Preparation of Nano Silver Paste and Applications in Transparent Electrodes via Electric-Field Driven Micro-Scale 3D Printing
Abstract
:1. Introduction
2. Experiment Details
2.1. Materials
2.2. Preparation of Nano-Silver Paste
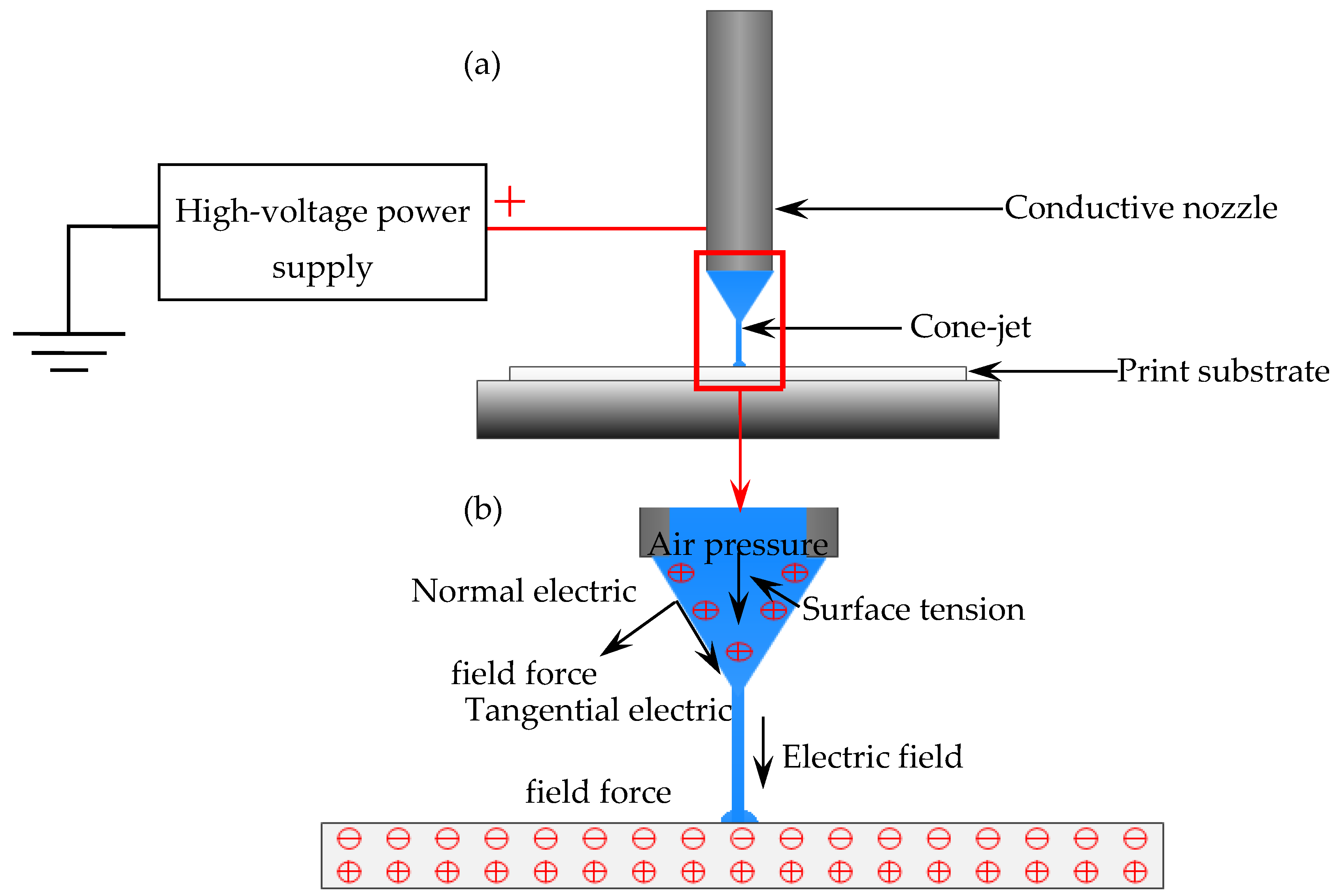
2.3. EFD Micro-Scale 3D Printing Technology
