Mathematical Modeling: Global Stability Analysis of Super Spreading Transmission of Respiratory Syncytial Virus (RSV) Disease
Abstract
:1. Introduction
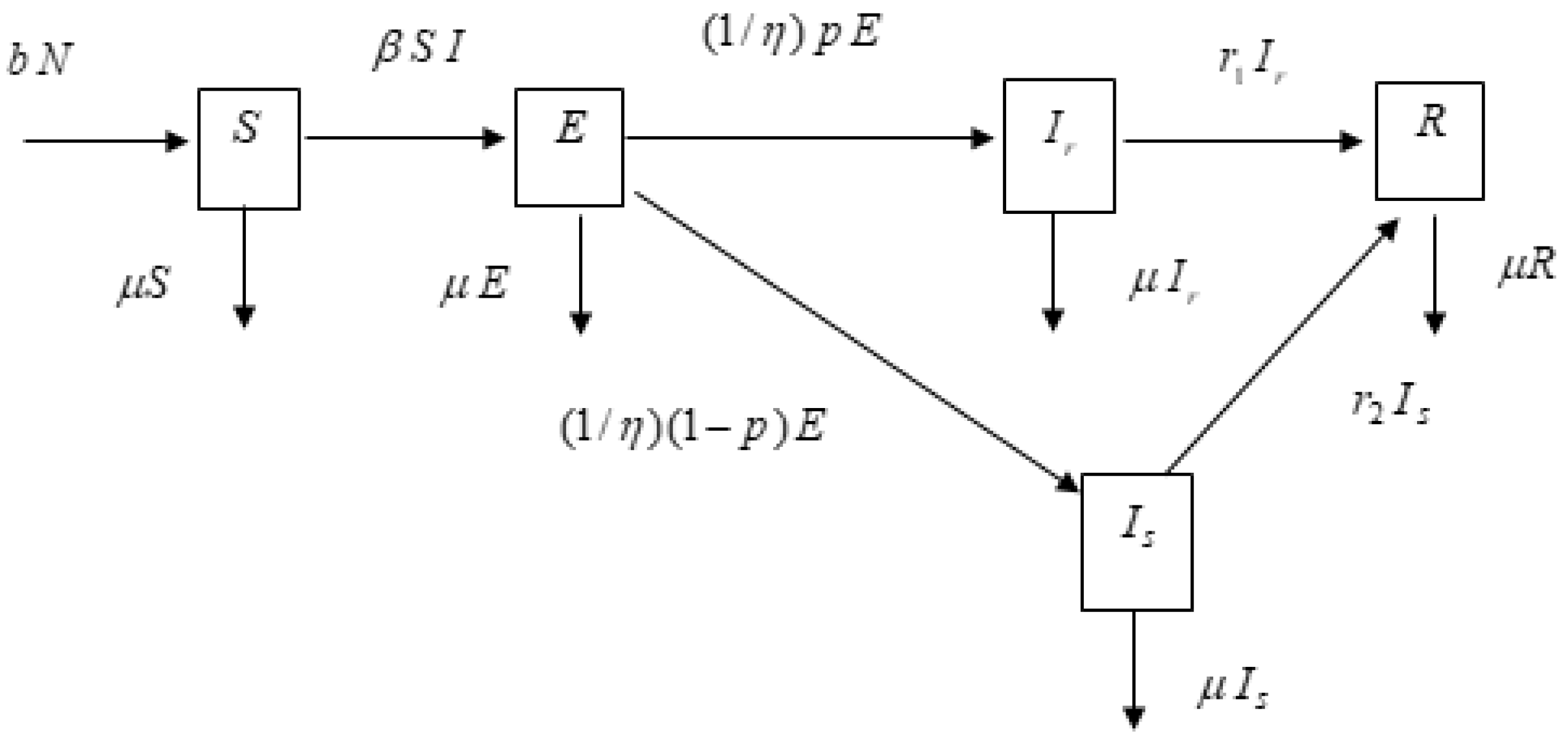
2. Materials and Methods
3. Analysis of the Model
3.1. Equilibrium Points
