Ultrashort Echo Time and Fast Field Echo Imaging for Spine Bone Imaging with Application in Spondylolysis Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
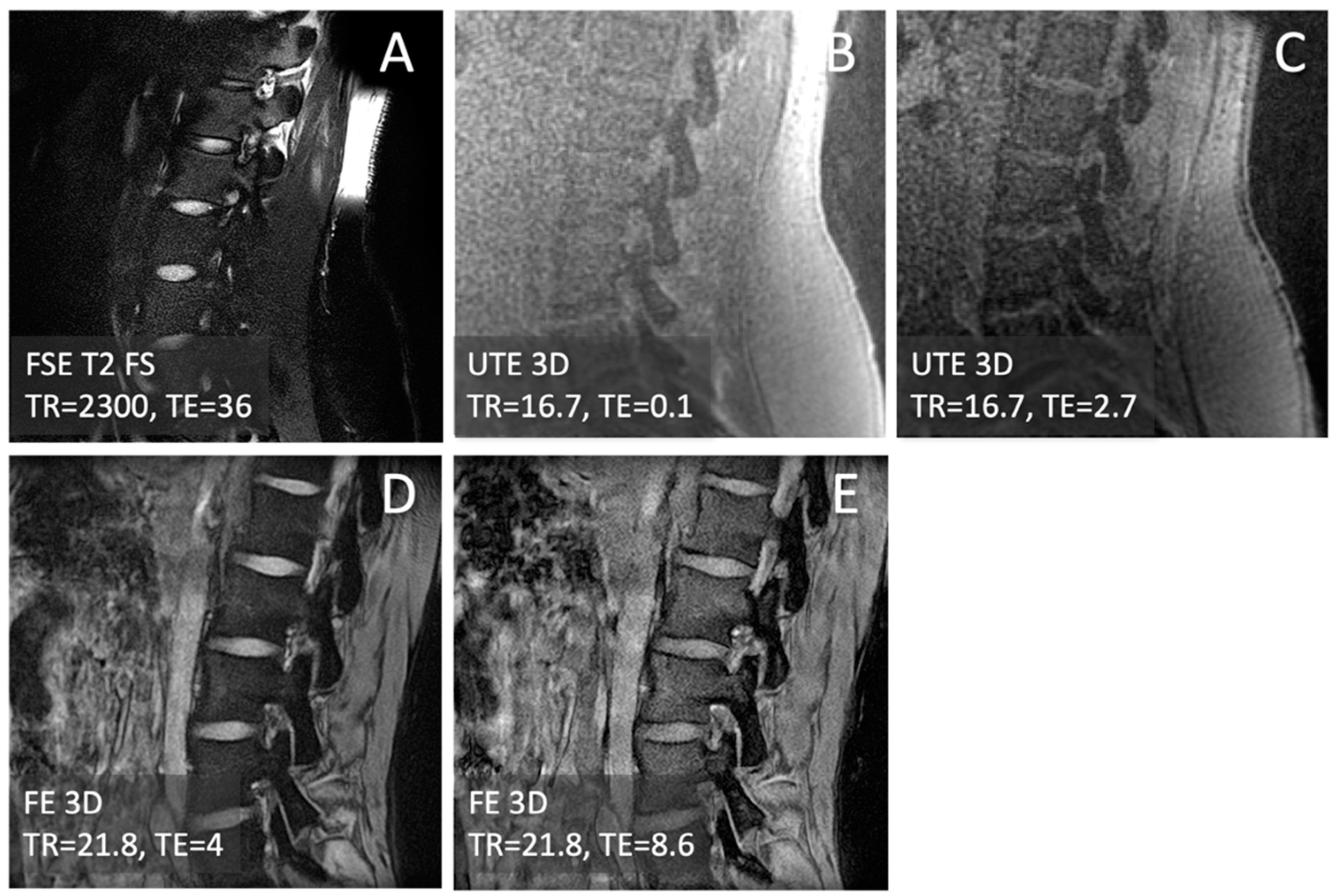
2.2. MRI
2.3. CT-like Image Processing
2.4. Signal-to-Noise Ratio (SNR) and Contrast-to-Noise Ratio (CNR)
2.5. Statistics
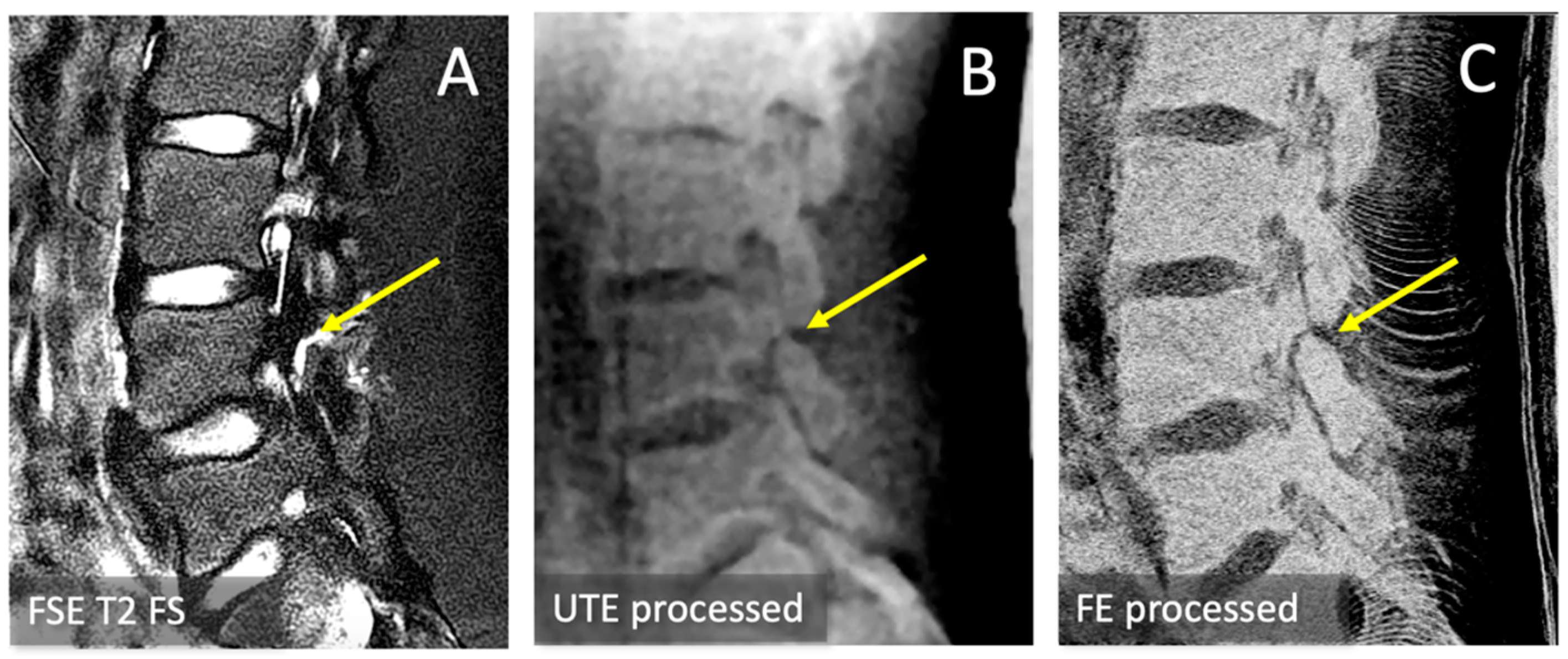
2.6. Spondylolysis Evaluation
3. Results
3.1. Observations
3.2. SNR and CNR
3.3. Spondylolysis Depiction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olsen, T.L.; Anderson, R.L.; Dearwater, S.R.; Kriska, A.M.; Cauley, J.A.; Aaron, D.J.; LaPorte, R.E. The epidemiology of low back pain in an adolescent population. Am. J. Public Health 1992, 82, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Wiltse, L.L.; Newman, P.H.; Macnab, I. Classification of spondylolisis and spondylolisthesis. Clin. Orthop. Relat. Res. 1976, 117, 23–29. [Google Scholar] [CrossRef]
- Fredrickson, B.E.; Baker, D.; McHolick, W.J.; Yuan, H.A.; Lubicky, J.P. The natural history of spondylolysis and spondylolisthesis. J. Bone Jt. Surg. Am. 1984, 66, 699–707. [Google Scholar] [CrossRef]
- Bechtel, W.; Griffiths, H.; Eisenstadt, R. The Pathogenesis of Spondylolysis. Investig. Radiol. 1982, 17, S29. [Google Scholar] [CrossRef]
- Li, J.; Liang, J.; Xu, Y.; Du, D.; Feng, F.; Shen, J.; Cui, Y. Incidence of lumbar spondylolysis in athletes with low back pain: A systematic evaluation and single-arm meta-analysis. Medicine 2023, 102, e34857. [Google Scholar] [CrossRef]
- West, A.M.; d’Hemecourt, P.A.; Bono, O.J.; Micheli, L.J.; Sugimoto, D. Diagnostic Accuracy of Magnetic Resonance Imaging and Computed Tomography Scan in Young Athletes With Spondylolysis. Clin. Pediatr. 2019, 58, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Yoshida, T.; Mimatsu, K. Early diagnosis of lumbar spondylolysis by MRI. J. Bone Jt. Surg. Br. 1993, 75, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.L.; Mathews, V.P.; Elster, A.D.; Mark, L.P.; Daniels, D.L.; Mueller, W. MR imaging of lumbar spondylolysis: The importance of ancillary observations. AJR Am. J. Roentgenol. 1997, 169, 233–239. [Google Scholar] [CrossRef]
- Leone, A.; Cianfoni, A.; Cerase, A.; Magarelli, N.; Bonomo, L. Lumbar spondylolysis: A review. Skelet. Radiol. 2011, 40, 683–700. [Google Scholar] [CrossRef]
