Unseen Drivers of Antimicrobial Resistance: The Role of Industrial Agriculture and Climate Change in This Global Health Crisis
Abstract
1. Introduction
2. Background
2.1. The Study of AMR and Importance of Education
2.2. Industrial Agricultural Practices and Their Impact on the Environment
2.3. Soil Microbiota: The Unseen Ecosystems
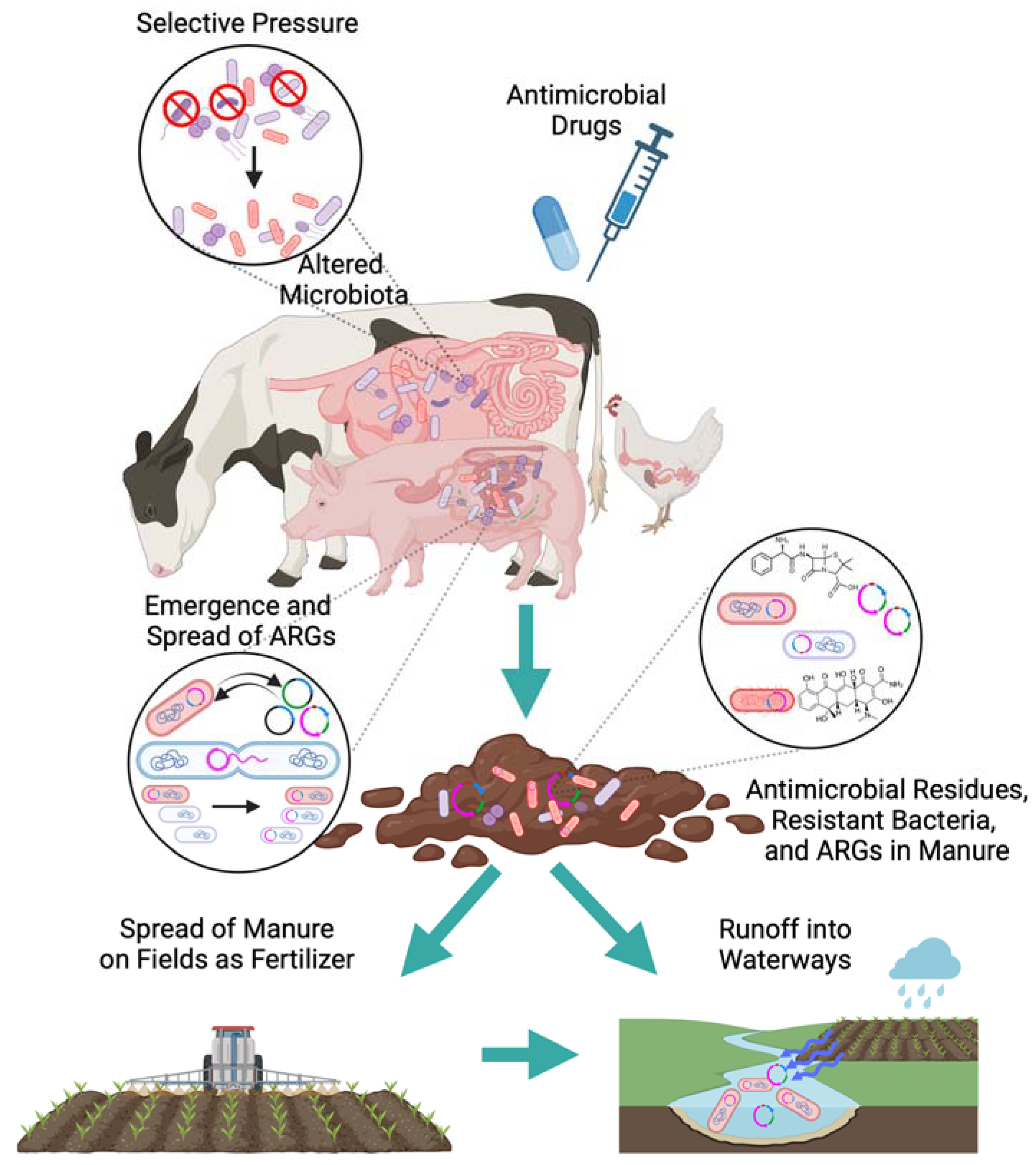
2.4. Industrial Livestock Production
3. Review of Current Sources and Drivers of AMR in the Environment
3.1. Influence of Industrial Crop Production on Soil Microbial Diversity and AMR
3.2. Influence of Conventional Livestock Production on AMR
3.3. Effects of Global Climate Change on AMR and Food Systems
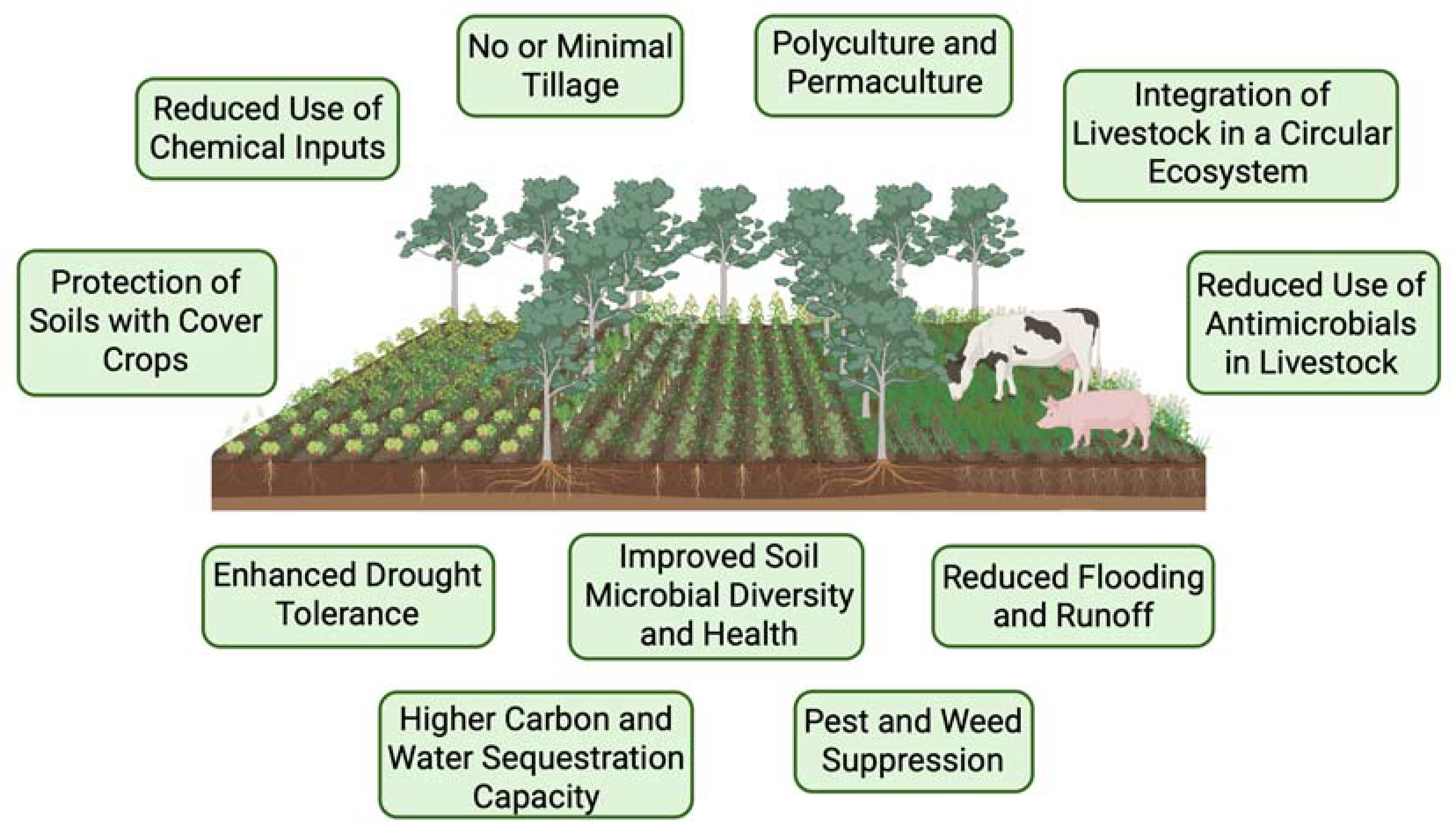
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2013; 2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Mudenda, S.; Daka, V.; Matafwali, S.K. World Health Organization AWaRe framework for antibiotic stewardship: Where are we now and where do we need to go? An expert viewpoint. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e84. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Ho, B.T. Revenge of the Microbes, 2nd ed.; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar]
- CDC. Antimicrobial Resistance Threats in the United States, 2021–2022 [Fact Sheet]; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2024. [Google Scholar]
- Goryluk-Salmonowicz, A.; Popowska, M. Factors promoting and limiting antimicrobial resistance in the environment—Existing knowledge gaps. Front. Microbiol. 2022, 13, 992268. [Google Scholar] [CrossRef]
- Liu, C.M.; Aziz, M.; Park, D.E.; Wu, Z.; Stegger, M.; Li, M.; Wang, Y.; Schmidlin, K.; Johnson, T.J.; Koch, B.J. Using source-associated mobile genetic elements to identify zoonotic extraintestinal E. coli infections. One Health 2023, 16, 100518. [Google Scholar] [CrossRef]
- Adisasmito, W.B.; Almuhairi, S.; Behravesh, C.B.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Cediel Becerra, N.; Charron, D.F.; Chaudhary, A.; Ciacci Zanella, J.R.; et al. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022, 18, e1010537. [Google Scholar] [CrossRef]
- Rogers Van Katwyk, S.; Hoffman, S.J.; Mendelson, M.; Taljaard, M.; Grimshaw, J.M. Strengthening the science of addressing antimicrobial resistance: A framework for planning, conducting and disseminating antimicrobial resistance intervention research. Health Res. Policy Syst. 2020, 18, 60. [Google Scholar] [CrossRef]
- Calvo-Villamañán, A.; San Millán, Á.; Carrilero, L. Tackling AMR from a multidisciplinary perspective: A primer from education and psychology. Int. Microbiol. 2023, 26, 1–9. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Van Boeckel, T.; Frost, I.; Kariuki, S.; Khan, E.A.; Limmathurotsakul, D.; Larsson, D.G.J.; Levy-Hara, G.; Mendelson, M.; Outterson, K.; et al. The Lancet Infectious Diseases Commission on antimicrobial resistance: 6 years later. Lancet Infect. Dis. 2020, 20, e51–e60. [Google Scholar] [CrossRef]
- CDC. About Antimicrobial Resistance Investments & Action; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2024. [Google Scholar]