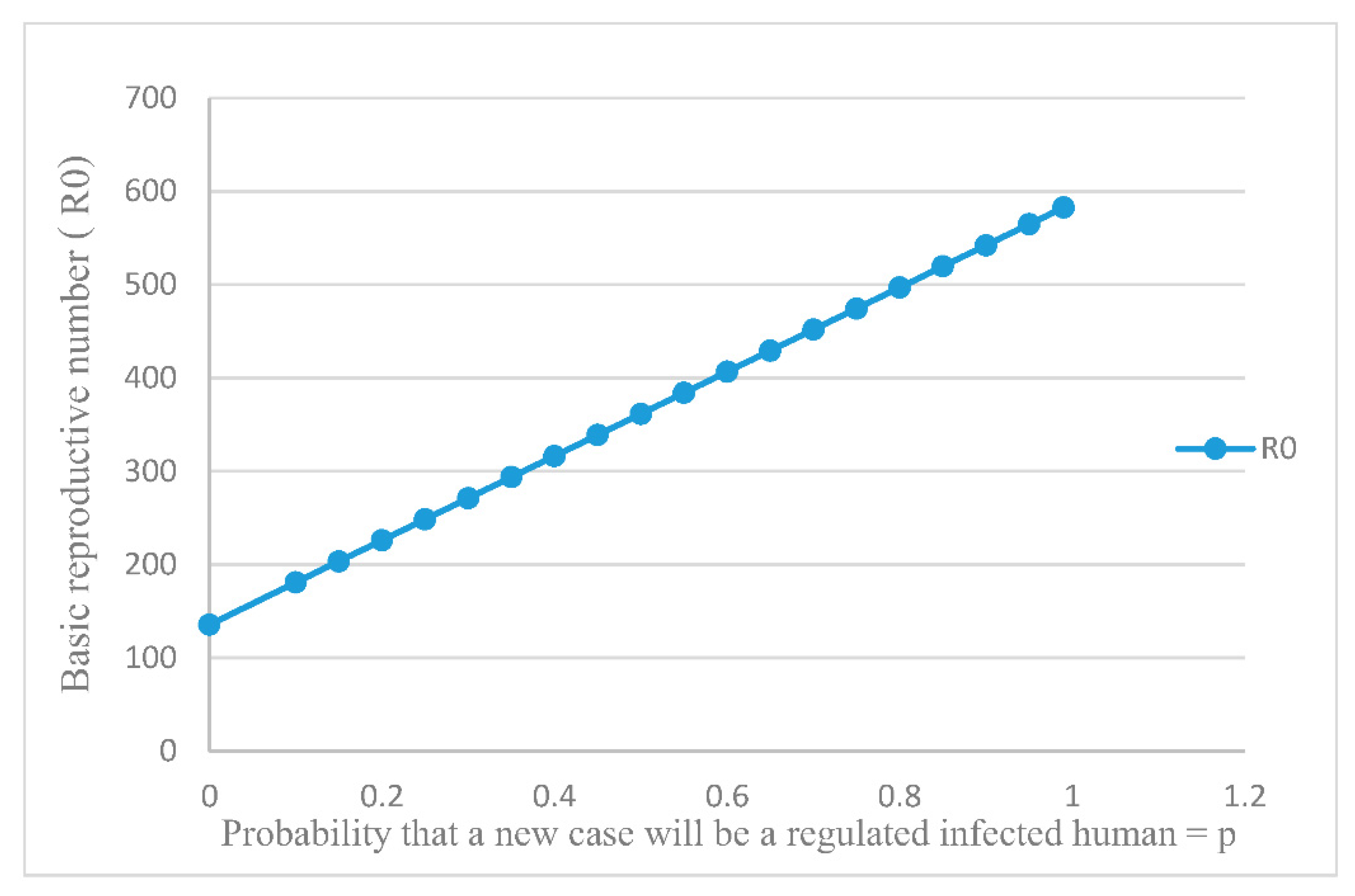
3.2. Basic Reproductive Number
3.3. Local Asymptotical Stability
3.4. Global Stability of the Equilibrium States