- Journy, N.; Roue, T.; Cardis, E.; Le Pointe, H.D.; Brisse, H.; Chateil, J.F.; Laurier, D.; Bernier, M.O. Childhood CT scans and cancer risk: Impact of predisposing factors for cancer on the risk estimates. J. Radiol. Prot. 2016, 36, N1–N7. [Google Scholar] [CrossRef]
- Berrington de Gonzalez, A.; Pasqual, E.; Veiga, L. Epidemiological studies of CT scans and cancer risk: The state of the science. Br. J. Radiol. 2021, 94, 20210471. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Sung, C.Y.; Wang, S.C.; Shao, Y.J. Risks of leukemia, intracranial tumours and lymphomas in childhood and early adulthood after pediatric radiation exposure from computed tomography. CMAJ 2023, 195, E575–E583. [Google Scholar] [CrossRef]
- Carey, T.S.; Garrett, J.M.; Jackman, A.; Hadler, N. Recurrence and care seeking after acute back pain: Results of a long-term follow-up study. North Carolina Back Pain Project. Med. Care 1999, 37, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.C.; Biswas, R.; Chen, K.; Chang, E.Y.; Chung, C.B. UTE MRI of the Osteochondral Junction. Curr. Radiol. Rep. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.U.; Coy, A.; Motamedi, D.; Sun, D.; Joseph, G.B.; Krug, R.; Link, T.M. CT-like MRI: A qualitative assessment of ZTE sequences for knee osseous abnormalities. Skelet. Radiol. 2022, 51, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.Y.; Moazamian, D.; Ma, Y.; Jang, H.; Jerban, S.; Du, J.; Chung, C.B. Clinical application of ultrashort echo time (UTE) and zero echo time (ZTE) magnetic resonance (MR) imaging in the evaluation of osteoarthritis. Skelet. Radiol. 2023, 52, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Breighner, R.E.; Endo, Y.; Konin, G.P.; Gulotta, L.V.; Koff, M.F.; Potter, H.G. Technical Developments: Zero Echo Time Imaging of the Shoulder: Enhanced Osseous Detail by Using MR Imaging. Radiology 2018, 286, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Achar, S.; Hwang, D.; Finkenstaedt, T.; Malis, V.; Bae, W.C. Deep-Learning-Aided Evaluation of Spondylolysis Imaged with Ultrashort Echo Time Magnetic Resonance Imaging. Sensors 2023, 23, 8001. [Google Scholar] [CrossRef]
- Jang, U.; Hwang, D. High-quality multiple T2(*) contrast MR images from low-quality multi-echo images using temporal-domain denoising methods. Med. Phys. 2012, 39, 468–474. [Google Scholar] [CrossRef]