- Cole, J.; Eskdale, A.; Paul, J.D. Tackling AMR: A Call for a(n Even) More Integrated and Transdisciplinary Approach between Planetary Health and Earth Scientists. Challenges 2022, 13, 66. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance [Fact Sheet]; Wold Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria. Advancing Interprofessional Education and Practice to Combat Antimicrobial Resistance; The Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria, Ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2021. [Google Scholar]
- Wang, R.; Degnan, K.O.; Luther, V.P.; Szymczak, J.E.; Goren, E.N.; Logan, A.; Shnekendorf, R.; Hamilton, K.W. Development of a Multifaceted Antimicrobial Stewardship Curriculum for Undergraduate Medical Education: The Antibiotic Stewardship, Safety, Utilization, Resistance, and Evaluation (ASSURE) Elective. Open Forum Infect. Dis. 2021, 8, ofab231. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.F. Fighting Back against Antimicrobial Resistance with Comprehensive Policy and Education: A Narrative Review. Antibiotics 2022, 11, 644. [Google Scholar] [CrossRef]
- Augie, B.M.; Miot, J.; van Zyl, R.L.; McInerney, P.A. Educational antimicrobial stewardship programs in medical schools: A scoping review. JBI Evid. Synth. 2021, 19, 2906–2928. [Google Scholar] [CrossRef]
- Luther, V.P.; Ohl, C.A.; Hicks, L.A. Antimicrobial Stewardship Education for Medical Students. Clin. Infect. Dis. 2013, 57, 1366. [Google Scholar] [CrossRef]
- Luther, V.P.; Ohl, C. Get Smart About Antibiotics: An Antibiotic Stewardship Curriculum; Wake Forest University School of Medicine: Winston-Salem, NC, USA, 2012. [Google Scholar]
- Espinosa-Gongora, C.; Jessen, L.R.; Dyar, O.J.; Bousquet-Melou, A.; González-Zorn, B.; Pulcini, C.; Re, G.; Schwarz, S.; Timofte, D.; Toutain, P.L.; et al. Towards a Better and Harmonized Education in Antimicrobial Stewardship in European Veterinary Curricula. Antibiotics 2021, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Granick, J.L.; Fellman, C.L.; DeStefano, I.M.; Diaz-Campos, D.; Janovyak, E.; Beaudoin, A.L.; Bollig, E.R. A survey of US and Caribbean veterinary schools reveals strengths and opportunities in antimicrobial stewardship and infection prevention and control activities. J. Am. Vet. Med. Assoc. 2024, 262, 1485–1490. [Google Scholar] [CrossRef]
- Goldman, M.; Vaidyam, A.; Parupalli, S.; Rosencranz, H.; Ramkumar, D.; Ramkumar, J. Food Systems and Planetary Health Nexus Elective: A Novel Approach to A Medical Education Imperative for the 21st Century. Challenges 2024, 15, 6. [Google Scholar] [CrossRef]
- Cozim-Melges, F.; Ripoll-Bosch, R.; Veen, G.F.; Oggiano, P.; Bianchi, F.J.; Van Der Putten, W.H.; Van Zanten, H.H. Farming practices to enhance biodiversity across biomes: A systematic review. NPJ Biodivers. 2024, 3, 1. [Google Scholar] [CrossRef]
- Struik, P.C.; Kuyper, T.W. Sustainable intensification in agriculture: The richer shade of green. A review. Agron. Sustain. Dev. 2017, 37, 39. [Google Scholar] [CrossRef]
- Lin, B.B.; Chappell, M.J.; Vandermeer, J.; Smith, G.; Quintero, E.; Bezner-Kerr, R.; Griffith, D.M.; Ketcham, S.; Latta, S.C.; McMichael, P. Effects of industrial agriculture on climate change and the mitigation potential of small-scale agro-ecological farms. Cabi Rev. 2011, 6, 1–18. [Google Scholar] [CrossRef]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef]
