Accumulation of Trace Metal in Sediment and Soft Tissue of Strombus canarium in a Tropical Remote Island of Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Storage
2.2.1. Sediment Samples
2.2.2. Biological Samples
2.3. Digestion Procedure
2.3.1. Sediment Samples
2.3.2. Biological Samples
2.4. Analysis of Metals
2.5. Data Processing and Statistical Analysis
2.5.1. The Enrichment Factor (EF)
2.5.2. The Geoaccumulation Index (Igeo)
2.5.3. Biota-Sediment Accumulation Factor (BSAF)
2.5.4. The Conversion Factor (CF)
2.5.5. Estimated Daily Intake (EDI)
2.5.6. The Target Hazard Quotient (THQ)
3. Results and Discussion
3.1. Concentrations of Trace Metals in Surface Sediment
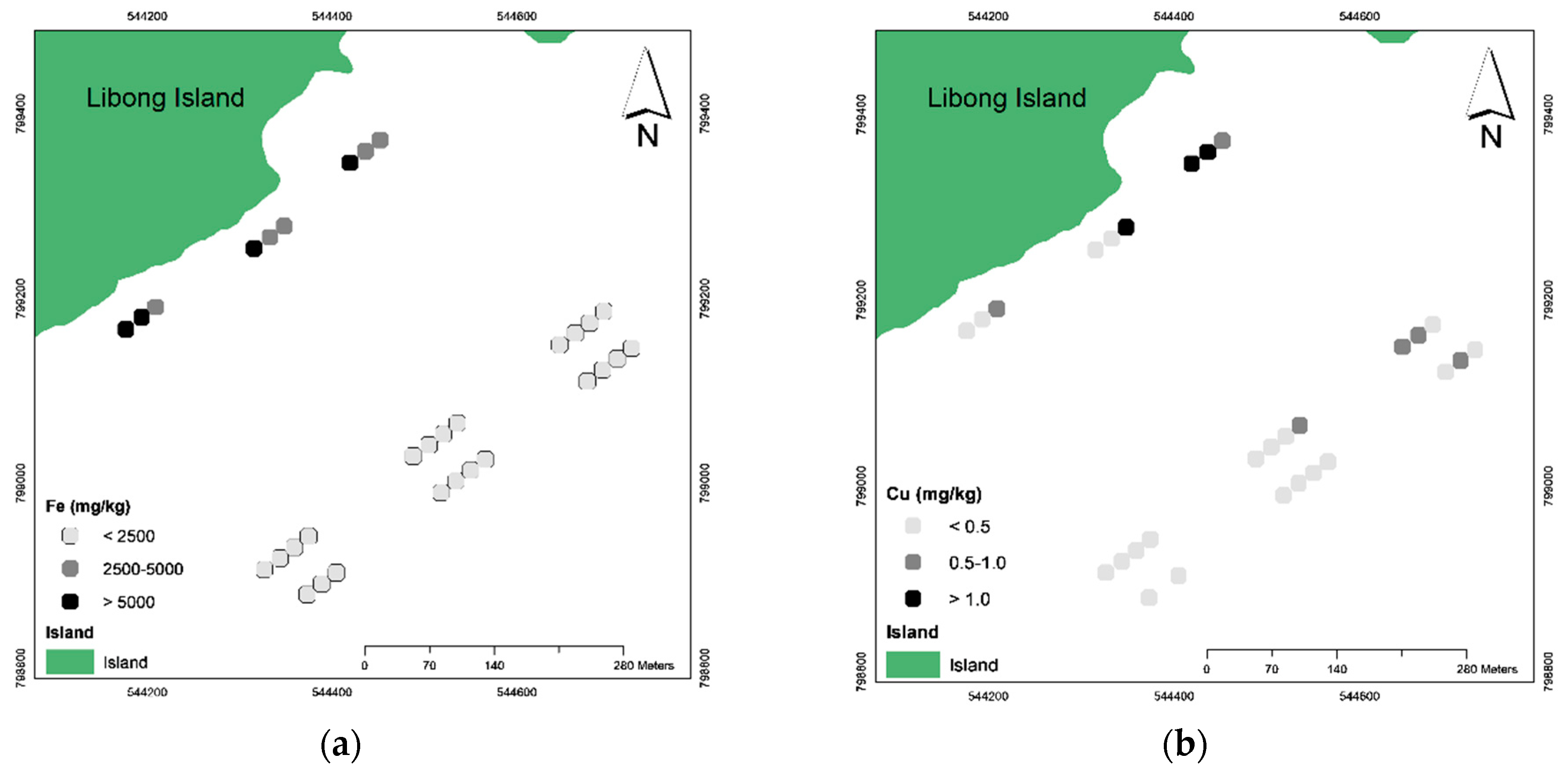
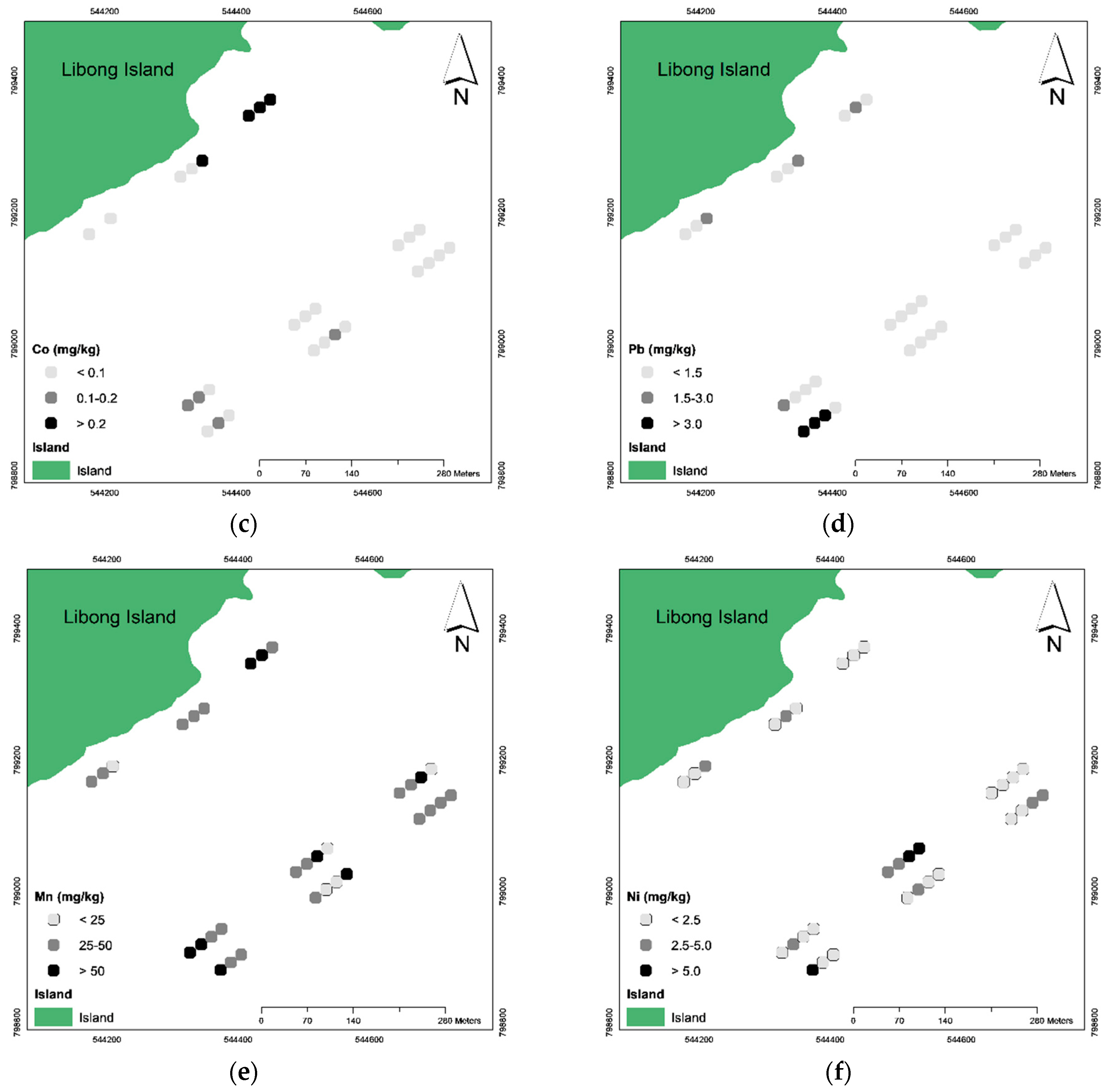
3.2. The Spatial Distribution of Trace Metals
3.3. Environmental Risk Assessment of Trace Metals in Surface Sediment
3.4. Concentrations of Trace Metals in Strombus canarium
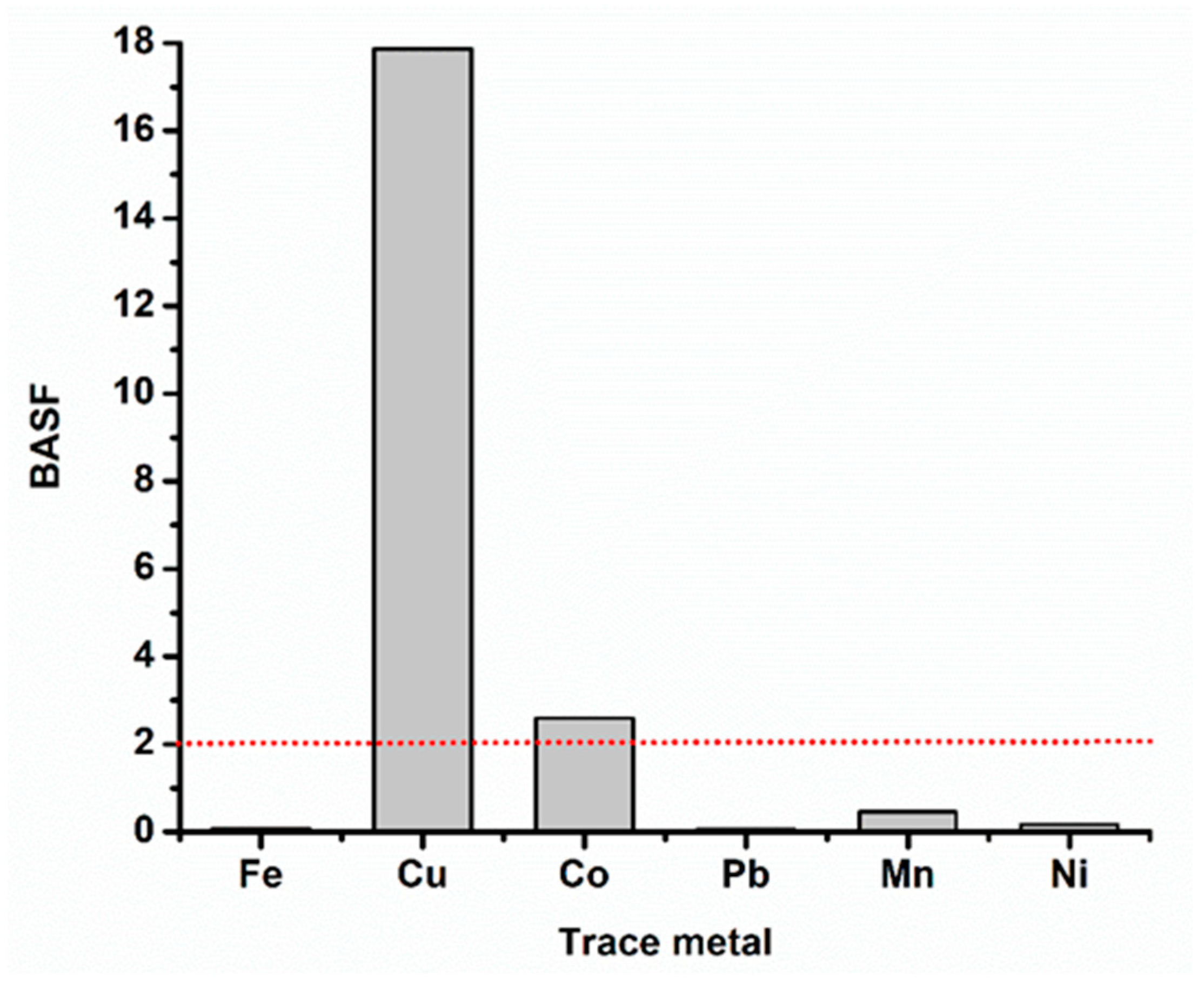
3.5. Biota Sediment Accumulation Factor (BSAF)
3.6. The Relationship between Trace Metal Concentrations in Sediment and Soft Tissue of Shellfish
3.7. The Relationship between Trace Metal Concentrations in the Soft Tissues of Strombus canarium and Weight
3.8. Health Risk Assessment of the Trace Metals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, J.; Maboob, S.; Jagadheesan, R.; Elumalai, K.; Krishnappa, K.; Al-Misned, F.; Kaimkhani, Z.A.; Govindarajan, M. A novel approach to assess the heavy metal content in the feathers of shorebirds: A perspective of environmental research. J. King Saud Univ. Sci. 2020, 32, 3065–3071. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, X.; Xia, L. Heavy metal ion adsorption by permeable oyster shell bricks. Constr. Build. Mater. 2021, 275, 122128. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Liu, L.-Z.; Shi, W.-L.; Meng, X.-Z. Comprehensive risk assessment of heavy metals in lake sediment from public parks in Shanghai. Ecotoxicol. Environ. Saf. 2014, 102, 129–135. [Google Scholar] [CrossRef]
- US EPA. Framework for Metals Risk Assessment. EPA-120/R-07/001; U.S. Environmental Protection Agency, Office of the Science Advisor: Washington, DC, USA, 2007; pp. 1–172.
- Pereira, P.; Korbas, M.; Pereira, V.; Cappello, T.; Maisano, M.; Canario, J.; Almeida, A.; Pacheco, M. A multidimensional concept for mercury neuronal and sensory toxicity in fish—From toxicokinetics and biochemistry to morphometry and behavior. BBA Gen. Subj. 2019, 1863, 129298. [Google Scholar] [CrossRef]
- WHO/FAO/IAEA. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, 1996; pp. 6–10. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalgan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen: London, UK, 2019; pp. 1–24. [Google Scholar]
- Chon, H.-S.; Ohandja, D.-G.; Voulvoulis, N. The role of sediments as a source of metals in river catchments. Chemosphere 2012, 88, 1250–1256. [Google Scholar] [CrossRef]
- Amin, B.; Ismail, A.; Arshad, A.; Yap, C.K.; Kamarudin, M.S. Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai, Indonesi. Environ. Monit. Assess. 2009, 148, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Naung, N. Distribution of the genus Strombus Linnaeus 1758 (Gastropoda: Strombidae) in some coastal areas of Myanmar. J. Aquac. Mar. Biol. 2018, 7, 258–263. [Google Scholar] [CrossRef]