2.4. Characterization and Measurements
3. Results and Discussion
3.1. Testing Results of the Nano-Silver Paste
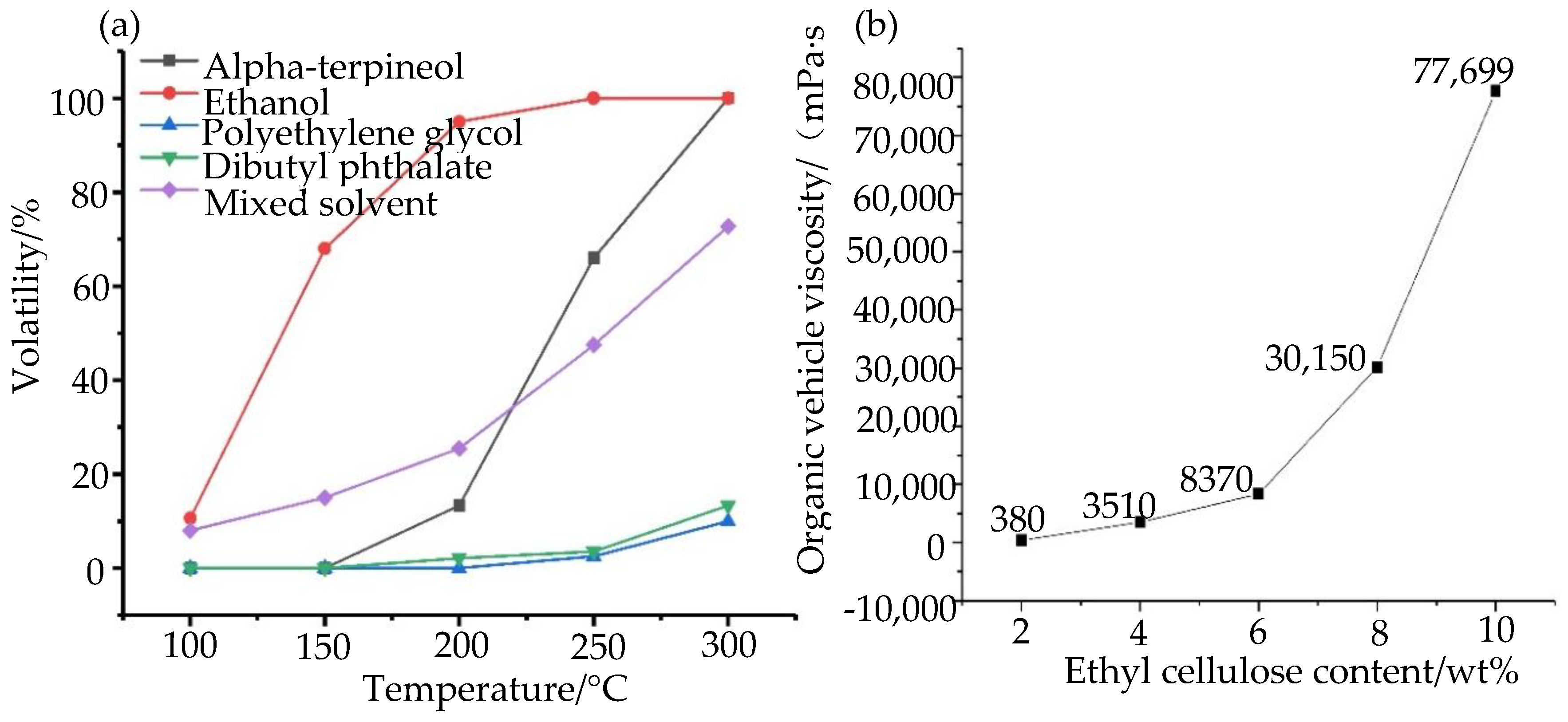
