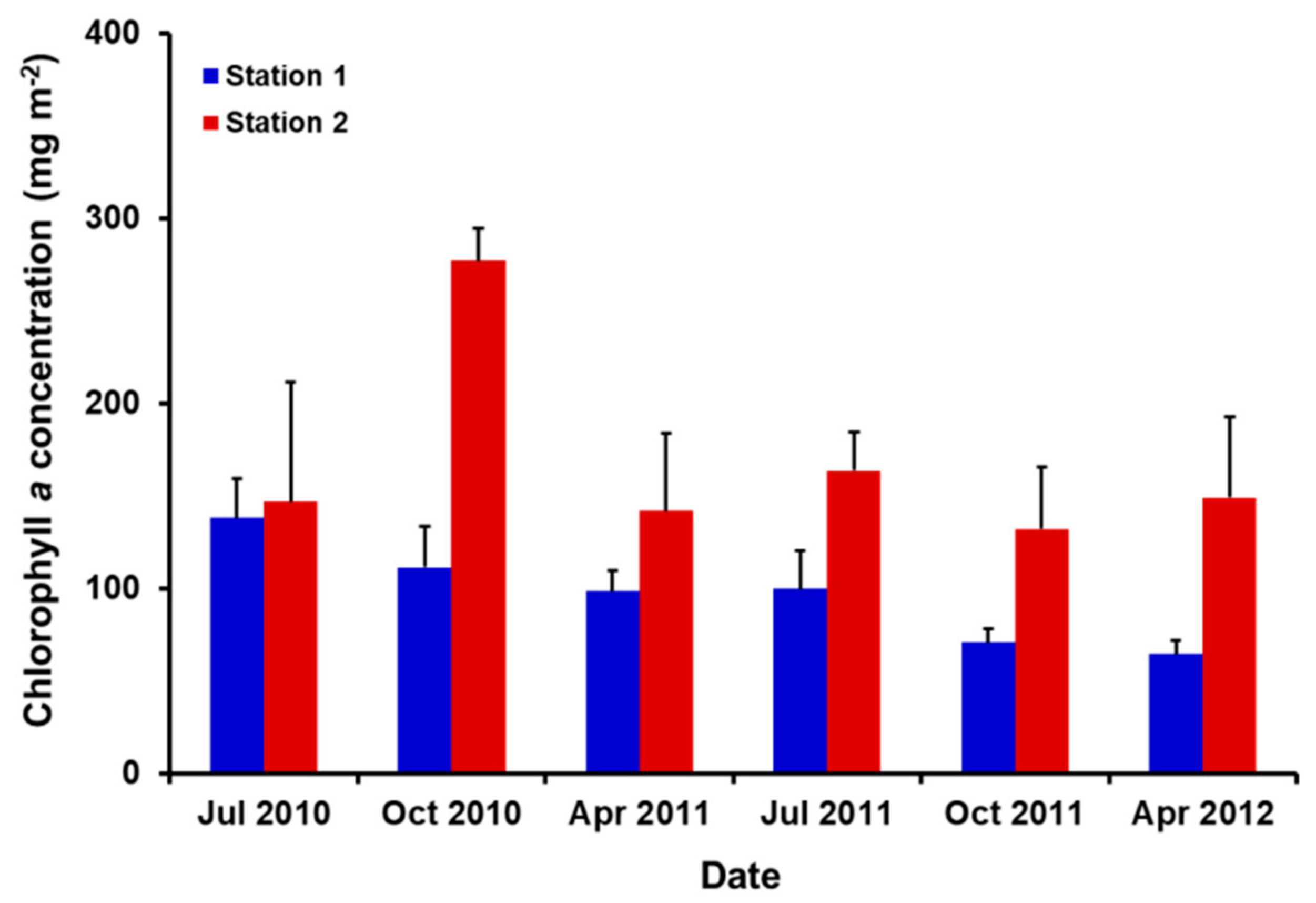
3.2. Biomass of Benthic Diatoms
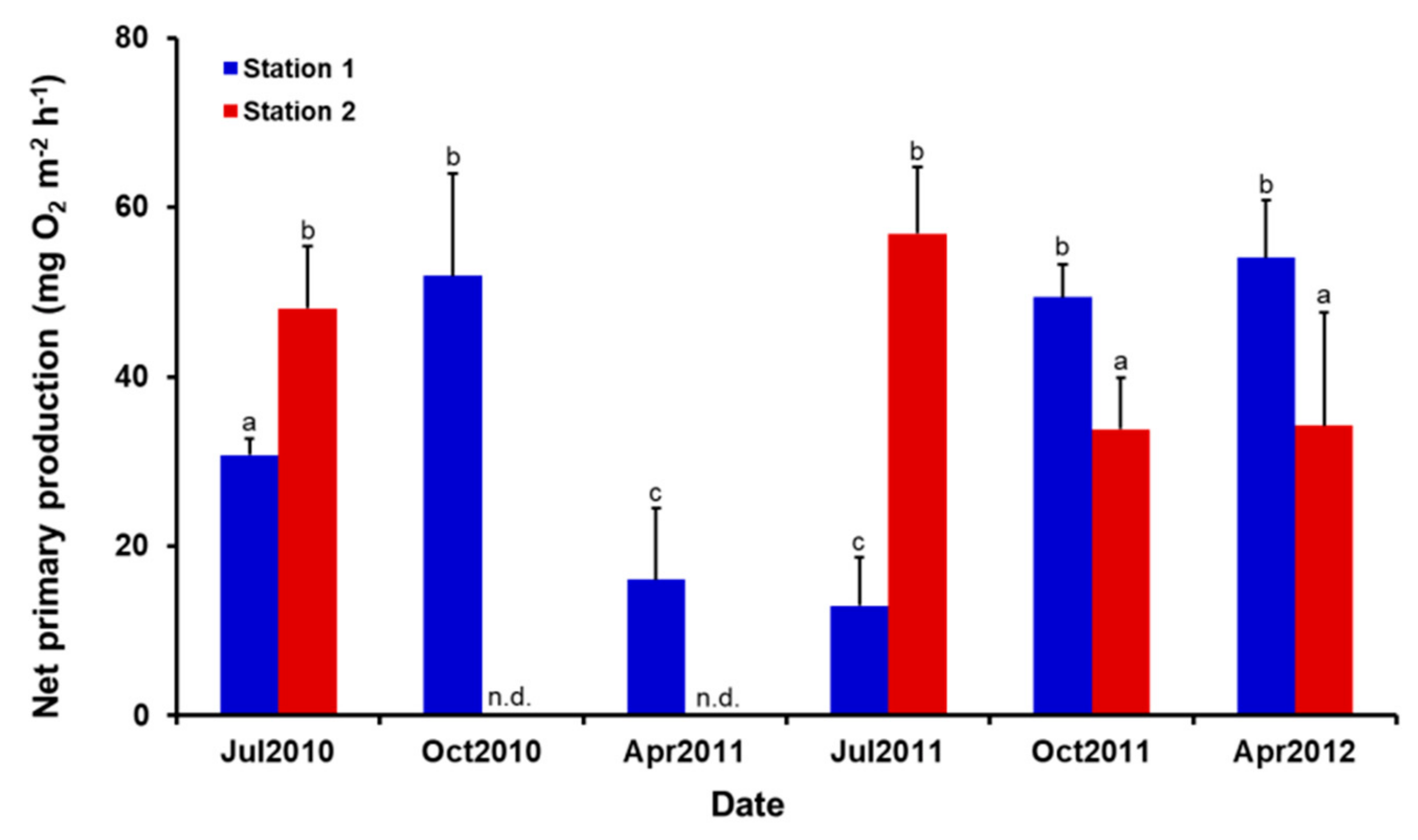
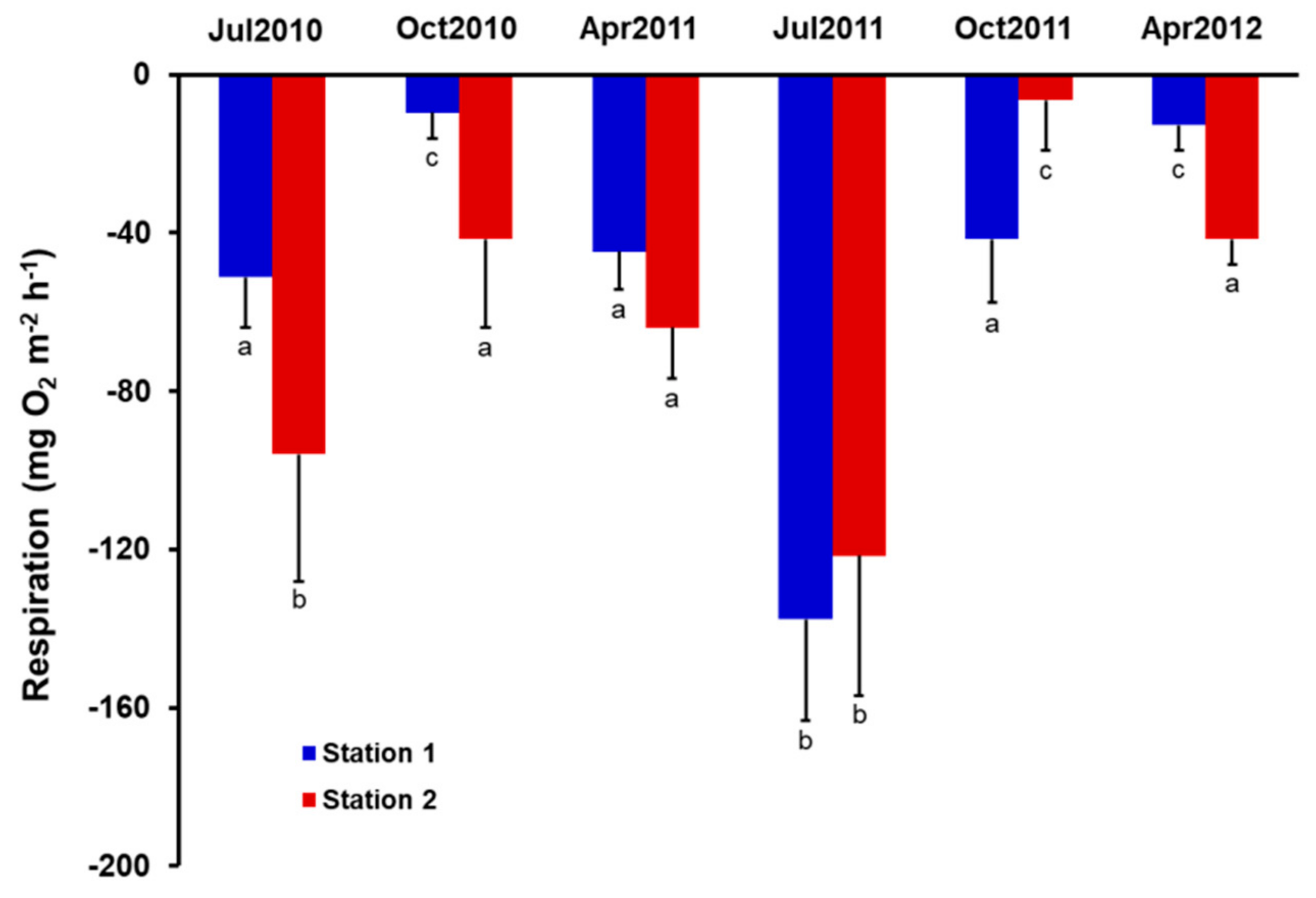
3.3. Benthic Diatom Primary Production and Respiration
3.4. Benthic Diatoms Attached to Sand Grains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurasinski, G.; Janssen, M.; Voss, M.; Böttcher, M.E.; Brede, M.; Burchard, H.; Forster, S.; Gosch, L.; Gräwe, U.; Gründling-Pfaff, S.; et al. Understanding the coastal ecocline: Assessing sea-land-interactions at non-tidal, low-lying coasts through interdisciplinary research. Front. Mar. Sci. 2019, 5, 342. [Google Scholar] [CrossRef] [Green Version]
- Wolski, T.; Wiśniewski, B. Long-term, seasonal and short-term fluctuations in the water level of the southern Baltic Sea. Quaest. Geogr. 2014, 33, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Lass, H.U.; Magaard, L. Wasserstandsschwankungen und Seegang. In Meereskunde der Ostsee; Rheinheimer, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 68–74. [Google Scholar]
- Schwarzer, K. Dynamik der Küste 1996. In Meereskunde der Ostsee; Rheinheimer, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 25–33. [Google Scholar]
- Karsten, U.; Baudler, H.; Himmel, B.; Jaskulke, R.; Ewald, H.; Schumann, R. Short-term measurements of exposure and inundation of sediment areas in a tide-less wind flat system at the Southern Baltic Sea coast. J. Mar. Syst. 2012, 105–108, 187–193. [Google Scholar] [CrossRef]
- Cahoon, L.B. The role of benthic microalgae in neritic ecosystems. Oceanogr. Mar. Biol. 1999, 37, 47–86. [Google Scholar]