- Cob, Z.C.; Arshad, A.; Bujang, J.S.; Ghaffar, M.A. Seasonal variation in growth and survival of Strombus canarium (Linnaeus, 1758) larvae. Pak. J. Biol. Sci. 2009, 12, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poutiers, J.M. Gastropods. In The Living Marine Resources of the Western Central Pacific; Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 1998; pp. 363–646. ISBN 92-5-104051-6. [Google Scholar]
- Kronenberg, G. Strombus (Canarium) Ochroglottis Betuleti a new subspecies from Sri Lanka with a short note on the distribution of S. (C.) Mutabilis Swainson, 1821. Gloria Maris 1991, 30, 53–58. [Google Scholar]
- Nateewathana, A. Taxonomic account of commercially and edible mollusks, excluding cephalopods, of Thailand. Spec. Publ. Phuket Mar. Biol. Cent. 1995, 15, 93–116. [Google Scholar]
- Hossen, F.M.; Hamdan, S.; Rahman, R.M. Cadmium and lead in blood cockle (Anadara granosa) from Asajaya, Sarawak. Malays. Sci. World J. 2014, 2014, 924360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradit, S.; Nitiratsuwan, T.; Towatana, P.; Jualaong, S.; Sornplang, K.; Noppradit, P.; Jirajarus, M.; Darakai, Y.; Weeerawong, C. Marine debris accumulation on the beach in Libong, a small island in Andaman sea, Thailand. Appl. Ecol. Environ. Res. 2020, 18, 5461–5474. [Google Scholar] [CrossRef]
- US EPA. QA/QC Guidance for Sampling and Analysis of Sediments, Water, and Tissues for Dredged for Material Evaluations; EPA-823-B95-001; U.S. Environmental Protection Agency, Office of the Science Advisor: Washington, DC, USA, 1995; pp. 1–144.