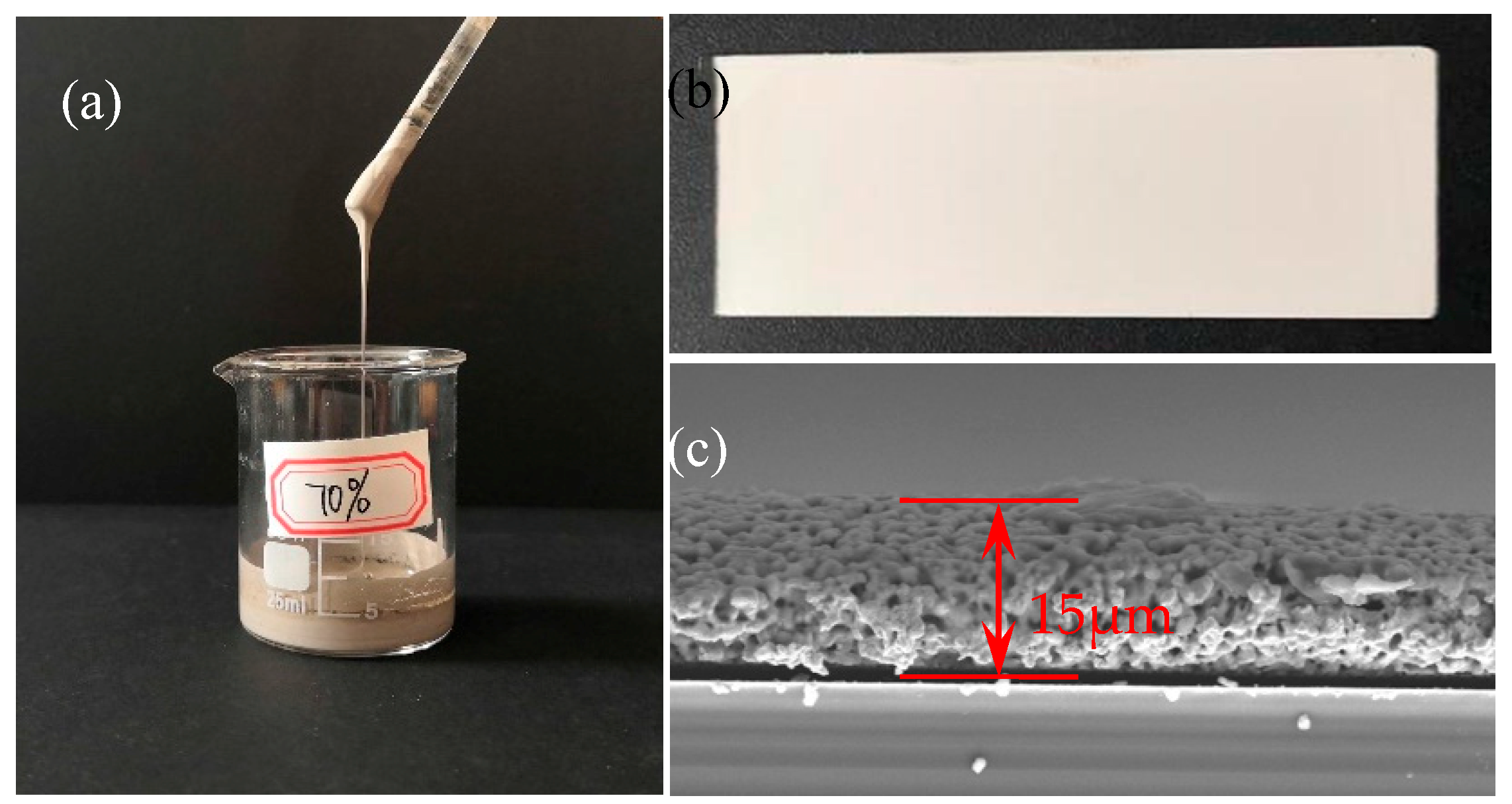
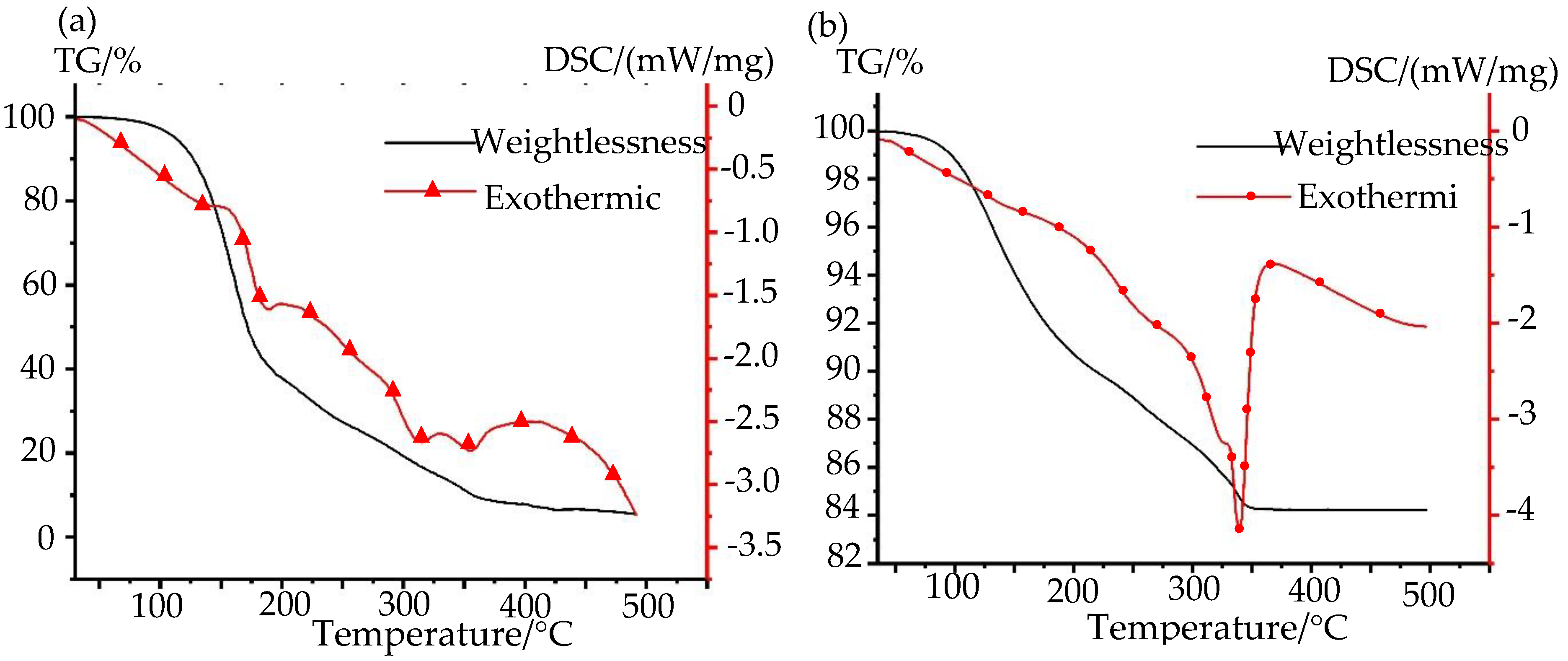
3.1.1. Stability of Nano-Silver Paste