- Ask, J.; Rowe, O.; Brugel, S.; Strömgren, M.; Byström, P.; Andersson, A. Importance of coastal primary production in the northern Baltic Sea. Ambio 2016, 45, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Middleburg, J.J.; Barranguet, C.; Boschker, H.T.S.; Herman, P.M.J.; Moens, T.; Heip, C.H.R. The fate of intertidal microphytobenthos carbon: An in situ 13C-labeling study. Limnol. Oceanogr. 2000, 5, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- Oakes, J.M.; Connolly, R.M.; Revill, A.T. Isotope enrichment in mangrove forests separates microphytobenthos and detritus as carbon sources for animals. Limnol. Oceanogr. 2010, 55, 393–402. [Google Scholar] [CrossRef]
- Aslam, S.N.; Cresswell-Maynard, T.; Thomas, D.N.; Underwood, G.J.C. Production and characterization of the intra- and extracellular carbohydrates and polymeric substances (EPS) of three sea-ice diatom species, and evidence for a cryoprotective role for EPS. J. Phycol. 2012, 48, 1494–1509. [Google Scholar] [CrossRef]
- De Brouwer, J.F.C.; Wolfstein, K.; Ruddy, G.K.; Jones, T.E.R.; Stal, L.J. Biogenic stabilization of intertidal sediments: The importance of extracellular polymeric substances produced by benthic diatoms. Microb. Ecol. 2005, 49, 501–512. [Google Scholar] [CrossRef]
- Risgaard-Petersen, N.; Rysgaard, S.; Nielsen, L.P.; Revsbech, N.P. Diurnal variation of denitrification and nitrification in sediments colonized by benthic microphytes. Limnol. Oceanogr. 1994, 39, 573–579. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthörn tidal flat (southern North Sea) in relation to varying culture conditions. Diatom Res. 2012, 27, 65–73. [Google Scholar] [CrossRef]
