Acidification and Deoxygenation of the Northwestern Japan/East Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Analytical Methods
2.3. Data Processing
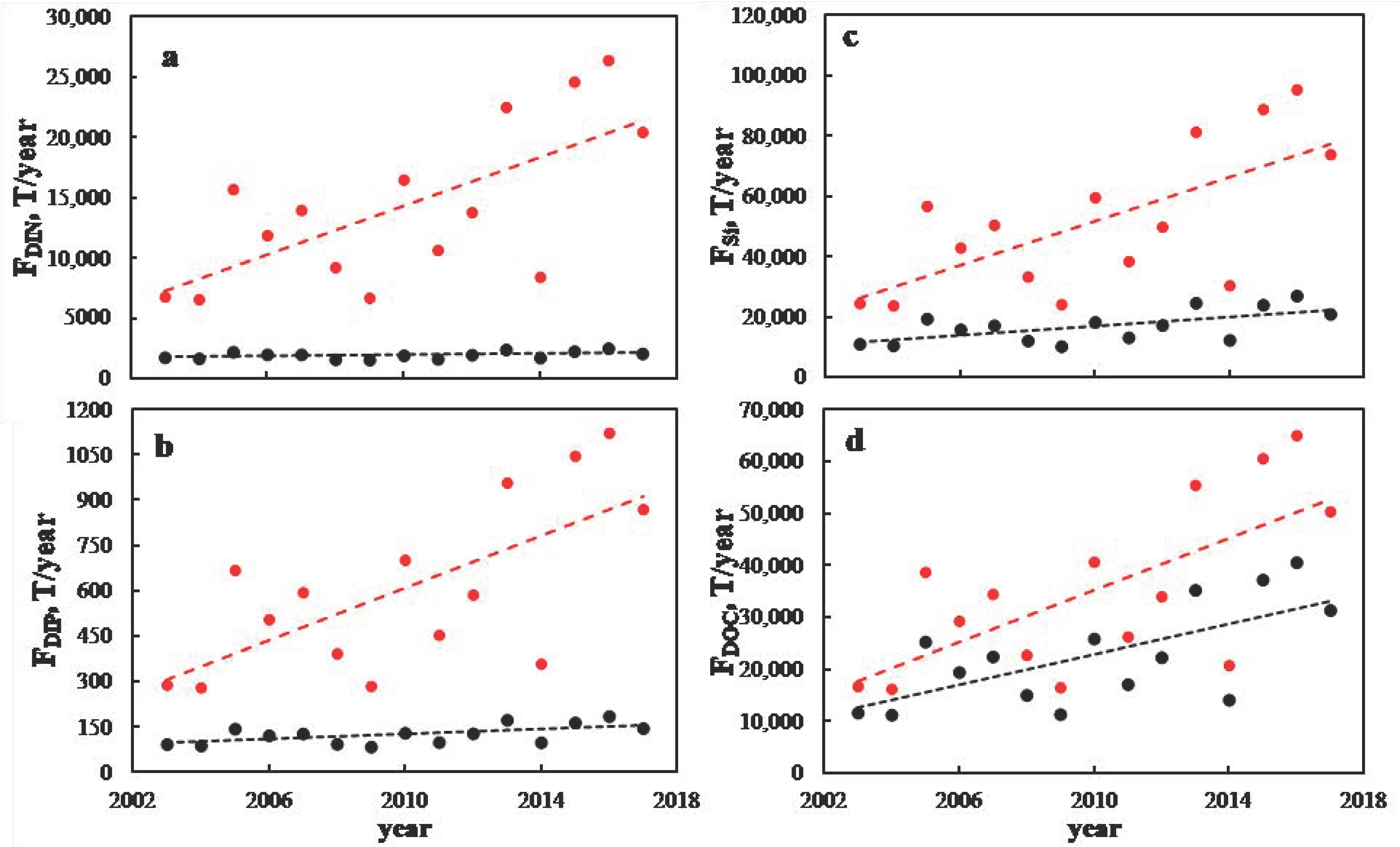
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talley, L.; Min, D.-H.; Lobanov, V.; Luchin, B.A.; Ponomarev, V.I.; Salyuk, A.; Shcherbina, A.; Tishchenko, P.; Zhabin, I. Japan/East Sea Water Masses and their Relation to the Sea’s Circulation. Oceanography 2006, 19, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Talley, L.D.; Lobanov, V.; Ponomarev, V.; Salyuk, A.; Tishchenko, P.; Zhabin, I.; Riser, S. Deep convection and brine rejection in the Japan Sea. Geophys. Res. Lett. 2003, 30, 1159. [Google Scholar] [CrossRef] [Green Version]
- Gamo, T.; Nozaki, Y.; Sakai, H.; Nakai, T.; Tsubota, H. Spatial and temporal variations of water characteristics in the Japan Sea bottom layers. J. Mar. Res. 1986, 44, 781–793. [Google Scholar] [CrossRef]
- Kim, K.-R.; Kim, K. What is happening in the East Sea (Japan Sea)?: Recent chemical observations from CREAMS 93–96. J. Korean Soc. Oceanogr. 1996, 31, 164–172. [Google Scholar]
- Kim, K.-R.; Kim, K.; Kang, D.-J.; Park, S.Y.; Park, M.-K.; Kim, Y.-G.; Min, H.-S.; Min, D. The East Sea (Japan Sea) in change: A story of dissolved oxygen. MTS J. 1999, 33, 15–22. [Google Scholar] [CrossRef]
- Gamo, T. Global warming may have showed down the deep conveyor belt of a marginal sea of the northwestern Pacific: Japan Sea. Geophys. Res. Lett. 1999, 26, 3137–3140. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Bychkov, A.S.; Wang, S.L.; Pavlova, G.Y. An anoxic Sea of Japan by the year 2200? Mar. Chem. 1999, 67, 249–265. [Google Scholar] [CrossRef]
- Ponomarev, V.I.; Salyuk, A.N. The climate regime shifts and heat accumulation in the Sea of Japan. In Proceedings of the CREAMS’97 International Symposium, Fukuoka, Japan, 28–30 January 1997; pp. 157–161. [Google Scholar]
- Kim, K.; Kim, K.-R.; Min, D.-H.; Volkov, Y.; Yoon, J.-H.; Takematsu, M. Warming and structural changes in the East (Japan) Sea: A clue to future changes in global oceans? Geophys. Res. Lett. 2001, 28, 3293–3296. [Google Scholar] [CrossRef]
- Kang, D.-J.; Kim, J.-Y.; Lee, T.; Kim, K.-R. Will the East/Japan Sea become an anoxic sea in the next century? Mar. Chem. 2004, 91, 77–84. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.-R.; Kim, Y.-G.; Cho, Y.-K.; Kang, D.-J.; Takematsu, M.; Volkov, Y. Water mass and decadal variability in the East Sea (Sea of Japan). Prog. Oceanogr. 2004, 61, 157–174. [Google Scholar] [CrossRef]