- Blum, W.E.H.; Zechmeister-Boltenstern, S.; Keiblinger, K.M. Does Soil Contribute to the Human Gut Microbiome? Microorganisms 2019, 7, 287. [Google Scholar] [CrossRef]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health—A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Lyu, D.; Msimbira, L.A.; Nazari, M.; Antar, M.; Pagé, A.; Shah, A.; Monjezi, N.; Zajonc, J.; Tanney, C.A.S.; Backer, R.; et al. The Coevolution of Plants and Microbes Underpins Sustainable Agriculture. Microorganisms 2021, 9, 1036. [Google Scholar] [CrossRef]
- Jiao, S.; Wang, J.; Wei, G.; Chen, W.; Lu, Y. Dominant role of abundant rather than rare bacterial taxa in maintaining agro-soil microbiomes under environmental disturbances. Chemosphere 2019, 235, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Riesenfeld, C.S.; Goodman, R.M.; Handelsman, J. Uncultured soil bacteria are a reservoir of new antibiotic resistance genes. Environ. Microbiol. 2004, 6, 981–989. [Google Scholar] [CrossRef]
- Springael, D.; Top, E.M. Horizontal gene transfer and microbial adaptation to xenobiotics: New types of mobile genetic elements and lessons from ecological studies. TRENDS Microbiol. 2004, 12, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Matamoros, V.; Bayona, J.M.; Berendonk, T.U.; Elsinga, G.; Hornstra, L.M.; Piña, B. Antibiotic resistance gene distribution in agricultural fields and crops. A soil-to-food analysis. Environ. Res. 2019, 177, 108608. [Google Scholar] [CrossRef]
- Despotovic, M.; de Nies, L.; Busi, S.B.; Wilmes, P. Reservoirs of antimicrobial resistance in the context of One Health. Curr. Opin. Microbiol. 2023, 73, 102291. [Google Scholar] [CrossRef] [PubMed]
- Kaviani Rad, A.; Astaykina, A.; Streletskii, R.; Afsharyzad, Y.; Etesami, H.; Zarei, M.; Balasundram, S.K. An Overview of Antibiotic Resistance and Abiotic Stresses Affecting Antimicrobial Resistance in Agricultural Soils. Int. J. Environ. Res. Public Health 2022, 19, 4666. [Google Scholar] [CrossRef]
- Kelbrick, M.; Hesse, E.; O’Brien, S. Cultivating antimicrobial resistance: How intensive agriculture ploughs the way for antibiotic resistance. Microbiology 2023, 169, 001384. [Google Scholar] [CrossRef]
- Li, Y.; Song, D.; Liang, S.; Dang, P.; Qin, X.; Liao, Y.; Siddique, K.H.M. Effect of no-tillage on soil bacterial and fungal community diversity: A meta-analysis. Soil Tillage Res. 2020, 204, 104721. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Barros-Rodríguez, A.; Rangseekaew, P.; Lasudee, K.; Pathom-Aree, W.; Manzanera, M. Impacts of Agriculture on the Environment and Soil Microbial Biodiversity. Plants 2021, 10, 2325. [Google Scholar] [CrossRef]
- Klümper, U.; Gionchetta, G.; Catão, E.; Bellanger, X.; Dielacher, I.; Elena, A.X.; Fang, P.; Galazka, S.; Goryluk-Salmonowicz, A.; Kneis, D.; et al. Environmental microbiome diversity and stability is a barrier to antimicrobial resistance gene accumulation. Commun. Biol. 2024, 7, 706. [Google Scholar] [CrossRef]
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic Stewardship in Food-producing Animals: Challenges, Progress, and Opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Nehra, K.; Rana, J.S.; Dahiya, T. Antibiotic resistance in agriculture: Perspectives on upcoming strategies to overcome upsurge in resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef]