- Fredriksen, S.; Karsten, U.; Bartsch, I.; Woelfel, J.; Koblowsky, M.; Schumann, R.; Moy, S.; Steneck, B.; Hop, H.; Wiktor, J.; et al. Biodiversity of benthic micro- and macroalgae from Svalbard with special focus on Kongsfjorden. In The Ecosystem of Kongsfjorden, Svalbard; Advances in Polar Ecology; Hop, H., Wiencke, C., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 331–371. [Google Scholar]
- Kuriyama, K.; Gründling-Pfaff, S.; Diehl, N.; Woelfel, J.; Karsten, U. Microphytobenthic primary production on exposed coastal sandy sediments of the Southern Baltic Sea using ex-situ sediment cores and oxygen optodes. Oceanologia 2021, 63, 247–260. [Google Scholar] [CrossRef]
- Woelfel, J.; Schumann, R.; Adler, S.; Hübener, T.; Karsten, U. Diatoms inhabiting a wind flat of the Baltic Sea: Species diversity and seasonal succession. Estuar. Coast. Shelf Sci. 2007, 75, 296–307. [Google Scholar] [CrossRef]
- Sabbe, K. Short-term fluctuations in benthic diatom numbers on an intertidal sandflat in the Westerschelde estuary (Zeeland, The Netherlands). Hydrobiologia 1993, 269/270, 275–284. [Google Scholar] [CrossRef]
- Orvain, F.; Lefebvre, S.; Montepini, J.; Sébire, M.; Gangnery, A.; Sylvand, B. Spatial and temporal interaction between sediment and microphytobenthos in a temperate estuarine macro-intertidal bay. Mar. Ecol. Prog. Ser. 2012, 58, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Daggers, T.D.; Kromkamp, J.C.; Herman, P.M.J.; van der Wal, D. A model to assess microphytobenthic primary production in tidal systems using satellite remote sensing. Remote Sens. Environ. 2018, 211, 129–145. [Google Scholar] [CrossRef]