- Jang, U.; Nam, Y.; Kim, D.H.; Hwang, D. Improvement of the SNR and resolution of susceptibility-weighted venography by model-based multi-echo denoising. Neuroimage 2013, 70, 308–316. [Google Scholar] [CrossRef]
- Johnson, B.; Alizai, H.; Dempsey, M. Fast field echo resembling a CT using restricted echo-spacing (FRACTURE): A novel MRI technique with superior bone contrast. Skelet. Radiol. 2021, 50, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Goerner, F.L.; Clarke, G.D. Measuring signal-to-noise ratio in partially parallel imaging MRI. Med. Phys. 2011, 38, 5049–5057. [Google Scholar] [CrossRef]
- Edelstein, W.; Bottomley, P.; Hart, H.; Leue, W.; Schenck, J.; Redington, R. NMR imaging at 5.1 MHz: Work in progress. In International Symposium on NMR Imaging; Witcofski, R., Karstaedt, N., Partain, C., Eds.; Bowman Gray School of Medicine: Winston-Salem, NC, USA, 1982; pp. 139–145. [Google Scholar]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Multiple comparison analysis testing in ANOVA. Biochem. Med. 2011, 21, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Gomyo, M.; Katase, S.; Hiraoka, S.; Tateishi, H. Magnetic resonance bone imaging: Applications to vertebral lesions. Jpn. J. Radiol. 2023, 41, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, B.J.; Schneider, C.; Kronthaler, S.; Gassert, F.T.; Bohm, C.; Pfeiffer, D.; Baum, T.; Kirschke, J.S.; Karampinos, D.C.; Makowski, M.R.; et al. CT-like images based on T1 spoiled gradient-echo and ultra-short echo time MRI sequences for the assessment of vertebral fractures and degenerative bone changes of the spine. Eur. Radiol. 2021, 31, 4680–4689. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Bydder, M.; Takahashi, A.M.; Carl, M.; Chung, C.B.; Bydder, G.M. Short T2 contrast with three-dimensional ultrashort echo time imaging. Magn. Reson. Imaging 2011, 29, 470–482. [Google Scholar] [CrossRef]
- Largent, A.; Barateau, A.; Nunes, J.C.; Mylona, E.; Castelli, J.; Lafond, C.; Greer, P.B.; Dowling, J.A.; Baxter, J.; Saint-Jalmes, H.; et al. Comparison of Deep Learning-Based and Patch-Based Methods for Pseudo-CT Generation in MRI-Based Prostate Dose Planning. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Bourbonne, V.; Jaouen, V.; Hognon, C.; Boussion, N.; Lucia, F.; Pradier, O.; Bert, J.; Visvikis, D.; Schick, U. Dosimetric Validation of a GAN-Based Pseudo-CT Generation for MRI-Only Stereotactic Brain Radiotherapy. Cancers 2021, 13, 1082. [Google Scholar] [CrossRef]