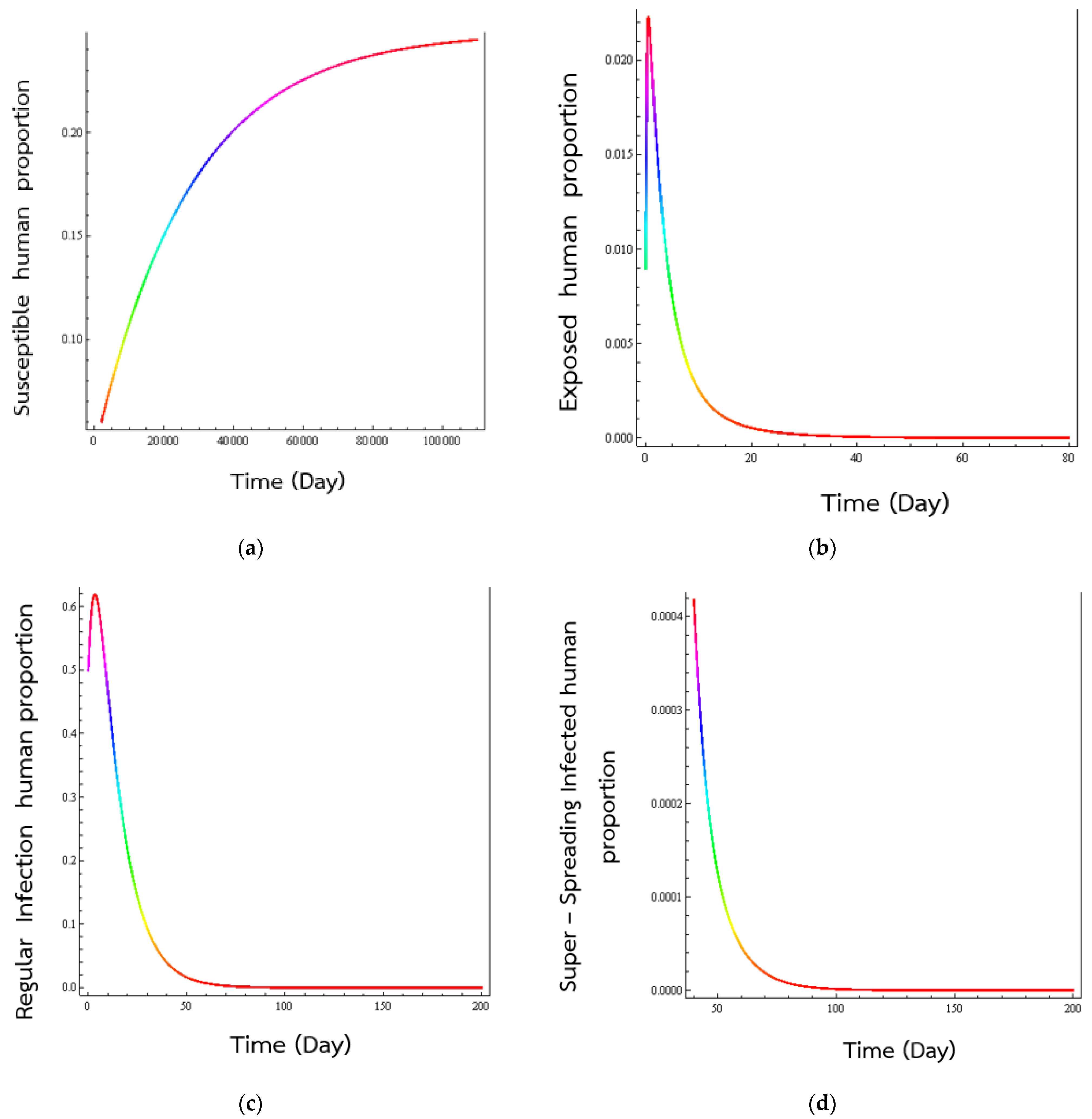
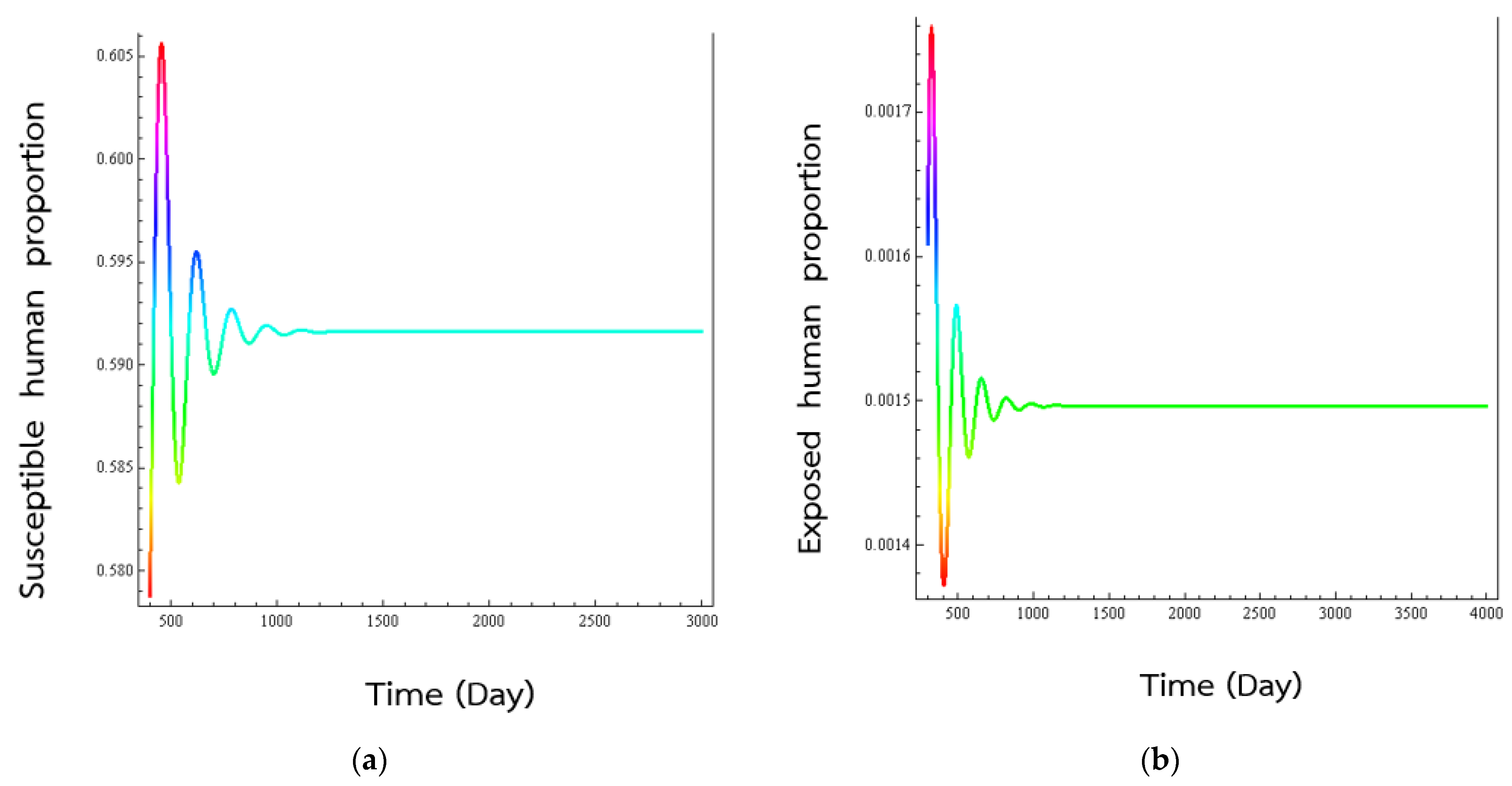
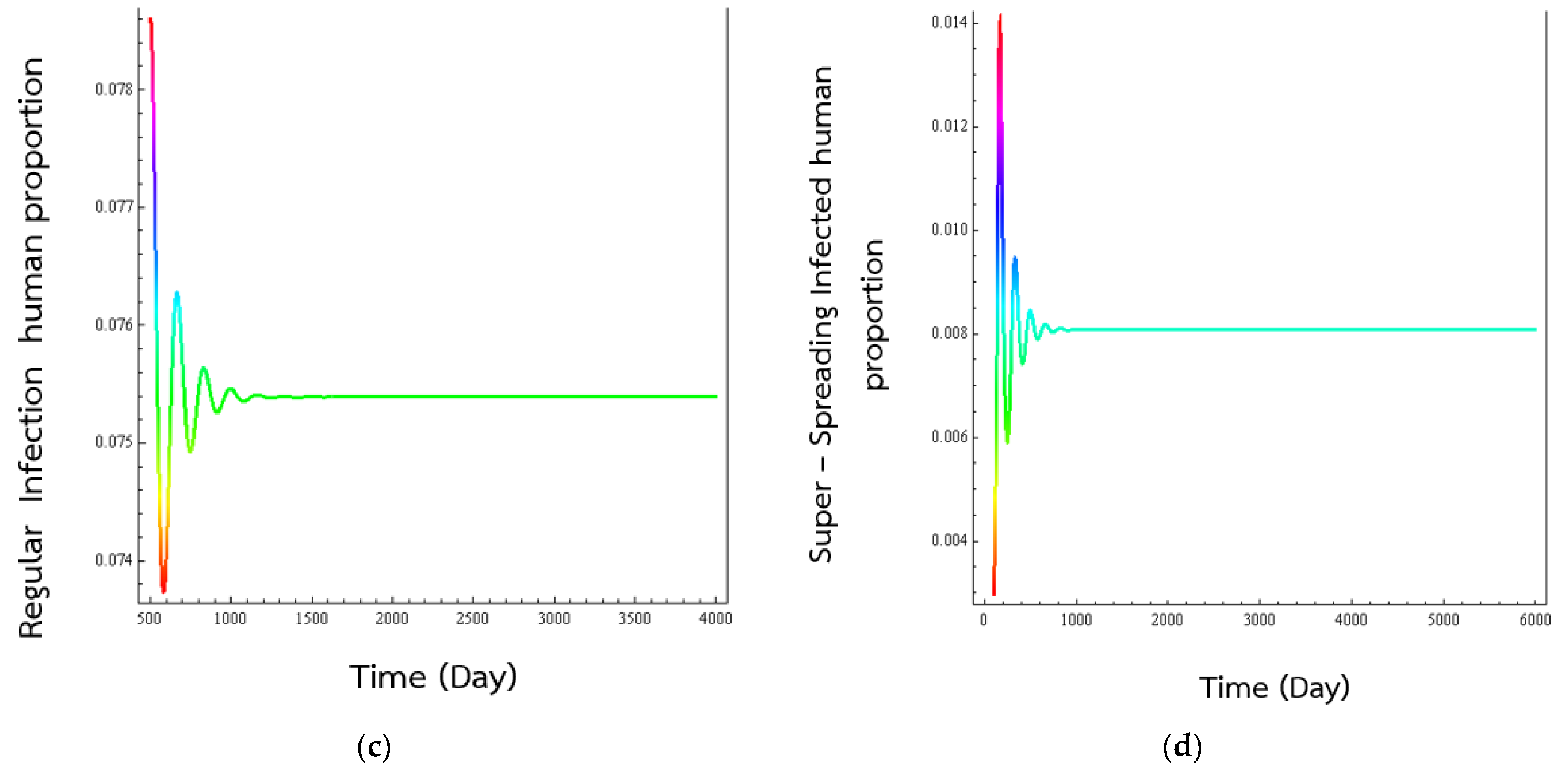