- US Food and Drug Administration (Ed.) FDA Releases Annual Summary Report on Antimicrobials Sold or Distributed in 2022 for Use in Food-Producing Animals; US FDA: Silver Spring, MD, USA, 2023. [Google Scholar]
- World Health Organization (Ed.) WHO’s List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- US Food and Drug Administration (Ed.) FDA Announces Transition of Over-the-Counter Medically Important Antimicrobials for Animals to Prescription Status; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2023. [Google Scholar]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Onwona-Kwakye, M.; Plants-Paris, K.; Keita, K.; Lee, J.; Brink, P.; Hogarh, J.N.; Darkoh, C. Pesticides Decrease Bacterial Diversity and Abundance of Irrigated Rice Fields. Microorganisms 2020, 8, 318. [Google Scholar] [CrossRef]
- Walder, F.; Schmid, M.W.; Riedo, J.; Valzano-Held, A.Y.; Banerjee, S.; Büchi, L.; Bucheli, T.D.; van der Heijden, M.G.A. Soil microbiome signatures are associated with pesticide residues in arable landscapes. Soil Biol. Biochem. 2022, 174, 108830. [Google Scholar] [CrossRef]
- Qiu, D.; Ke, M.; Zhang, Q.; Zhang, F.; Lu, T.; Sun, L.; Qian, H. Response of microbial antibiotic resistance to pesticides: An emerging health threat. Sci. Total Environ. 2022, 850, 158057. [Google Scholar] [CrossRef]
- Kurenbach, B.; Marjoshi, D.; Amábile-Cuevas, C.F.; Ferguson, G.C.; Godsoe, W.; Gibson, P.; Heinemann, J.A. Sublethal exposure to commercial formulations of the herbicides dicamba, 2, 4-dichlorophenoxyacetic acid, and glyphosate cause changes in antibiotic susceptibility in Escherichia coli and Salmonella enterica serovar Typhimurium. mBio 2015, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; An, X.-L.; Zheng, B.-X.; Gillings, M.; Peñuelas, J.; Cui, L.; Su, J.-Q.; Zhu, Y.-G. Loss of soil microbial diversity exacerbates spread of antibiotic resistance. Soil Ecol. Lett. 2019, 1, 3–13. [Google Scholar] [CrossRef]
- Roslund, M.I.; Laitinen, O.H.; Sinkkonen, A. Scoping review on soil microbiome and gut health—Are soil microorganisms missing from the planetary health plate? People Nat. 2024, 6, 1078–1095. [Google Scholar] [CrossRef]
- Peltoniemi, K.; Velmala, S.; Fritze, H.; Lemola, R.; Pennanen, T. Long-term impacts of organic and conventional farming on the soil microbiome in boreal arable soil. Eur. J. Soil Biol. 2021, 104, 103314. [Google Scholar] [CrossRef]
- Fess, T.L.; Benedito, V.A. Organic versus conventional cropping sustainability: A comparative system analysis. Sustainability 2018, 10, 272. [Google Scholar] [CrossRef]
- Montgomery, D.R.; Biklé, A.; Archuleta, R.; Brown, P.; Jordan, J. Soil health and nutrient density: Preliminary comparison of regenerative and conventional farming. PeerJ 2022, 10, e12848. [Google Scholar] [CrossRef]
- Ramkumar, D.; Marty, A.; Ramkumar, J.; Rosencranz, H.; Vedantham, R.; Goldman, M.; Meyer, E.; Steinmetz, J.; Weckle, A.; Bloedorn, K.; et al. Food for thought: Making the case for food produced via regenerative agriculture in the battle against non-communicable chronic diseases (NCDs). One Health 2024, 18, 100734. [Google Scholar] [CrossRef]
- Vandevijvere, S.; Pedroni, C.; De Ridder, K.; Castetbon, K. The Cost of Diets According to Their Caloric Share of Ultraprocessed and Minimally Processed Foods in Belgium. Nutrients 2020, 12, 2787. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A. Is the world converging to a ‘Western diet’? Public Health Nutr. 2021, 24, 309–317. [Google Scholar] [CrossRef]