- Florkow, M.C.; Willemsen, K.; Mascarenhas, V.V.; Oei, E.H.G.; van Stralen, M.; Seevinck, P.R. Magnetic Resonance Imaging Versus Computed Tomography for Three-Dimensional Bone Imaging of Musculoskeletal Pathologies: A Review. J. Magn. Reson. Imaging 2022, 56, 11–34. [Google Scholar] [CrossRef]
- Morbee, L.; Vereecke, E.; Laloo, F.; Chen, M.; Herregods, N.; Jans, L. MR Imaging of the Pelvic Bones: The Current and Cutting-Edge Techniques. J. Belg. Soc. Radiol. 2022, 106, 123. [Google Scholar] [CrossRef] [PubMed]


| Mean (+/− Std. Dev.) Values for Each Sequence | ||||
| Measurement | UTE 1st Echo | UTE Multi | FE 1st Echo | FE Multi |
| Bone SNR | 107 (66) | 89.8 (29.5) | 38.1 (10.3) | 53.3 (16.3) |
| Muscle SNR | 94.4 (65.4) | 74.0 (32.8) | 23.4 (7.0) | 31.9 (14.2) |
| Bone–Muscle CNR | 13.0 (5.2) | 15.7 (5.0) | 14.6 (4.2) | 21.5 (8.5) |
| Two-Way ANOVA: Effect of | ||||
| Measurement | UTE vs. FE | 1st- vs. Multi-echo | Interaction | |
| Bone SNR | 0.0004 | 0.9289 | 0.2235 | |
| Muscle SNR | 0.0002 | 0.6556 | 0.2864 | |
| Bone–Muscle CNR | 0.0935 | 0.0311 | 0.3327 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vucevic, D.; Malis, V.; Yamashita, Y.; Mesa, A.; Yamaguchi, T.; Achar, S.; Miyazaki, M.; Bae, W.C. Ultrashort Echo Time and Fast Field Echo Imaging for Spine Bone Imaging with Application in Spondylolysis Evaluation. Computation 2024, 12, 152. https://doi.org/10.3390/computation12080152
Vucevic D, Malis V, Yamashita Y, Mesa A, Yamaguchi T, Achar S, Miyazaki M, Bae WC. Ultrashort Echo Time and Fast Field Echo Imaging for Spine Bone Imaging with Application in Spondylolysis Evaluation. Computation. 2024; 12(8):152. https://doi.org/10.3390/computation12080152
Chicago/Turabian StyleVucevic, Diana, Vadim Malis, Yuichi Yamashita, Anya Mesa, Tomosuke Yamaguchi, Suraj Achar, Mitsue Miyazaki, and Won C. Bae. 2024. "Ultrashort Echo Time and Fast Field Echo Imaging for Spine Bone Imaging with Application in Spondylolysis Evaluation" Computation 12, no. 8: 152. https://doi.org/10.3390/computation12080152
APA StyleVucevic, D., Malis, V., Yamashita, Y., Mesa, A., Yamaguchi, T., Achar, S., Miyazaki, M., & Bae, W. C. (2024). Ultrashort Echo Time and Fast Field Echo Imaging for Spine Bone Imaging with Application in Spondylolysis Evaluation. Computation, 12(8), 152. https://doi.org/10.3390/computation12080152