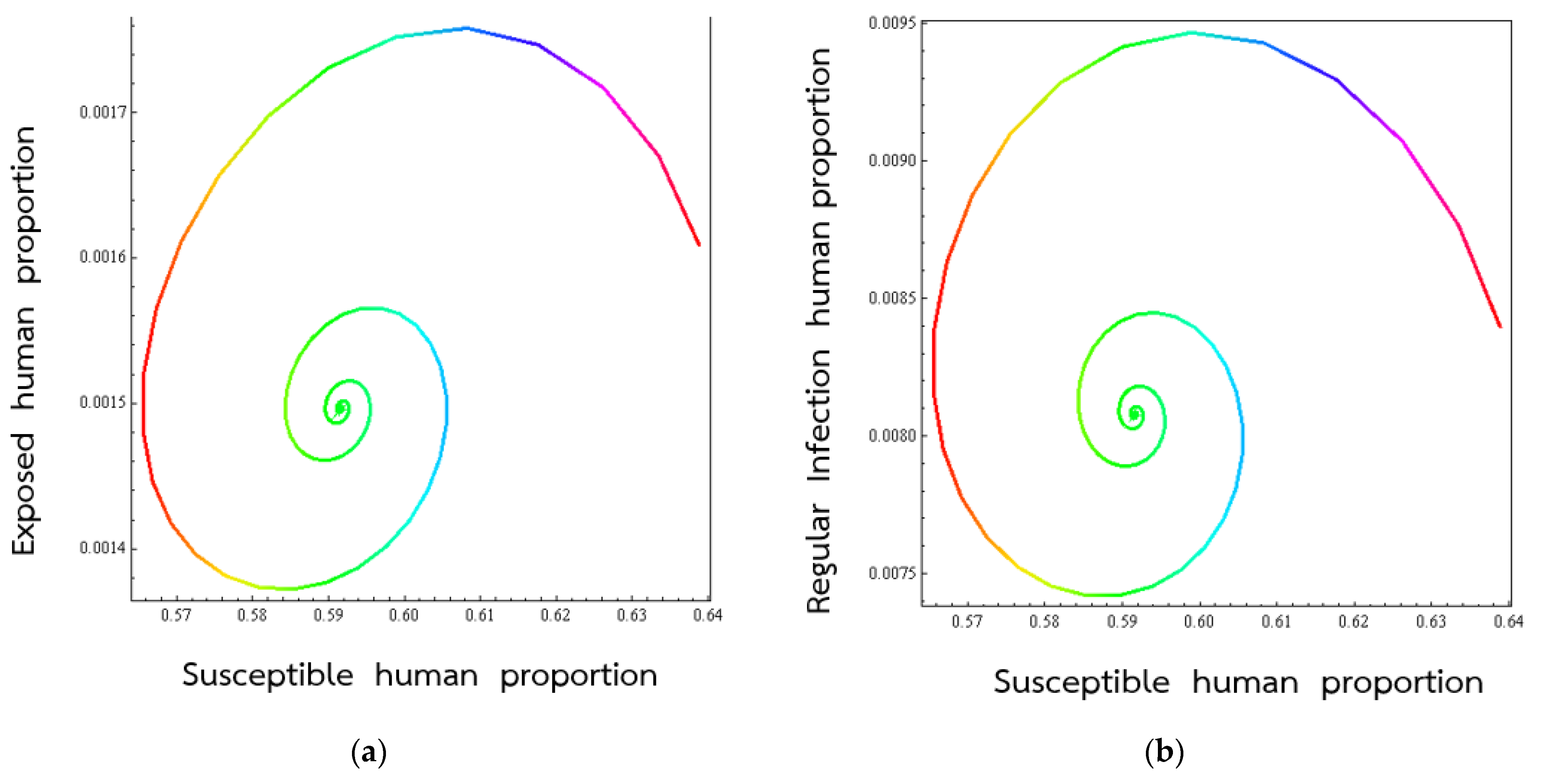
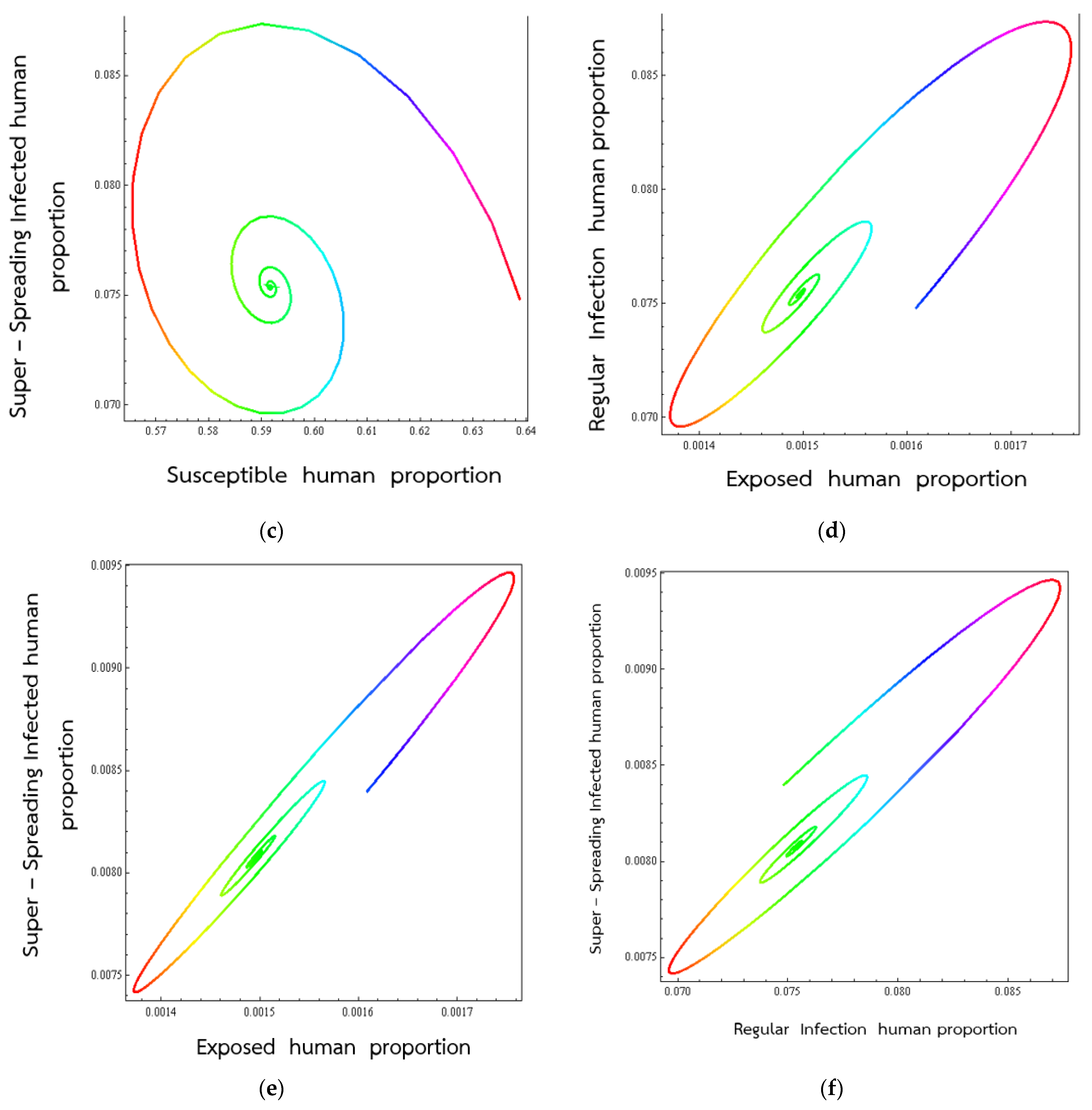
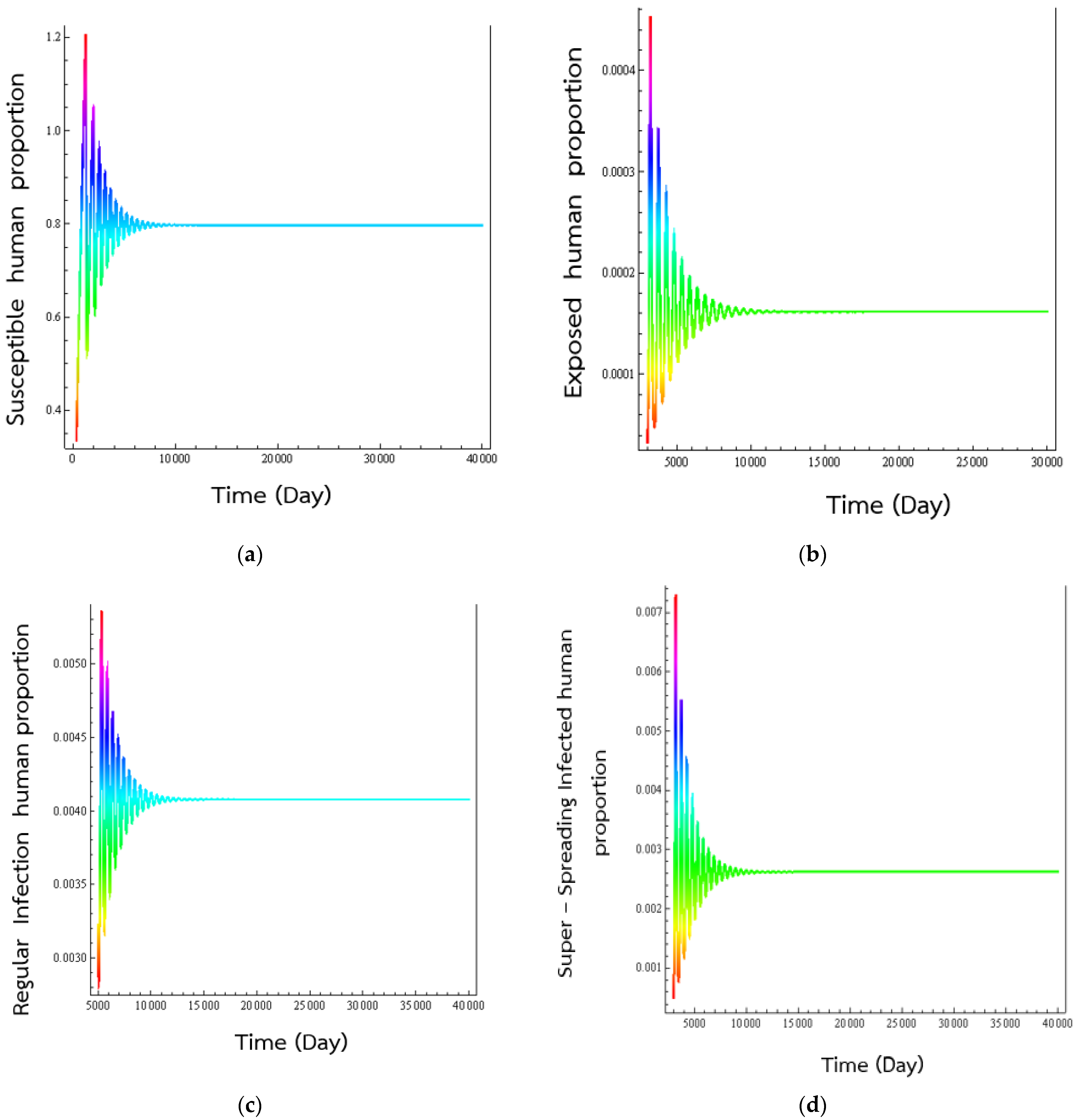
4. Numerical Results
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2019. Available online: https://www.who.int/influenza/rsv/en/ (accessed on 18 April 2019).