- Pradit, S.; Wattayakorn, G.; Angsupanich, S.; Baeyens, W.; Leermakers, M. Distribution of trace elements in sediments and biota of Songkhla Lake, Southern Thailand. Water Air Soil Pollut. 2010, 206, 155–174. [Google Scholar] [CrossRef]
- Zhao, W. Determination of six heavy metal elements such as Co in solid waste by ICP-MS. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 032108. [Google Scholar] [CrossRef]
- Astatkie, H.; Ambelu, A.; Mengistie, E. Contamination of stream sediment with heavy metals in the Awetu watershed of Southwestern Ethiopia. Front. Earth Sci. 2021, 9, 658737. [Google Scholar] [CrossRef]
- Pradit, S.; Shashili, N.A.M.; Towatana, P.; Saengmanee, W. Trace metals, grain size and organic matter in sediment from a coastal area of Thailand and Malaysia. Aquat Ecosyst. Health Manag. 2016, 19, 345–354. [Google Scholar] [CrossRef]
- Salomons, W.; Förstner, U. Metals in the Hydrocycle; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1984; pp. 1–360. [Google Scholar]
- Ghrefat, H.A.; Abu-Rukah, Y.; Rosen, M.A. Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environ. Monit. Assess. 2011, 178, 95–109. [Google Scholar] [CrossRef]
- Shazili, N.A.M.; Rashid, M.K.A.; Husain, M.L.; Nordin, A.; Ali, S. Trace metals in the surface sediments of the South China Sea, area I: Gulf of Thailand and the East Coast of Peninsular Malaysia. In Proceedings of the First Technical Seminar on Marine Fishery Resources Survey in the South China Sea, Area I: Gulf of Thailand and East Coast of Peninsular Malaysia; Training Department, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 1999; pp. 73–85. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The geochemical evolution of the continental crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Kouidri, M.; Youcef, N.D.; Benabdellah, I.; Ghoubali, R.; Bernoussi, A.; Lagha, A. Enrichment and geoaccumulation of heavy metals and risk assessment of sediments from coast of Ain Temouchent (Algeria). Arab. J. Geosci. 2016, 9, 354. [Google Scholar] [CrossRef]
- Shaari, H.; Azmi, S.N.H.; Sultan, K.; Bidai, J.; Mohamad, Y. Spatial distribution of selected heavy metals in surface sediments of the EEZ of the East Coast of Peninsular Malaysia. Int. J. Oceans Oceanogr. 2015, 2015, 618074. [Google Scholar] [CrossRef] [Green Version]
- Muller, G. Index of geoaccumulation in the sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Thongra-ar, W.; Musika, C.; Wongsudawan, W.; Munhapol, A. Heavy metals contamination in sediments along the Eastern Coast of the Gulf of Thailand. Environ. Asia 2008, 1, 37–45. [Google Scholar]
- Islam, M.S.; Habibullah-Al-Mamun, M. Accumulation of trace elements in sediment and fish species of Paira River Bangladesh. AIMS Environ. Sci. 2017, 4, 310–322. [Google Scholar] [CrossRef]
- Ju, Y.-R.; Chen, C.-W.; Chen, C.-F.; Chuang, X.-Y.; Dong, C.-D. Assessment of heavy metals in aquaculture fishes collected from southwest coast of Taiwan and human consumption risk. Int. Biodeter. Biodegr. 2017, 124, 314–325. [Google Scholar] [CrossRef]
- Dallinger, R. Strategies of metal detoxification in terrestrial invertebrates. Ecotoxicology of Metal in Invertebrates; Dallinger, R., Rainbow, P.S., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 246–289. [Google Scholar]
- Yap, C.K.; Wong, K.W.; Al-shami, S.A.; Nulit, R.; Cheng, W.H.; Aris, A.Z.; Sharifinia, M.; Bakhtiari, A.R.; Okamura, H.; Saleem, M.; et al. Human Health risk assessments of trace metals on the clam Corbicula javanica in a tropical river in Peninsular Malaysia. Int. J. Environ. Res. Public Health 2021, 18, 195. [Google Scholar] [CrossRef]
- Khandaker, M.U.; Asaduzzaman, K.h.; Nawi, S.M.; Usman, A.R.; Amin, Y.M.; Daar, E.; Bradley, D.A.; Ahmed, H.; Okhunov, A.A. Assessment of radiation and heavy metals risk due to the dietary intake of marine fishes (Rastrelliger kanagurta) from the Straits of Malacca. PLoS ONE 2015, 10, e0128790. [Google Scholar] [CrossRef] [Green Version]
- Rangkadilok, N.; Siripriwon, P.; Nookabkaew, S.; Suriyo, T.; Satayavivad, J. Arsenic, cadmium, and manganese levels in shellfish from Map Ta Phut, an industrial area in Thailand, and the potential toxic effects on human cells. Arch. Environ. Contam. Toxicol. 2015, 68, 169–180. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, F.; Yan, X. Concentrations of selected trace elements in marine bivalves and the dietary risk to residents of Dalian City, Northern China. Hum. Ecol. Risk Assess. 2013, 19, 145–150. [Google Scholar] [CrossRef]
- US EPA Regional Screening Level (RSL) Summary Table: November 2011. Available online: http://www.epa.gov//regshwmd/risk/human/Index.htm (accessed on 20 January 2014).