- Frankenbach, S.; Ezequiel, J.; Plecha, S.; Goessling, J.W.; Vaz, L.; Kühl, M.; Dias, J.M.; Vaz, N.; Serôdio, J. Synoptic spatio-temporal variability of the photosynthetic productivity of microphytobenthos and phytoplankton in a tidal estuary. Front. Mar. Sci. 2020, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Wasmund, N. Ecology and bioproduction in the microphytobenthos of the chain of shallow inlets (Boddens) south of the Darss-Zingst Peninsula (Southern Baltic Sea). Int. Rev. Ges. Hydrobiol. 1986, 71, 153–178. [Google Scholar] [CrossRef]
- Yap, H.T. Benthic energy dynamics in a southern Baltic ecosystem. Mar. Biol. 1991, 108, 477–484. [Google Scholar] [CrossRef]
- Meyercordt, J.; Meyer-Reil, L.A. Primary production of benthic microalgae in two shallow coastal lagoons of different trophic status in the southern Baltic Sea. Mar. Ecol. Prog. Ser. 1999, 178, 179–191. [Google Scholar] [CrossRef]
- Urban-Malinga, B.; Wiktor, J. Microphytobenthic primary production along a non-tidal sandy beach gradient: An annual study from the Baltic Sea. Oceanologia 2003, 45, 705–720. [Google Scholar]
- Woelfel, J.; Schoknecht, A.; Schaub, I.; Enke, N.; Schuhmann, R.; Karsten, U. Growth and photosynthesis characteristics of three benthic diatoms from the brackish southern Baltic Sea in relation to varying environmental conditions. Phycologia 2014, 53, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls A, B, C1 and C2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Rohde, K.H.; Nehring, D. Ausgewählte Methoden zur Bestimmung von Inhaltsstoffen im Meer- und Brackwasser. In Reihe IV/27, Physik der flüssigen Erde; Nationalkomitee Für Geodäsie und Geophysik der Akademie der Wissenschaften der DDR: Berlin, Germany, 1979; pp. 31–37. [Google Scholar]
- Malcom-Lawes, D.J.; Wong, K.H. Determination of orthophosphate in water and soil using a flow analyzer. Analyst 1990, 115, 65–67. [Google Scholar] [CrossRef]