- Respiratory Syncytial Virus RSV. 2019. Available online: https://www.webmd.com/lung/rsv-in-babies (accessed on 6 May 2019).
- Respiratory Syncytial Virus RSV. 2019. Available online: https://my.clevelandclinic.org/health/disease/8282respiratory-syncytial-virus-in-children-and-adults (accessed on 15 May 2019).
- Respiratory Syncytial Virus RSV. 2019. Available online: https://medlineplus.gov/respiratorysyncytialvirusinfections.html (accessed on 15 May 2019).
- Arenas, A.J.; Gonzalez, G.; Jodar, L. Existence of periodic solutions in a model of respiratory syncytial virus RSV. J. Math. Anal. Appl. 2008, 344, 969–980. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.; Weber, M.; Milligan, P. Modeling epidemics caused by Respiratory syncytial virus (RSV). Math. Biosci. 2001, 172, 95–113. [Google Scholar] [CrossRef]
- Sungchasit, R.; Pongsumpun, P.; Tang, I.M. Environmental Impact on the Spread of Dengue Virus when two Mosquito Species Circulate. Far East J. Math. Sci. FJMS 2017, 101, 137–170. [Google Scholar] [CrossRef]
- Acedo, L.; Diez Domingo, J.; Morano, J.A.; Villanueva, R.J. Mathematical modelling of respiratory syncytial virus (RSV): Vaccination strategies and budget applications. Epidemiol. Infect. 2001, 138, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Geiman, J.M.; Douglas, R.G., Jr.; Meagher, M.P. Control of nosocomial respiratory syncytial viral Infections. Pediatrics 1978, 62, 728–732. [Google Scholar] [CrossRef]
- Moore, H.C.; Jacoby, H.P.; Blyth, C.; Mercer, G. Modelling the seasonal epidemics of Respiratory Syncytial Virus in young children Submitted to Influenza and Other Respiratory Viruses among humans. Curr. Opin. Virol. 2013, 28, 142–151. [Google Scholar]
- Gonzalez-Parra, G.; Rodríguez, D.M.; Villanueva-Micó, R.J. Impact of a New SARS-CoV-2 Variant on the Population: A Mathematical Modeling Approach. Math. Comput. Appl. 2021, 26, 25. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, C.H.; Choi, Y.; Kim, J. The daily computed weighted averaging basic reproduction number Rn0,k,ω for MERS-CoV in South Korea. Phys. A Stat. Mech. Its Appl. 2016, 451, 190–197. [Google Scholar] [CrossRef]
- Pongsumpun, P.; Sungchasit, R.; Tang, I.M. Lyapunov Function for a Dengue Transmission Model where two Species of Mosquitoes are Present: Global Stability. Am. J. Appl. Sci. 2017, 14, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yu, C.; Qu, J.; Zhang, L.; Jiang, S.; Huang, D.; Chen, B.; Zhang, Z.; Guan, W.; Ling, Z.; et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Cha, M.J.; Chung, M.J.; Kim, K.; Lee, K.S.; Kim, T.J.; Kim, T.S. Clinical implication of radiographic scores in acute Middle East respiratory syndrome corona-virus pneumonia: Report from a single tertiary-referral center of South Korea. Eur. J. Radiol. 2018, 107, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohuma, E.O.; Okiro, E.; Ochola, R.; Sande, C.; Cane, P.A.; Medley, G.; Bottomley, C.; Nokes, D.J. The natural history of respiratory syncytial virus in a birth cohort: The influence of age and previous infection on reinfection and disease. Am. J. Epidemiol. 2012, 176, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Stechlinski, P. Infectious Disease Modeling; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Kim, Y.; Cho, N. A Simulation Study on Spread of Disease and Control Measures in Closed Population Using ABM. Computation 2022, 10, 2. [Google Scholar] [CrossRef]
- Van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Sungchasit, R.; Pongsumpun, P. Mathematical Model of Dengue Virus with Primary and Secondary Infection. Curr. Appl. Sci. Technol. 2019, 19, 154–178. [Google Scholar]
- Athithan, S.; Ghosh, M.; Li, X.-Z. Mathematical modelling and optimal control of corruption dynamics. Asian Eur. J. Math. 2018, 11, 12. [Google Scholar] [CrossRef]
- Liu, L.; Ren, X.; Liu, X. Dynamical Behaviours of an Influenza Epidemic Model with Virus Mutation. J. Biol. Syst. 2018, 26, 455–472. [Google Scholar] [CrossRef]