- Kirchhelle, C. Pharming animals: A global history of antibiotics in food production (1935–2017). Palgrave Commun. 2018, 4, 96. [Google Scholar] [CrossRef]
- Wallinga, D.; Smit, L.A.M.; Davis, M.F.; Casey, J.A.; Nachman, K.E. A Review of the Effectiveness of Current US Policies on Antimicrobial Use in Meat and Poultry Production. Curr. Environ. Health Rep. 2022, 9, 339–354. [Google Scholar] [CrossRef]
- Cazer, C.L.; Eldermire, E.R.B.; Lhermie, G.; Murray, S.A.; Scott, H.M.; Gröhn, Y.T. The effect of tylosin on antimicrobial resistance in beef cattle enteric bacteria: A systematic review and meta-analysis. Prev. Vet. Med. 2020, 176, 104934. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Tian, Y.; Shen, Y.; Wang, S.; Zhang, Y. Clinical Impact of Colistin Banning in Food Animal on mcr-1-Positive Enterobacteriaceae in Patients From Beijing, China, 2009–2019: A Long-Term Longitudinal Observational Study. Front. Microbiol. 2022, 13, 826624. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Hassan, B.; Farzana, R.; Ali, Q.; Sands, K.; Mathias, J.; Afegbua, S.; Haque, M.N.; Walsh, T.R.; Mohsin, M. International manufacturing and trade in colistin, its implications in colistin resistance and One Health global policies: A microbiological, economic, and anthropological study. Lancet Microbe 2023, 4, e264–e276. [Google Scholar] [CrossRef]
- Fenollar, A.; Doménech, E.; Ferrús, M.A.; Jiménez-Belenguer, A. Risk Characterization of Antibiotic Resistance in Bacteria Isolated from Backyard, Organic, and Regular Commercial Eggs. J. Food Prot. 2019, 82, 422–428. [Google Scholar] [CrossRef]
- de Alcântara Rodrigues, I.; Ferrari, R.G.; Panzenhagen, P.H.N.; Mano, S.B.; Conte-Junior, C.A. Chapter Four—Antimicrobial resistance genes in bacteria from animal-based foods. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 112, pp. 143–183. [Google Scholar]
- Hassani, S.; Moosavy, M.-H.; Gharajalar, S.N.; Khatibi, S.A.; Hajibemani, A.; Barabadi, Z. High prevalence of antibiotic resistance in pathogenic foodborne bacteria isolated from bovine milk. Sci. Rep. 2022, 12, 3878. [Google Scholar] [CrossRef] [PubMed]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- Prado, J.; Ribeiro, H.; Alvarenga, P.; Fangueiro, D. A step towards the production of manure-based fertilizers: Disclosing the effects of animal species and slurry treatment on their nutrients content and availability. J. Clean. Prod. 2022, 337, 130369. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.-G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef]
- Sanz, C.; Casado, M.; Navarro-Martin, L.; Cañameras, N.; Carazo, N.; Matamoros, V.; Bayona, J.M.; Piña, B. Implications of the use of organic fertilizers for antibiotic resistance gene distribution in agricultural soils and fresh food products. A plot-scale study. Sci. Total Environ. 2022, 815, 151973. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment-Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zheng, H.; Herrero-Fresno, A.; Olsen, J.E.; Dalsgaard, A.; Ding, Z. Co-occurrence of antimicrobial and metal resistance genes in pig feces and agricultural fields fertilized with slurry. Sci. Total Environ. 2021, 792, 148259. [Google Scholar] [CrossRef]