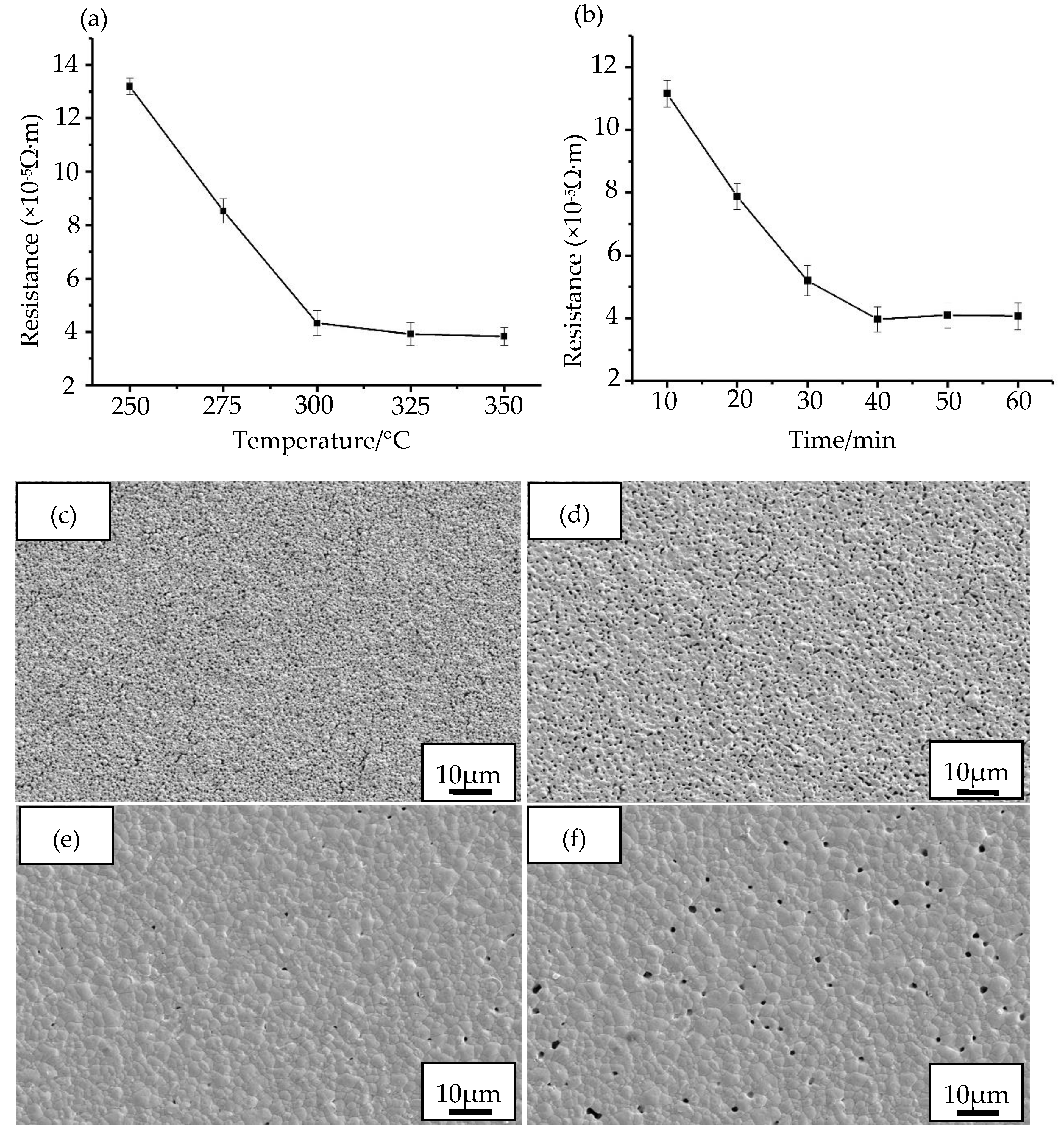
3.1.2. The Sintering and Curing of Nano-Silver Paste
3.2. EFD Micro-Scale 3D Printing Results of the Nano-Silver Paste