- Kim, T.-W.; Lee, K.; Feely, R.A.; Sabine, C.L.; Chen, C.-T.A.; Jeong, H.J.; Kim, K.Y. Prediction of Sea of Japan (East Sea) acidification over the past 40 years using a multiparameter regression model. Glob. Biogeochem. Cycles 2010, 24, GB3005. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kang, D.-J.; Lee, T.; Kim, K.-R. Long-term trend of CO2 and ocean acidification in the surface water of the Ulleung Basin, the East/Japan Sea inferred from the underway observational data. Biogeosciences 2014, 11, 2443–2454. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-T.A.; Lui, H.-K.; Hsieh, C.-H.; Yanagi, T.; Kosugi, N.; Ishii, M.; Gong, G.-C. Deep oceans may acidify faster than anticipated due to global warming. Nat. Clim. Chang. 2017, 7, 890–894. [Google Scholar] [CrossRef]
- Tishchenko, P.Y.; Tishchenko, P.P.; Lobanov, V.B.; Mikhaylik, T.A.; Sergeev, A.F.; Semkin, P.Y.; Shvetsova, M.G. Impact of the transboundary Razdolnaya and Tumannaya Rivers on deoxygenation of the Peter the Great Bay (Sea of Japan). Estuar. Coast. Shelf Sci. 2020, 239, 106731. [Google Scholar] [CrossRef]
- Tishchenko, P.Y.; Lobanov, V.B.; Zvalinsky, V.I.; Sergeev, A.F.; Koltunov, A.; Mikhailik, T.A.; Tishchenko, P.P.; Shvetsova, M.G.; Sagalaev, S.; Volkova, T.I. Seasonal Hypoxia of Amursky Bay in the Japan Sea: Formation and Destruction. Terr. Atmos. Ocean. Sci. 2013, 24, 1033–1050. [Google Scholar] [CrossRef] [Green Version]
- Tishchenko, P.P.; Tishchenko, P.Y.; Lobanov, V.B.; Sergeev, A.F.; Semkin, P.Y.; Zvalinsky, V.I. Summertime in situ monitoring of oxygen depletion in Amursky Bay (Japan/East Sea). Cont. Shelf Res. 2016, 118, 77–87. [Google Scholar] [CrossRef]
- Tishchenko, P.Y.; Sergeev, A.F.; Lobanov, V.B.; Zvalinsky, V.I.; Koltunov, A.M.; Mikhajlik, T.A.; Tishchenko, P.P.; Shvetsova, M.G. Hypoxia in near-bottom waters of the Amursky Bay. Bull. Far East. Branch Russ. Acad. Sci. 2008, 6, 115–125. [Google Scholar]
- Mikhaylik, T.A.; Nedashkovsky, A.P.; Khodorenko, N.D.; Tishchenko, P.Y. Peculiarities of the eutrophication of the Amur Bay (Japan Sea) by Razdolnaya River. Izvetiya TINRO 2020, 200, 401–411. (In Russian) [Google Scholar] [CrossRef]
- Tishchenko, P.Y.; Talley, L.D.; Lobanov, V.B.; Zhabin, I.A.; Luchin, V.A.; Nedashkovskii, A.P.; Sagalaev, S.G.; Chichkin, R.V.; Shkirnikova, E.M.; Ponomarev, V.I.; et al. Seasonal Variability of the hydrochemical conditions in the Sea of Japan. Oceanology 2003, 43, 643–655. [Google Scholar]