- Yao, G.; Xiaoming, S.; Xiaodong, J.; Rina, S.; Li, Z.; Yi, H.; Yating, L.; Xiaojie, L.; Rongfei, L.; Chi, W. The effect of Fe-Mn minerals and seawater interface and enrichment mechanism of ore-forming elements of polymetallic crusts and nodules from the South China Sea. Acta Oceanol. Sin. 2017, 36, 34–46. [Google Scholar]
- Ranibar Jafarabadi, A.; Riyahi Bakhtiari, A.; Maisano, M.; Pereira, P.; Cappello, T. First record of bioaccumulation and bioconcentration of metals in Scleractinian corals and their algal symbionts from Kharg and Lark coral reefs (Persian Gulf, Iran). Sci. Total Environ. 2018, 640–641, 1500–1511. [Google Scholar] [CrossRef]
- Ranibar Jafarabadi, A.; Riyahi Bakhtiari, A.; Spano, N.; Capello, T. First report of geochemical fractionation distribution, bioavailability and risk assessment of potentially toxic inorganic elements in sediments of coral reef Islands of the Persian Gulf, Iran. Mar. Pollut. Bull. 2018, 137, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Perumal, K.; Antony, J.; Muthuramalingam, S. Heavy metal pollutants and their spatial distribution in surface sediments from Thondi coast, Palk Bay. South India. Environ. Sci. Eur. 2021, 33, 63. [Google Scholar] [CrossRef]
- Cai, P.; Cai, G.; Chen, X.; Li, S.; Zhao, L. The concentration distribution and biohazard assessment of heavy metal elements in surface sediments from the continental shelf of Hainan Island. Mar. Pollut. Bull. 2021, 166, 112254. [Google Scholar] [CrossRef] [PubMed]
- El-Sorogy, A.; Al-Kahtany, K.; Youssef, M.; Al-Kahtany, F.; Al-Malky, M. Distribution and metal contamination in the coastal sediments of Dammam Al-Jubail area, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull. 2018, 128, 8–16. [Google Scholar] [CrossRef]
- Liu, S.; Shi, X.; Yang, G.; Khokiattiwong, S.; Kornkanitnan, N. Concentration distribution and assessment of heavy metals in the surface sediments of the western Gulf of Thailand. Environ. Earth Sci. 2016, 75, 346. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, G.; Yu, R.; He, H.; Lin, C. Distribution, fractionation, and contamination assessment of heavy metals in offshore surface sediments from western Xiamen Bay, China. Acta Geochim. 2016, 35, 355–367. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, X.; Liu, Q.; Teng, A.; Xu, W. Distribution and pollution assessment of heavy metals in surface sediments in the central Bohai Sea, China: A case study. Environ. Earth Sci. 2016, 75, 364. [Google Scholar] [CrossRef]
- Potipat, J.; Tangkrock-Olan, N.; Helander, H.F. Bioconcentration factor (BCF) and depuration of heavy metals of oysters (Saccostrea cucullata) and mussels (Perna viridis) in the river basins of coastal area of Chanthaburi Province, Gulf of Thailand. Environ. Asia 2015, 8, 118–128. [Google Scholar]
- Okbah, M.A.; Nasr, S.M.; Soliman, N.F.; Khairy, M.A. Distribution and contamination status of trace metals in the mediterranean coastal sediments, Egypt. Soil Sediment Contam. 2014, 23, 656–676. [Google Scholar] [CrossRef]
- Salem, D.M.S.A.; Khaled, A.; El Nemr, A.; El-Sikaily, A. Comprehensive risk assessment of heavy metals in surface sediments along the Egyptian Red Sea coast. Egypt. J. Aquat. Res. 2014, 40, 349–362. [Google Scholar] [CrossRef] [Green Version]
- Tayakoly Sany, S.B.; Salleh, A.; Rezayi, M.; Saadati, N.; Narimany, L.; Tehrani, G.M. Distribution and contamination of heavy metal in the coastal sediments of port Klang, Selangor, Malaysia. Water Air Soil Pollut. 2013, 224, 1476. [Google Scholar] [CrossRef]
- Ünlü, S.; Topçuoğlu, S.; Alpar, B.; Kırbaşoğlu, C.; Yılmaz, Y.Z. Heavy metal pollution in surface sediment and mussel samples in the Gulf of Gemlik. Environ. Monit. Assess. 2008, 144, 169–178. [Google Scholar] [CrossRef]
- Duarte, C.M.; Martín, M.; Margarita, G. Evidence of iron deficiency in seagrasses growing above carbonate sediments. Limnol. Oceanogr. 1995, 40, 1153–1158. [Google Scholar] [CrossRef]
- Tahril; Taba, P.; la Nafie, N.; Noor, A.; Ratna; Muzakkar, M.Z. Iron, manganese and copper metals in seagrass ecology in Donggala district, Indonesia. Asian J. Appl. Sci. 2020, 13, 1–7. [Google Scholar]
- Filho, G.M.A.; Creed, J.C.; Andrade, L.R.; Pfeiffer, W.C. Metal accumulation by Halodule wrightii populations. Aquat. Bot. 2004, 80, 241–251. [Google Scholar] [CrossRef]
- Thangaradjpu, T.; Nobi, E.P.; Dilipan, E.; Sivakumar, K.; Susila, S. Heavy metal enrichment in seagrasses of Andaman Islands and its implication to the health of the coastal ecosystem. Indian J. Mar. Sci. 2010, 39, 85–91. [Google Scholar]
- Chen, Y.-M.; Gao, J.-B.; Yuan, Y.-Q.; Ma, J.; Yu, S. Relationship between heavy metal contents and clay mineral properties in surface sediments: Implications for metal pollution assessment. Cont. Shelf Res. 2016, 124, 125–133. [Google Scholar] [CrossRef]
- US EPA. Guidance for the Pollution Classification of Great Lakes Harbor Sediments; U.S. Environmental Protection Agency: Chicago, IL, USA, 1997; pp. 1–8.