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Verlag Chemie: Weinheim, Germany, 1983; pp. 159–226. [Google Scholar]
- Kühl, M.; Polerecky, L. Functional and structural imaging of phototrophic microbial communities and symbioses. Aquat. Microb, Ecol. 2008, 53, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Warkentin, M.; Freese, H.; Karsten, U.; Schumann, R. New and fast method to quantify respiration rates of bacterial and plankton communities in freshwater ecosystems by using optical oxygen sensor spots. Appl. Environ. Microbiol. 2007, 73, 6722–6729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woelfel, J.; Schumann, R.; Peine, F.; Flohr, A.; Kruss, A.; Tegowski, J.; Blondel, P. Microphytobenthos of Arctic Kongsfjorden (Svalbard, Norway): Biomass and potential primary production along the shore line. Polar Biol. 2010, 33, 1239–1253. [Google Scholar] [CrossRef] [Green Version]
- Hargrave, B.T.; Prouse, N.J.; Phillips, G.A.; Neame, P.A. Primary production and respiration in the pelagic and benthic communities at two intertidal sites in the upper Bay of Fundy. Can. J. Fish. Aquat. Sci. 1983, 40, 229–243. [Google Scholar] [CrossRef]
- Levene, H.; Olkin, I.; Hotelling, H. Robust tests for equality of variances. In Contributions to Probability and Statistics. Essays in Honor of Harold Hotelling; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 78–92. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Webb, W.L.; Newton, M.; Starr, D. Carbon dioxide exchange of Alnus rubra—A mathematical model. Oecologia 1974, 17, 281–291. [Google Scholar] [CrossRef]
- HELCOM. Available online: https://helcom.fi/wp-content/uploads/2020/07/BSEFS-Sea-Surface-Temperature-in-the-Baltic-Sea-2018.pdf (accessed on 31 August 2021).