- Talley, L.D.; Tishchenko, P.; Luchin, V.; Nedashkovskiy, A.; Sagalaev, S.; Kang, D.-J.; Warner, W.; Min, D.-H. Atlas of Japan (East) Sea hydrographic properties in summer, 1999. Prog. Oceanogr. 2004, 61, 277–348. [Google Scholar] [CrossRef]
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis, 3rd ed.; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Willey-VCH: Weinheim, Germany; New York, NY, USA; Chichester, UK; Brisban, Australia; Singapore; Toronto, ON, Canada, 1999; pp. 159–251. [Google Scholar]
- Tishchenko, P.Y.; Kang, D.-J.; Chichkin, R.V.; Lazaruk, A.Y.; Wong, C.S.; Johnson, W.K. Application of potentiometric method using a cell without liquid junction to underway pH measurements in surface seawater. Deep Sea Res. Part I 2011, 58, 778–786. [Google Scholar] [CrossRef]
- Dickson, A.G. pH scales and proton-transfer reactions in saline media such as sea water. Geochim. Cosmochim. Acta 1984, 48, 2299–2308. [Google Scholar] [CrossRef]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.-C.; Zhai, W.; Hollibaugh, J.T.; Wang, Y.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Zhai, W.D.; Zhao, H.D.; Zheng, N.; Xu, Y. Coastal acidification in summer bottom oxygen-depleted waters in northwestern-northern Bohai Sea from June to August in 2011. Chin. Sci. Bull. 2012, 57, 1062–1068. [Google Scholar] [CrossRef] [Green Version]
- Stunzhas, P.A.; Tishchenko, P.Y.; Ivin, V.V.; Barabanshchikov, Y.A.; Volkova, T.I.; Vishkvartcev, D.I.; Zvalinsky, V.I.; Mikhailik, T.A.; Semkin, P.J.; Tishchenko, P.P.; et al. The First Case of Anoxia in Waters of the Far East Marine Biosphere Reserve. Dokl. Earth Sci. 2016, 467 Pt 1, 295–298. [Google Scholar] [CrossRef]
- Shulkin, V.; Tishchenko, P.; Semkin, P.; Shvetsova, M. Influence of river discharge and phytoplankton on the distribution of nutrients and trace metals in Razdolnaya River estuary, Russia. Estuar. Coast. Shelf Sci. 2018, 211, 166–176. [Google Scholar] [CrossRef]
- Tishchenko, P.Y.; Semkin, P.Y.; Pavlova, G.Y.; Tishchenko, P.P.; Lobanov, V.B.; Marjash, A.A.; Mikhailik, T.A.; Sagalaev, S.G.; Sergeev, A.F.; Tibenko, E.Y.; et al. Hydrochemistry of the Tumen River Estuary, Sea of Japan. Oceanology 2018, 58, 175–186. [Google Scholar] [CrossRef]
- REGIONAL OVERVIEW on River and Direct Inputs of Contaminants into the Marine and Coastal Environment in NOWPAP Region. With Special Focus on the Land Based Sources of Pollution. NOWPAP POMRAC, Vladivostok. 2009. Available online: http://www.pomrac.tigdvo.ru/Pub/DOC/PUBL/POMRAC_RDI_2009.pdf (accessed on 25 August 2021).