- NOAA. Sediment Quality Guidelines Developed for the National Status and Trends Program. 1999. Available online: http://response.restoration.noaa.gov/book_shelf/121_sedi_qual_guide.pdf (accessed on 20 July 2010).
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Con. Tox. 2000, 39, 20–31. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.L. Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuar. Coast. Shelf Sci. 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Boudissa, S.M.; Lambert, J.; Mu¨ller, C.; Kennedy, G.; Gareau, L.; Zayed, J. Manganese concentrations in the soil and air in the vicinity of a closed manganese alloy production plant. Sci. Total Environ. 2006, 361, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, Q.; Jiang, T.; Wang, H.; Xie, L.; Zhang, R. A novel ferritin subunit involved in shell formation from the pearl oyster (Pinctada fucata). Comp. Biochem. Physiol. B 2003, 135, 43–54. [Google Scholar] [CrossRef]
- Bai, Z.; Yuan, Y.; Yue, G.; Li, J. Molecular cloning and copy number variation of a ferritin subunit (Fth1) and its association with growth in freshwater pearl mussel Hyriopsis cumingii. PLoS ONE 2011, 6, e22886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P. Manganese metabolism in humans. Front. Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [Green Version]
- Albretsen, J. The toxicity of iron, an essential element. Vet. Med. 2006, 101, 82–90. [Google Scholar]
- Forti, E.; Salovaara, S.; Cetin, Y.; Bulgheroni, A.; Tessadri, R.; Jennings, P.; Prieto, P. In vitro evaluation of the toxicity induced by nickel soluble and particulate forms in human airway epithelial cells. Toxicol. In Vitro 2011, 25, 454–461. [Google Scholar] [CrossRef]
- Saha, N.; Zaman, M.R. Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City Bangladesh. Environ. Monit. Assess. 2013, 185, 3867–3878. [Google Scholar] [CrossRef]
- Angeloval, M.; Asenova, S.; Nedkova, V.; Koleva-Kolarova, R. Copper in the human organism. Trakia J. Sci. 2011, 9, 88–98. [Google Scholar]
- Attri, S.; Sharma, N.; Jahagirdar, S.; Thapa, B.R.; Prasad, R. Erythrocyte metabolism and antioxidant status of patients with Wilson disease with hemolytic anemia. Pediatr. Res. 2006, 59, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, I. Cobalt poisoning: A comprehensive review of the literature. Int. J. Med. Toxicol. Forensic Med. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Ismail, T.H.T.; Adnan, N.A.F.; Samah, M.A.A. Study on accumulation of Fe, Pb, Zn, Ni and Cd in Nerita lineata and Thais bitubercularis from Tanjung Harapan and Teluk Kemang, Malaysia. J. Clean WAS 2017, 1, 6–16. [Google Scholar] [CrossRef]
- García-lestón, J.; Mendez, J.; Pásaro, E.; Laffon, B. Author’s personal copy genotoxic effects of lead: An updated review. Environ. Int. 2010, 36, 623–636. [Google Scholar] [CrossRef]
- Sabri, S.; Said, M.I.M.; Azman, S. Lead (Pb) And Zinc (Zn) concentrations in marine gastropod Strombus canarium in Jahor coastal areas. Malays. J. Anal. Sci. 2014, 18, 37–42. [Google Scholar]
- Abdel Gawad, S.S. Concentrations of heavy metals in water, sediment and mollusk gastropod, Lanistes carinatus from Lake Manzala, Egypt. Egypt. J. Aquat. Res. 2018, 44, 77–82. [Google Scholar] [CrossRef]
- Ragi, A.S.; Leena, P.P.; Cheriyan, E.; Nair, S.M. Heavymetal concentrations in some gastropods and bivalves collected from the fishing zone of South India. Mar. Pollut. Bull. 2017, 118, 452–458. [Google Scholar] [CrossRef]
- Duysak, Ö.; Ersoy, B. A Biomonitoring study: Heavy metals in Monodonta turbinate (Mollusca: Gastropoda) from Iskenderun bay, North-Eastern Mediterranean. Pakistan J. Zool. 2014, 46, 1317–1322. [Google Scholar]
- Madkour, H.A. Distribution and relationships of heavy metals in the giant clam (Tridacna mamima) and associated sediments from different sites in the Egyptian Red Sea Coast. Egypt. J. Aquat. Res. 2005, 31, 45–59. [Google Scholar]