- Blot, K.; Hammami, N.; Blot, S.; Vogelaers, D.; Lambert, M.-L. Seasonal variation of hospital-acquired bloodstream infections: A national cohort study. Infect. Control Hosp. Epidemiol. 2022, 43, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Kito, Y.; Kuwabara, K.; Ono, K.; Kato, K.; Yokoi, T.; Horiguchi, K.; Kato, K.; Hirose, M.; Ohara, T.; Goto, K.; et al. Seasonal variation in the prevalence of Gram-negative bacilli in sputum and urine specimens from outpatients and inpatients. Fujita Med. J. 2022, 8, 46–51. [Google Scholar] [CrossRef] [PubMed]
- van der Wiel, K.; Bintanja, R. Contribution of climatic changes in mean and variability to monthly temperature and precipitation extremes. Commun. Earth Environ. 2021, 2, 1. [Google Scholar] [CrossRef]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Chapter 4—Climate change and future of agri-food production. In Future Foods; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 49–79. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Change 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Ho, H.C.; Shi, L.; Zeng, Y.; Yang, X.; Huang, Q.; Pei, Y.; Huang, C.; Yang, L. Association between antibiotic resistance and increasing ambient temperature in China: An ecological study with nationwide panel data. Lancet Reg. Health–West. Pac. 2023, 30, 100628. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, W.; Zhao, M.; Li, J.; Liu, X.; Shi, L.; Yang, X.; Xia, H.; Yang, S.; Yang, L. The association between ambient temperature and antimicrobial resistance of Klebsiella pneumoniae in China: A difference-in-differences analysis. Front. Public Health 2023, 11, 1158762. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Change 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Semenza, J.C.; Ko, A.I. Waterborne Diseases That Are Sensitive to Climate Variability and Climate Change. N. Engl. J. Med. 2023, 389, 2175–2187. [Google Scholar] [CrossRef]
- Chua, P.L.C.; Ng, C.F.S.; Tobias, A.; Seposo, X.T.; Hashizume, M. Associations between ambient temperature and enteric infections by pathogen: A systematic review and meta-analysis. Lancet Planet. Health 2022, 6, e202–e218. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Loya, M.; Tekin, E.; Kang, T.M.; Cardona, N.; Lozano-Huntelman, N.; Rodriguez-Verdugo, A.; Savage, V.M.; Yeh, P.J. Antibiotics Shift the Temperature Response Curve of Escherichia coli Growth. mSystems 2021, 6, e0022821. [Google Scholar] [CrossRef] [PubMed]
- Burnham, J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021, 8, 2049936121991374. [Google Scholar] [CrossRef] [PubMed]
- Ebi, K.L.; Vanos, J.; Baldwin, J.W.; Bell, J.E.; Hondula, D.M.; Errett, N.A.; Hayes, K.; Reid, C.E.; Saha, S.; Spector, J.; et al. Extreme Weather and Climate Change: Population Health and Health System Implications. Annu. Rev. Public Health 2021, 42, 293–315. [Google Scholar] [CrossRef]
- Lynch, V.D.; Shaman, J. Waterborne Infectious Diseases Associated with Exposure to Tropical Cyclonic Storms, United States, 1996–2018. Emerg. Infect. Dis. 2023, 29, 1548–1558. [Google Scholar] [CrossRef]
- Yang, S.-H.; Chen, C.-H.; Chu, K.-H. Fecal indicators, pathogens, antibiotic resistance genes, and ecotoxicity in Galveston Bay after Hurricane Harvey. J. Hazard. Mater. 2021, 411, 124953. [Google Scholar] [CrossRef]