- Bernt, A. Trophische Beziehungen Zwischen Benthischen Diatomeen (Bacillariophyceae) und Amphichaeta sannio (Oligochaeta, Naididae). PhD Dissertation, Universität Rostock, Rostock, Germany, 2001; p. 111. [Google Scholar]
- Lehmann, A.; Myrberg, K. Upwelling in the Baltic Sea—A review. J. Mar. Syst. 2008, 74, 3–12. [Google Scholar] [CrossRef]
- Prelle, L.R.; Graiff, A.; Gründling-Pfaff, S.; Sommer, V.; Kuriyama, K.; Karsten, U. Photosynthesis and respiration of Baltic Sea benthic diatoms to changing environmental conditions and growth responses of selected species as affected by an adjacent peatland (Hütelmoor). Front. Microbiol. 2019, 10, 1500. [Google Scholar] [CrossRef]
- Ezequiel, J.; Laviale, M.; Frankenbach, S.; Cartaxana, P.; Serôdio, J. Photoacclimation state determines the photobehaviour of motile microalgae: The case of a benthic diatom. J. Exp. Mar. Biol. Ecol. 2015, 468, 11–20. [Google Scholar] [CrossRef]
- Cartaxana, P.; Ruivo, M.; Hubas, C.; Davidson, I.; Serôdio, J.; Jesus, B. Physiological versus behavioral photoprotection in intertidal epipelic and epipsammic benthic diatom communities. J. Exp. Mar. Biol. Ecol. 2011, 405, 120–127. [Google Scholar] [CrossRef]
- Glud, R.N.; Kühl, M.; Wenzhöfer, F.; Rysgaard, S. Benthic diatoms of a high Arctic fjord (Young Sound, NE Greenland): Importance for ecosystem primary production. Mar. Ecol. Prog. Ser. 2002, 238, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Richardson, K.; Beardall, J.; Raven, J.A. Adaptation of unicellular algae to irradiance: An analysis of strategies. New Phytol. 1983, 93, 157–191. [Google Scholar] [CrossRef]
- Billerbeck, M.; Roy, H.; Bosselmann, K.; Huettel, M. Benthic photosynthesis in submerged Wadden Sea intertidal flats. Estuar. Coast. Shelf Sci. 2007, 71, 704–716. [Google Scholar] [CrossRef]
- Colijn, F.; De Jonge, V.N. Primary production of microphytobenthos in the Ems-Dollard Estuary. Mar. Ecol. Prog. Ser. 1984, 14, 185–196. [Google Scholar] [CrossRef]
- Schiewer, U. Ecology of Baltic Coastal Waters; Ecological Studies 197; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Borck, D.; Frenzel, P. Micro-habitats of brackish water ostracods from Poel Island, southern Baltic Sea coast. Senck. Marit. 2006, 36, 99–107. [Google Scholar] [CrossRef]
| Station | Depth | Month, Year | Temperature | Salinity | Median | Water | Organic | C:N ratio | |
|---|---|---|---|---|---|---|---|---|---|
| No. | (m) | (°C) | (SA) | Grain Size | Content | Content | Content | ||