- El Nemr, A.; Khaled, A.; Moneer, A.A.; Sikaily, A.E. Risk probability due to heavy metals in bivalve from Egyptian Mediterranean coast. Egypt. J. Aquat. Res. 2012, 38, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Denil, D.J.; Fui, C.F.; Ransangan, J. Health risk assessment due to heavy metals exposure via consumption of bivalves harvested from Marudu Bay, Malaysia. Open J. Mar. Sci. 2017, 7, 494–510. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.N.; He, B.; Jiang, G.B.; Chen, D.Y.; Yao, Z.W. Evaluation of mollusks as biomonitors to investigate heavy metal contaminations along the Chinese Bohai Sea. Sci. Total Environ. 2004, 324, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Niedzielski, P.; Klimaszyk, P.; Poniedzialek, B. Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ. Monit. Assess. 2014, 186, 3199–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nourozifard, P.; Mortazavi, S.; Asad, S.; Hassanzadeh, N. Using Saccostrea cucullata as a biomonitor of heavy metals (Cu, Pb, Zn, Cd, Ni, and Cr) in water and sediments of Qeshm Island, Persian Gulf. Ecopersia 2020, 8, 181–190. [Google Scholar]
- Nam, Y.K.; Kim, E.J. Diversification and domain evolution of molluskan metallothioneins: A mini review. Fish. Aquatic Sci. 2017, 20, 2–18. [Google Scholar] [CrossRef]
- Samsi, A.N.; Karim, S. The relationship between the length and weight of snail Nerita lineata Gmelin 1791 on environmental factors in the mangrove ecosystem. J. Phys. Conf. Ser. 2019, 1341, 022022. [Google Scholar] [CrossRef] [Green Version]
- Aljahdali, M.O.; Alhassan, A.B. Spatial variation of metallic contamination and its ecological risk in sediment and freshwater mollusk: Melanoides tuberculata (Müller, 1774) (Gastropoda: Thiaridae). Water 2020, 12, 206. [Google Scholar] [CrossRef] [Green Version]
- Łuczyńska, J.; Tońska, E.; Paszczyk, B.; Łuczyński, M.J. The relationship between biotic factors and the content of chosen heavy metals (Zn, Fe, Cu and Mn) in six wild freshwater fish species collected from two lakes (Łańskie and Pluszne) located in northeastern Poland. Iran. J. Fish. Sci. 2020, 19, 421–442. [Google Scholar]
- Yap, C.K.; Ismail, A.; Tan, S.G. Effect of body size on heavy metal contents and concentrations in green-lipped mussel perna viridis (Linnaeus) from Malaysian coastal waters. Pertanika J. Sci. Technol. 2009, 17, 61–68. [Google Scholar]
- Williamson, P. Variables affecting body burdens of lead, zinc and cadmium in a roadside population of the snail Cepaea hortensis muller. Oecologia 1980, 44, 213–220. [Google Scholar] [CrossRef]

| Locations | Values | Metal Concentration (µg/g, Dry Weight) | Ref | |||||
|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Co | Pb | Mn | Ni | |||
| Libong Island, Thailand | Mean | 2711.94 | 0.47 | 0.11 | 1.32 | 40.41 | 2.75 | This study |
| S.D. | 1428.83 | 0.37 | 0.09 | 1.48 | 16.12 | 4.04 | ||
| Min | 1264.00 | 0.10 | 0.10 | 0.18 | 20.06 | 0.42 | ||
| Max | 5711.00 | 1.34 | 0.42 | 7.69 | 82.79 | 22.88 | ||
| Kharg coral, Iran Lark coral, Iran | Mean Mean | - - | 16.40 5.00 | 6.60 4.00 | 0.72 0.35 | 14.40 5.00 | 18.20 8.40 | [41] |
| Hormuz, Iran Lark, Iran Qeshm, Iran Hengam, Iran | Mean Mean Mean Mean | 32,555.00 33,699.00 34,935.00 30,553.00 | 2.03 3.93 2.44 4.78 | 1.09 2.10 1.31 2.32 | 0.49 0.63 0.53 0.75 | 4.87 7.52 5.41 7.92 | 17.25 33.91 19.49 28.07 | [42] |
| Thondi Coast, India | Mean | 52,802.30 | 54.70 | - | 14.1 | 686.1 | 27.7 | [43] |
| Hainan island, china | Mean | - | 14.97 | 13.21 | 26.82 | - | 25.26 | [44] |
| Arabian Gulf, Saudi Arabia | Mean | 8474.21 | 297.29 | 4.01 | 5.25 | 111.57 | 77.07 | [45] |
| Gulf of Thailand | Mean | - | 12.25 | - | 21.35 | - | - | [46] |
| Xiamen Bay, China | Mean | 32,783.00 | 52.51 | 19.87 | 37.49 | 674.00 | 23.93 | [47] |
| Bohai Sea, china | Mean | 13,000.00 | 16.84 | 7.69 | 11.36 | 502.96 | 18.50 | [48] |
| Gulf of Thailand | Mean | 17,860.00 | 7.41 | - | 1.81 | - | - | [49] |
| Mediterranean Sea, Egypt | Mean | 13,256.00 | 8.46 | 8.24 | 13.17 | 381.00 | 25.93 | [50] |
| Egyptian Red Sea Coast | Mean | 3490.00 | 1.94 | 9.69 | 3.26 | 127.08 | 11.40 | [51] |
| Selangor, Malaysia | Mean | - | 24.89 | - | 96.02 | 219.10 | 13.90 | [52] |
| Gulf of Gemlik, Turkey | Mean | 44,000.00 | 41.00 | 19.00 | 29.00 | 634.00 | 110.00 | [53] |