- Tkalin, A.V.; Belan, T.A.; Shapovalov, E.N. The state of marine environment near Vladivostok, Russia. Mar. Pollut. Bull. 1993, 26, 418–422. [Google Scholar] [CrossRef]
- Belan, T.A. Benthos abundance pattern and species composition in conditions of pollution in Amursky Bay (Peter the Great Bay, Sea of Japan). Mar. Pollut. Bull. 2003, 46, 1111–1119. [Google Scholar] [CrossRef]
- Oleinik, E.V.; Moshchenko, A.V.; Lishavskaya, T.S. Impact of Polluted Bottom Deposits on the Species Structure and Abundance of Bivalves in Peter the Great Bay, Sea of Japan. Russ. J. Mar. Biol. 2004, 30, 20–27. [Google Scholar] [CrossRef]
- Silina, A.V.; Ovsyannikova, I.I. Long-term changes in a community of Japanese scallop and its epibionts in the polluted area of Amurskii Bay, Sea of Japan. Russ. J. Mar. Biol. 1995, 21, 54–60. [Google Scholar]
- Sudo, H. A note on the Japan Sea Proper Water. Prog. Oceanogr. 1986, 17, 313–336. [Google Scholar] [CrossRef]
- Gamo, T.; Nakayama, N.; Takahata, N.; Sano, Y.; Zhang, J.; Yamazaki, E.; Taniyasu, S.; Yamashita, N. The Sea of Japan and its Unique Chemistry Reveald by Time-series Observations over the Last 30 years. Monogr. Environ. Earth Planets 2014, 2, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Dore, J.E.; Lukas, R.; Sadler, D.W.; Church, M.J.; Karl, D.M. Physical and biochemical modulation of ocean acidification in the central North Pacific. Proc. Natl. Acad. Sci. USA 2009, 106, 12235–12240. [Google Scholar] [CrossRef] [Green Version]
- Midorikawa, T.; Midorikawa, T.; Ishii, M.; Saito, S.; Sasano, D.; Kosugi, D.; Motoi, T.; Kamiya, H.; Nakadate, A.; Nemoto, K.; et al. Decreasing pH trend estimated from 25-yr time series of carbonate parameters in the western North Pacific. Tellus 2010, 62B, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Byrne, B.H.; Mecking, S.; Feely, R.A.; Liu, X. Direct observations of basin-wide acidification of the North Pacific. Geophys. Res. Lett. 2010, 37, L02601. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Davila, M.; Santano-Casiano, J.M.; Rueda, M.J.; Llinas, O. The water column distribution of carbonate system variables at the ESTOC site from 1995 to 2004. Biogeosciences 2010, 7, 3067–3081. [Google Scholar] [CrossRef] [Green Version]
- Bates, N.R.; Best, M.H.P.; Neely, K.; Garley, R.; Dickson, A.G.; Johnson, R.J. Detecting anthropogenic carbon dioxide uptake and ocean acidification in the North Atlantic Ocean. Biogeosciences 2012, 9, 2509–2522. [Google Scholar] [CrossRef] [Green Version]
- Olafsson, J.; Olafsdottir, S.R.; Benoit-Cattin, A.; Danielsen, M.; Arnarson, T.S.; Takahashi, T. Rate of Iceland Sea acidification from time series measurements. Biogeosciences 2012, 6, 2661–2668. [Google Scholar] [CrossRef] [Green Version]
- Doney, S.C. The Growing Human Footprint on Coastal and Open-Ocean Biogeochemistry. Science 2010, 328, 1512–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of Anthropogenic CO2 on the CaCO3 System in the Oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Hendriks, I.E.; Moore, T.S.; Olsen, Y.S.; Steckbauer, A.; Ramajo, L.; Carstensen, J.; Trotter, J.A.; McCulloch, M. Is Ocean Acidification an Open-Ocean Syndrome? Understanding Anthropogenic Impacts on Seawater pH. Estuaries Coasts 2013, 36, 221–236. [Google Scholar] [CrossRef] [Green Version]