| (µm) | (% FW) | (mg g−1 DW) | |||||||
| Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| 1 | 0.11–0.49 | Jul 2010 | 25 | 10 | 131 | 31.0 ± 3.0 a | 11.3 ± 2.3 a | 7.9 ± 0.2 a | |
| Oct 2010 | 9.1 | 13.4 | 36.5 ± 1.0 b | 25.0 ± 1.8 b | 8.3 ± 0.6 a | ||||
| Apr 2011 | 12.9 | 12.6 | 27.6 ± 4.2 a | 17.4 ± 1.7 c | 8.6 ± 0.3 a | ||||
| Jul 2011 | 23.3 | 11.8 | 33.2 ± 2.2 a | 15.8 ± 0.8 c | 7.8 ± 0.2 a | ||||
| Oct 2011 | 13 | 11.4 | 25.8 ± 0.8 c | 7.9 ± 0.4 d | 7.4 ± 0.4 b | ||||
| Apr 2012 | 7.8 | 12.4 | 31.1 ± 1.2 a | 12.9 ± 1.6 a | 8.0 ± 0.3 a | ||||
| Mean | 30.9 ± 3.8 A | 15.1 ± 5.9 A | 8.0 ± 0.4 A | ||||||
| 2 | 0.02–0.12 | Jul 2010 | 26.1 | 10.7 | 138 | 44.0 ± 3.9 c | 19.9 ± 3.1 c | 8.1 ± 0.2 a | |
| Oct 2010 | 12.2 | 13.4 | 37.3 ± 3.6 b | 21.1 ± 2.4 c | 7.7 ± 0.1 a | ||||
| Apr 2011 | 16.9 | 12.5 | 37.3 ± 2.4 b | 19.4 ± 2.1 c | 7.5 ± 0.4 a | ||||
| Jul 2011 | 25.3 | 12.2 | 43.6 ± 0.9 c | 23.3 ± 0.9 d | 7.5 ± 0.2 a | ||||
| Oct 2011 | 13.1 | 11.2 | 42.0 ± 2.6 c | 20.1 ± 1.8 c | 7.5 ± 0.4 a | ||||
| Apr 2012 | 9.9 | 12.1 | 45.1 ± 4.2 c | 26.5 ± 1.1 d | 7.2 ± 0.3 b | ||||
| Mean | 41.6 ± 3.4 B | 21.7 ± 2.8 A | 7.6 ± 0.3 A | ||||||
| Station No. | DIN (µmol L−1) | SRP (µmol L−1) | Silicate (µmol L−1) |
|---|---|---|---|
| 1 | 54–212 | 5–31 | 11–113 |
| 2 | 81–361 | 7–51 | 19–134 |
| Surface water | 9–26 | 0.5–2.2 | 2–53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karsten, U.; Kuriyama, K.; Hübener, T.; Woelfel, J. Benthic Diatoms on Sheltered Coastal Soft Bottoms (Baltic Sea)—Seasonal Community Production and Respiration. J. Mar. Sci. Eng. 2021, 9, 949. https://doi.org/10.3390/jmse9090949
Karsten U, Kuriyama K, Hübener T, Woelfel J. Benthic Diatoms on Sheltered Coastal Soft Bottoms (Baltic Sea)—Seasonal Community Production and Respiration. Journal of Marine Science and Engineering. 2021; 9(9):949. https://doi.org/10.3390/jmse9090949
Chicago/Turabian StyleKarsten, Ulf, Kana Kuriyama, Thomas Hübener, and Jana Woelfel. 2021. "Benthic Diatoms on Sheltered Coastal Soft Bottoms (Baltic Sea)—Seasonal Community Production and Respiration" Journal of Marine Science and Engineering 9, no. 9: 949. https://doi.org/10.3390/jmse9090949
APA StyleKarsten, U., Kuriyama, K., Hübener, T., & Woelfel, J. (2021). Benthic Diatoms on Sheltered Coastal Soft Bottoms (Baltic Sea)—Seasonal Community Production and Respiration. Journal of Marine Science and Engineering, 9(9), 949. https://doi.org/10.3390/jmse9090949