| Metals | US EPA | Canadian Sediment Quality Guidelines | NoAA | ||||
|---|---|---|---|---|---|---|---|
| Non-Polluted | Moderately Polluted | Heavily Polluted | TEL | PEL | ERL | ERM | |
| Fe (%) | - | - | - | - | - | - | - |
| Cu | <25 | 25–50 | >50 | 18.7 | 108.2 | 34 | 270 |
| Co | - | - | - | - | - | - | - |
| Pb | <40 | 40–60 | >60 | 30.2 | 112.0 | 46.7 | 218.0 |
| Mn | <300 | 300–500 | >500 | - | - | - | - |
| Ni | <20 | 20–50 | >50 | 15.9 | 42.8 | 20.9 | 15.9 |
| Values | Enrichment Factor (EF) | Geoaccumulation Index (Igeo) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Co | Pb | Mn | Ni | Cu | Co | Pb | Mn | Ni | |
| Mean | 0.18 | 0.06 | 0.50 | 2.48 | 1.16 | 0.04 | 0.01 | 0.10 | 0.50 | 0.18 |
| S.D. | 0.13 | 0.03 | 0.55 | 1.13 | 1.78 | 0.03 | 0.01 | 0.11 | 0.23 | 0.36 |
| Min | 0.02 | 0.00 | 0.06 | 0.63 | 0.07 | 0.00 | 0.00 | 0.01 | 0.13 | 0.00 |
| Max | 0.52 | 0.12 | 2.68 | 5.46 | 10.13 | 0.10 | 0.03 | 0.54 | 1.10 | 2.03 |
| Locations | Species | Values | Dry Weight Fraction (µg/g) | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Co | Pb | Mn | Ni | ||||
| Libong Island, Thailand | Gastropod (Strombus canarium) | Mean | 200.86 | 8.40 | 0.29 | 0.08 | 18.30 | 0.46 | This study |
| S.D. | 227.83 | 10.56 | 0.21 | 0.37 | 18.47 | 0.47 | |||
| Min | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Max | 864.50 | 58.93 | 0.92 | 2.06 | 67.72 | 1.38 | |||
| Lake Manzala, Egypt | Gastropod (Lanistes carinatus) | Mean | 567.28 | 2.65 | 3.08 | 0.37 | 24.91 | 6.19 | [76] |
| South India | Gastropod (Tibia curta) | Mean | 76.85 | 37.02 | 0.35 | 2.64 | 8.50 | 3.21 | [77] |
| North–Eastern Mediterranean | Gastropod (Monodonta turbinata) | Mean | 3176.85 | 334.47 | 12.76 | 90.80 | 49.40 | 26.93 | [78] |
| Red Sea coast | Bivalve (Tridacna maxima) | Mean | 600.00 | 39.73 | 1.63 | 33.66 | 41.77 | 38.88 | [79] |
| El-Mex, Mediterranean coast | Bivalve (Mytilidae) | Mean | - | 11.85 | 4.25 | 0.43 | - | 14.95 | [80] |
| Marudu Bay, Malaysia | Bivalve (Meretrix meretrix) | Mean | 285.50 | 74.90 | - | 2.77 | 20.30 | 4.23 | [81] |
| Fe | Cu | Co | Pb | Mn | Ni | |
|---|---|---|---|---|---|---|
| Fe | 1 | |||||
| Cu | 0.279 | 1 | ||||
| Co | −0.116 | 0.390 * | 1 | |||
| Pb | 0.310 | −0.063 | −0.272 | 1 | ||
| Mn | 0.770 ** | 0.283 | −0.015 | 0.061 | 1 | |
| Ni | 0.073 * | −0.174 | −0.033 | 0.036 | 0.084 | 1 |
| Weight | Fe | Cu | Co | Pb | Mn | Ni | |
|---|---|---|---|---|---|---|---|
| Weight | 1 | ||||||
| Fe | 0.559 ** | 1 | |||||
| Cu | 0.127 | 0.279 | 1 | ||||
| Co | −0.045 | −0.116 | 0.390 * | 1 | |||
| Pb | −0.042 | 0.310 | −0.063 | −0.272 | 1 | ||
| Mn | 0.426 * | 0.770 ** | 0.283 | −0.015 | 0.061 | 1 | |
| Ni | −0.086 | 0.073 | −0.174 | −0.033 | 0.036 | 0.084 | 1 |
| Metal | Mean Metal Concentration (mg/kg) ww | EDI (mg/kg bw/day) | Rfd (mg/kg bw/day) | THQ |
|---|---|---|---|---|
| Fe | 52.22 | 0.1175 | 0.70 | 0.1679 |
| Cu | 2.18 | 0.0049 | 0.04 | 0.1229 |
| Co | 0.08 | 0.0002 | 0.03 | 0.0057 |
| Pb | 0.02 | 0.0001 | 0.004 | 0.0117 |
| Mn | 4.76 | 0.0107 | 0.14 | 0.0765 |
| Ni | 0.12 | 0.0003 | 0.02 | 0.0135 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobkeatthawin, T.; Sirivithayapakorn, S.; Nitiratsuwan, T.; Muenhor, D.; Loh, P.-S.; Pradit, S. Accumulation of Trace Metal in Sediment and Soft Tissue of Strombus canarium in a Tropical Remote Island of Thailand. J. Mar. Sci. Eng. 2021, 9, 991. https://doi.org/10.3390/jmse9090991
Kobkeatthawin T, Sirivithayapakorn S, Nitiratsuwan T, Muenhor D, Loh P-S, Pradit S. Accumulation of Trace Metal in Sediment and Soft Tissue of Strombus canarium in a Tropical Remote Island of Thailand. Journal of Marine Science and Engineering. 2021; 9(9):991. https://doi.org/10.3390/jmse9090991
Chicago/Turabian StyleKobkeatthawin, Thawanrat, Sanya Sirivithayapakorn, Thongchai Nitiratsuwan, Dudsadee Muenhor, Pei-Sun Loh, and Siriporn Pradit. 2021. "Accumulation of Trace Metal in Sediment and Soft Tissue of Strombus canarium in a Tropical Remote Island of Thailand" Journal of Marine Science and Engineering 9, no. 9: 991. https://doi.org/10.3390/jmse9090991