- Tishchenko, P.Y.; Talley, L.D.; Nedashkovsky, A.P.; Sagalaev, S.G.; Zvalinsky, V.I. Temporal Variability of the Hydrochemical Properties of the Waters of the Sea of Japan. Oceanology 2002, 42, 795–803. [Google Scholar]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A. The influence of organisms on the composition of sea-water. In The Composition of Seawater: Comparative and Descriptive Oceanography; Hill, M.N., Ed.; The Sea Interscience: New York, NY, USA, 1963; Volume 2, pp. 26–77. [Google Scholar]
- Nixon, S.F. Eutrophycation and macroscope. Hydrobiologia 2009, 629, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.-T.; Son, S.H.; Park, J.W.; Kang, J.J.; Jeong, J.-Y.; Lee, J.I.; Kang, C.-K.; Lee, S.H. Long-Term Pattern of Primary Productivity in the East/Japan Sea Based on Ocean Color Data Derived from MODIS-Aqua. Remote Sens. 2016, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Ishizaka, J.; Yamada, K. Phytoplankton and Primary Production in the Japan Sea. In Remote Sensing of the Asian Seas; Barale, V., Gade, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 177–189. [Google Scholar] [CrossRef]
- Lee, S.H.; Son, S.; Dahms, H.U.; Park, J.W.; Lim, J.H.; Noh, J.H.; Kwon, J.I.; Joo, H.T.; Jeong, J.Y.; Kang, C.K. Decadal changes of phytoplankton chlorophyll-a in the East Sea/Sea of Japan. Oceanology 2014, 54, 771–779. [Google Scholar] [CrossRef]
- Konovalova, G.V.; Orlova, T.Y.; Pautova, L.A. Atlas of Phytoplankton of the Sea of Japan; Nauka: Leningrad, Russia, 1989; p. 160. (In Russian) [Google Scholar]
- Lobanov, V.B.; Ponomarev, V.I.; Salyuk, A.N.; Tishchenko, P.Y.; Talley, L.D. Structure and dynamics of synoptic scale eddies in the northern Japan Sea. In Far Eastern Seas of Russia; Oceanographic Research; Nauka: Moscow, Russia, 2007; Volume 1, pp. 450–473. (In Russian) [Google Scholar]
- Andreev, A.G. Interannual Variations of Sea Water Parameters and Chlorophyll a Concentration in the Japan Sea in Autumn. Russ. Meteorol. Hydrol. 2014, 39, 542–549. [Google Scholar] [CrossRef]
- Yoo, S.; An, Y.-R.; Bae, S.; Choi, S.; Ishizaka, J.; Kang, Y.-S.; Kim, Z.G.; Lee, C.; Lee, J.B.; Li, R.; et al. Status and trends in the Yellow Sea and East China Sea region. In Marine Ecosystems of the North Pacific Ocean, 2003–2008; McKinnell, S.M., Dagg, M.J., Eds.; PICES Special Publication: Sidney, BC, Canada, 2010; Volume 4, pp. 360–393. [Google Scholar]
- Ning, X.; Lin, C.; Su, J.; Liu, C.; Hao, Q.; Leet, F. Long-term changes of dissolved oxygen, hypoxia, and the responses of the ecosystems in the East China Sea from 1975 to 1995. J. Oceanogr. 2011, 67, 59–75. [Google Scholar] [CrossRef]
- Wang, B.; Xin, M.; Wei, Q.; Xie, L. A historical overview of coastal eutrophication in the China Seas. Mar. Pollut. Bull. 2018, 136, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Sand-Jensen, K. CO2 increases oceanic primary production. Nature 1997, 388, 526–527. [Google Scholar] [CrossRef]