- Pérez-Valdespino, A.; Pircher, R.; Pérez-Domínguez, C.Y.; Mendoza-Sanchez, I. Impact of flooding on urban soils: Changes in antibiotic resistance and bacterial community after Hurricane Harvey. Sci. Total Environ. 2021, 766, 142643. [Google Scholar] [CrossRef]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Chang 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Brumfield Kyle, D.; Usmani, M.; Santiago, S.; Singh, K.; Gangwar, M.; Hasan Nur, A.; Netherland, M.; Deliz, K.; Angelini, C.; Beatty Norman, L.; et al. Genomic diversity of Vibrio spp. and metagenomic analysis of pathogens in Florida Gulf coastal waters following Hurricane Ian. mBio 2023, 14, e01476-23. [Google Scholar] [CrossRef]
- Ngcamu, B.S. Climate change effects on vulnerable populations in the Global South: A systematic review. Nat. Hazards 2023, 118, 977–991. [Google Scholar] [CrossRef]
- Schmitt, J.; Offermann, F.; Söder, M.; Frühauf, C.; Finger, R. Extreme weather events cause significant crop yield losses at the farm level in German agriculture. Food Policy 2022, 112, 102359. [Google Scholar] [CrossRef]
- IPCC. Food, Fibre, and Other Ecosystem Products. In Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; United Nations Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2023; pp. 713–906. [Google Scholar] [CrossRef]
- Hasegawa, T.; Wakatsuki, H.; Ju, H.; Vyas, S.; Nelson, G.C.; Farrell, A.; Deryng, D.; Meza, F.; Makowski, D. A global dataset for the projected impacts of climate change on four major crops. Sci. Data 2022, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-C.; Hsiao, Y.-H.; Chang, C.-W.; Chen, Y.-M.; Lin, L.-Y. Agriculture Adaptation Options for Flood Impacts under Climate Change—A Simulation Analysis in the Dajia River Basin. Sustainability 2021, 13, 7311. [Google Scholar] [CrossRef]
- UN. World Population Prospects 2022; United Nations Department of Economic and Social Affairs, Ed.; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Ortiz, A.M.D.; Outhwaite, C.L.; Dalin, C.; Newbold, T. A review of the interactions between biodiversity, agriculture, climate change, and international trade: Research and policy priorities. One Earth 2021, 4, 88–101. [Google Scholar] [CrossRef]
- Bassitta, R.; Nottensteiner, A.; Bauer, J.; Straubinger, R.K.; Hölzel, C.S. Spread of antimicrobial resistance genes via pig manure from organic and conventional farms in the presence or absence of antibiotic use. J. Appl. Microbiol. 2022, 133, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Ager, E.O.; Carvalho, T.; Silva, E.M.; Ricke, S.C.; Hite, J.L. Global trends in antimicrobial resistance on organic and conventional farms. Sci. Rep. 2023, 13, 22608. [Google Scholar] [CrossRef]
- Quandt, A.; Neufeldt, H.; Gorman, K. Climate change adaptation through agroforestry: Opportunities and gaps. Curr. Opin. Environ. Sustain. 2023, 60, 101244. [Google Scholar] [CrossRef]
- Huss, C.P.; Holmes, K.D.; Blubaugh, C.K. Benefits and Risks of Intercropping for Crop Resilience and Pest Management. J. Econ. Entomol. 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- Beule, L.; Vaupel, A.; Moran-Rodas, V.E. Abundance, Diversity, and Function of Soil Microorganisms in Temperate Alley-Cropping Agroforestry Systems: A Review. Microorganisms 2022, 10, 616. [Google Scholar] [CrossRef]