- Esteva, L.; Vargas, C. Analysis of a dengue disease transmission model. Math. Biosci. 1998, 150, 131–151. [Google Scholar] [CrossRef]
- Jin, Z.; Haque, M. Global Stability Analysis of an Eco—Epidemiological Model of the Salton Sea. J. Biol. Syst. 2006, 14, 373–385. [Google Scholar] [CrossRef]
- Prathumwan, D.; Trach, K.; Chaiya, I. Mathematical Modeling for Prediction Dynamics of the Coronavirus Disease 2019 (COVID-19) Pandemic, Quarantine Control Measures. Symmetry 2020, 12, 1404. [Google Scholar] [CrossRef]
- Diekmann, D.; Heesterbeek, J. Mathematical epidemiology of infectious disease: Model building, analysis and interpretation. In Wiley Series in Mathematical and Computational Biology; Wiley: Chichester, UK, 2000. [Google Scholar]
- Lenhart, S.; Workman, J.T. Optimal Control Applied to Biological Models. In Mathematical and Computational Biology Series; Chapman & Hall/CRC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hogan, A.B.; Glass, K.; Moore, H.C.; Anderssen, R.S. Exploring the dynamics of respiratory syncytial virus 2 (RSV) transmission in children. Theor. Popul. Biol. 2016, 110, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.C.; Jacoby, P.; Hogan, A.B.; Blyth, C.C.; Mercer, G.N. Modelling the Seasonal Epidemics of Respiratory Syncytial Virus in Young Children. PLoS ONE 2014, 9, e100422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeling, J.M.; Rohani, P. Modeling Infectious Diseases in Humans and Animals; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Paynter, S.; Yakob, L.; Simões, E.A.F.; Lucero, M.G.; Tallo, V.; Nohynek, H.; Ware, R.S.; Weinstein, P.; Williams, G.; Sly, P.D. Using Mathematical Transmission Modelling to Investigate Drivers of Respiratory Syncytial Virus Seasonality in Children in the Philippines. PLoS ONE 2014, 9, e90094. [Google Scholar] [CrossRef]
- Hogan, A.B.; Glass, K.; Moore, H.C.; Anderssen, R.S. Age Structures in Mathematical Models for Infectious Diseases, with a Case Study of Respiratory Syncytial Virus. In Applications + Practical Conceptualization + Mathematics = fruitful Innovation; Springer: Tokyo, Japan, 2015; pp. 105–116. [Google Scholar] [CrossRef]
- Hattaf, K.; Yousfi, N. Modeling the Adaptive Immunity and Both Modes of Transmission in HIV Infection. Computation 2018, 6, 37. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Biological Meaning | Value |
|---|---|---|
| birth rate of human population | 1/(365 × 75.65) per day [1] | |
| death rate of human population | 1/(365 × 75.65) per day [1] | |
| transmission rate of virus between humans | 0.1–0.9 per day [1] or [11] or [20,21] | |
| incubation time of virus in humans | 0.1–0.9 per day [1] or [11] or [20,21] | |
| P | probability that a new case will be a regulated infected human | 0.01–0.0009 [20,21,22] |
| probability that a new case will be a super-spreading infected human | 0.1–0.9999 [20,21,22] | |
| r1 | recovery rate of regular infected humans | 0.01–0.9 [20,21,22] |
| recovery rate of super-spreading infected humans | 0.1–0.7 [20,21,22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sungchasit, R.; Tang, I.-M.; Pongsumpun, P. Mathematical Modeling: Global Stability Analysis of Super Spreading Transmission of Respiratory Syncytial Virus (RSV) Disease. Computation 2022, 10, 120. https://doi.org/10.3390/computation10070120
Sungchasit R, Tang I-M, Pongsumpun P. Mathematical Modeling: Global Stability Analysis of Super Spreading Transmission of Respiratory Syncytial Virus (RSV) Disease. Computation. 2022; 10(7):120. https://doi.org/10.3390/computation10070120
Chicago/Turabian StyleSungchasit, Rattiya, I-Ming Tang, and Puntani Pongsumpun. 2022. "Mathematical Modeling: Global Stability Analysis of Super Spreading Transmission of Respiratory Syncytial Virus (RSV) Disease" Computation 10, no. 7: 120. https://doi.org/10.3390/computation10070120