- Riebesell, U.; Schulz, K.G.; Bellerby, R.G.J.; Botros, M.; Fritsche, P.; Meyerhöfer, M.; Neill, C.; Nondal, G.; Oschlies, A.; Wohlers, J.; et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature 2007, 450, 545–549. [Google Scholar] [CrossRef]
- Kim, T.-W.; Lee, K.; Najjar, R.G.; Jeong, H.-D.; Jeong, H.J. Increasing N Abundance in the Northwestern Pacific Ocean Due to Atmospheric Nitrogen Deposition. Science 2011, 334, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-N.; Lee, K.; Gruber, N.; Karl, D.M.; Bullister, J.L.; Yang, S.; Kim, T.-W. Increasing anthropogenic nitrogen in the North Pacific Ocean. Science 2014, 346, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Onitsuka, G.; Uno, I.; Yana, T.; Yoon, J.-H. Modeling the Effects of Atmospheric Nitrogen Input on Biological Production in the Japan Sea. J. Oceanogr. 2009, 65, 433–438. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, S. Interannual variability of the phytoplankton community by the changes in vertical mixing and atmospheric deposition in the Ulleung Basin, East Sea: A modelling study. Ecol. Model. 2016, 322, 31–47. [Google Scholar] [CrossRef] [Green Version]
- Boesch, D.F. Challenges and Opportunities for Science in Reducing Nutrient Over-enrichment of Coastal Ecosystems. Estuaries 2002, 25, 886–900. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Diaz, R.J.; Levin, L.A.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef] [Green Version]
- Schmodtko, S.; Stramma, L.; Visbeck, M. Decline in global oceanic oxygen content during the past five decades. Nature 2017, 542, 335–339. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Gregoire, M.; Chavez, F.P.; Conley, D.J.; Garcon, V.; Gilbert, D.; Gutierrez, D.; Isensee, K.; et al. Declining oxygen in the global and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [Green Version]










| R/V | Cruise | Month | Year | Number of Stations |
|---|---|---|---|---|
| Roger Revelle | HNRO7 | June–July | 1999 | 7 |
| Professor Khromov | Kh36 | July–August | 1999 | 16 |
| Professor Gagarinskiy | Ga31a | April | 2001 | 11 |
| Professor Gagarinskiy | Ga31b | May-June | 2001 | 11 |
| Akademik M.A. Lavrentyev | La33 | May | 2004 | 10 |
| Akademik M.A. Lavrentyev | La35 | March | 2005 | 6 |
| Professor Gagarinskiy | Ga43 | May | 2007 | 13 |
| Akademik M.A. Lavrentyev | La46 | July | 2009 | 10 |
| Professor Gagarinskiy | Ga47 | May | 2010 | 5 |
| Akademik M.A. Lavrentyev | La66 | April | 2014 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tishchenko, P.; Lobanov, V.; Kaplunenko, D.; Sagalaev, S.; Tishchenko, P. Acidification and Deoxygenation of the Northwestern Japan/East Sea. J. Mar. Sci. Eng. 2021, 9, 953. https://doi.org/10.3390/jmse9090953
Tishchenko P, Lobanov V, Kaplunenko D, Sagalaev S, Tishchenko P. Acidification and Deoxygenation of the Northwestern Japan/East Sea. Journal of Marine Science and Engineering. 2021; 9(9):953. https://doi.org/10.3390/jmse9090953
Chicago/Turabian StyleTishchenko, Pavel, Vyacheslav Lobanov, Dmitry Kaplunenko, Sergey Sagalaev, and Petr Tishchenko. 2021. "Acidification and Deoxygenation of the Northwestern Japan/East Sea" Journal of Marine Science and Engineering 9, no. 9: 953. https://doi.org/10.3390/jmse9090953