- Ghale, B.; Mitra, E.; Sodhi, H.S.; Verma, A.K.; Kumar, S. Carbon Sequestration Potential of Agroforestry Systems and Its Potential in Climate Change Mitigation. Water Air Soil Pollut. 2022, 233, 228. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Ashworth, A.J.; O’Brien, P.L.; Thomas, A.L.; Runkle, B.R.K.; Philipp, D. Temperate silvopastures provide greater ecosystem services than conventional pasture systems. Sci. Rep. 2023, 13, 18658. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidance on wastewater and solid waste management for manufacturing of antibiotics. In UN Environment Program; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Wang, K.; Zhuang, T.; Su, Z.; Chi, M.; Wang, H. Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: Occurrence, removal and environmental impacts. Sci. Total Environ. 2021, 788, 147811. [Google Scholar] [CrossRef]
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment—A review. J. Water Process Eng. 2022, 45, 102474. [Google Scholar] [CrossRef]





| Antibiotic Used in Livestock | Medically Important Antibiotics That It Selects for Resistance to | Use in Human Medicine |
|---|---|---|
| Tylosin | Macrolides | Postsurgical infections, sexually transmitted infections, bacterial upper respiratory infections, bacterial pneumonia |
| Quinupristin/dalfopristin combination therapy | Multidrug-resistant bacterial infections, postsurgical infection, systemic bacterial infections | |
| Oxytetracycline | Tetracyclines | Bacterial pneumonia, methicillin-resistant Staphylococcus aureus, Lyme disease, intracellular bacterial infections |
| Sulfamethazine | Sulfonamides | Urinary tract infections, otitis media, used in combination with trimethoprim and as monotherapy |
| Enrofloxacin | Fluoroquinolones | Urinary tract infections, bacterial enteric infections, sexually transmitted infections, bacterial pneumonia |
| Avoparcin | Vancomycin | Postsurgical infections, systemic bacterial infections, bacterial pneumonia, bacterial endocarditis |
| Colistin | Colistin, polymyxin E | Multidrug-resistant Gram-negative infections, bacterial pneumonia, bacterial meningitis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graham, M.E.; Wilson, B.A.; Ramkumar, D.; Rosencranz, H.; Ramkumar, J. Unseen Drivers of Antimicrobial Resistance: The Role of Industrial Agriculture and Climate Change in This Global Health Crisis. Challenges 2025, 16, 22. https://doi.org/10.3390/challe16020022
Graham ME, Wilson BA, Ramkumar D, Rosencranz H, Ramkumar J. Unseen Drivers of Antimicrobial Resistance: The Role of Industrial Agriculture and Climate Change in This Global Health Crisis. Challenges. 2025; 16(2):22. https://doi.org/10.3390/challe16020022
Chicago/Turabian StyleGraham, Madeline E., Brenda A. Wilson, Davendra Ramkumar, Holly Rosencranz, and Japhia Ramkumar. 2025. "Unseen Drivers of Antimicrobial Resistance: The Role of Industrial Agriculture and Climate Change in This Global Health Crisis" Challenges 16, no. 2: 22. https://doi.org/10.3390/challe16020022
APA StyleGraham, M. E., Wilson, B. A., Ramkumar, D., Rosencranz, H., & Ramkumar, J. (2025). Unseen Drivers of Antimicrobial Resistance: The Role of Industrial Agriculture and Climate Change in This Global Health Crisis. Challenges, 16(2), 22. https://doi.org/10.3390/challe16020022