3.2.1. Geometric Morphology of the Printed Silver Wires
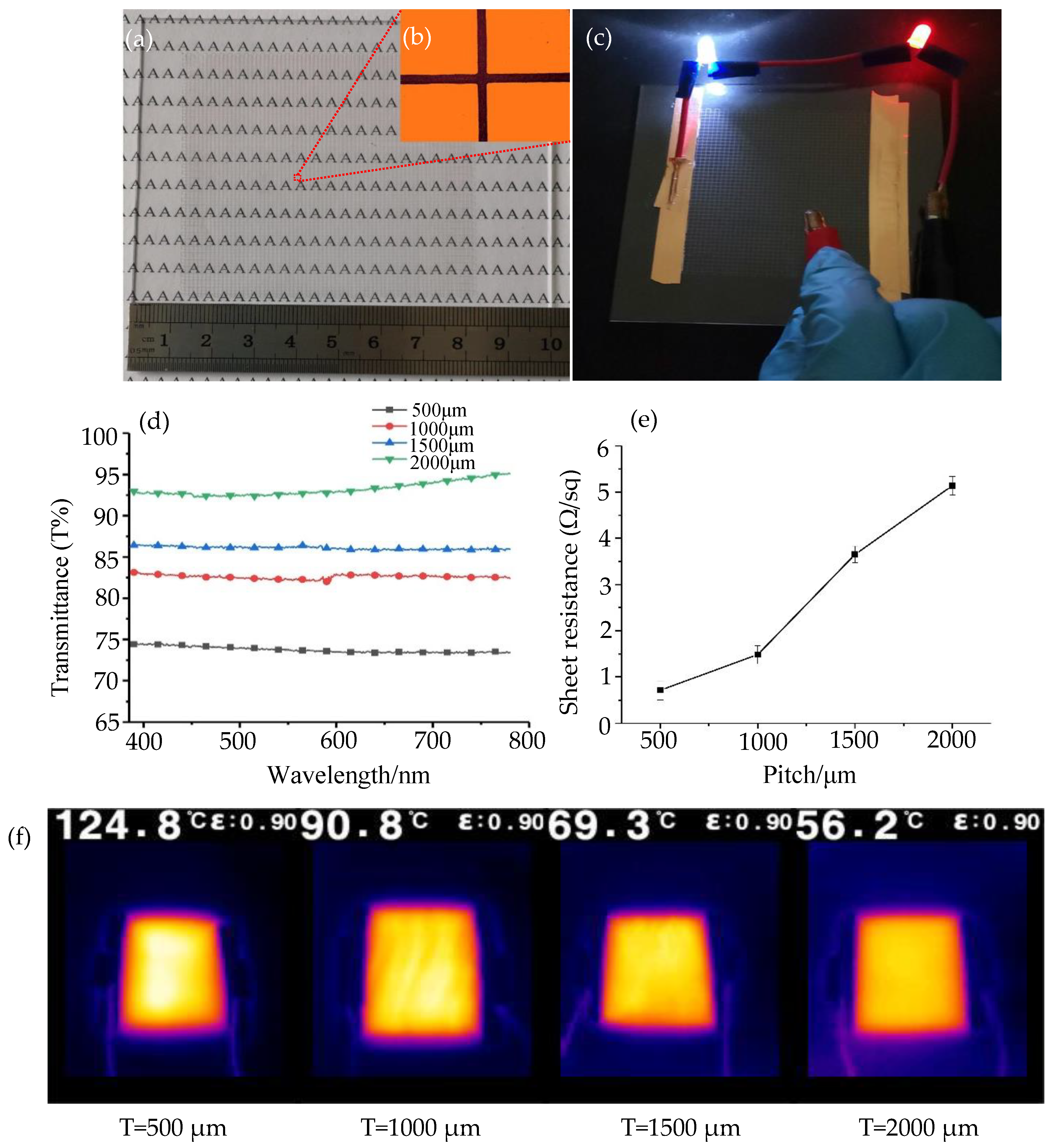
3.2.2. Photoelectric Properties of the Printed Silver Mesh
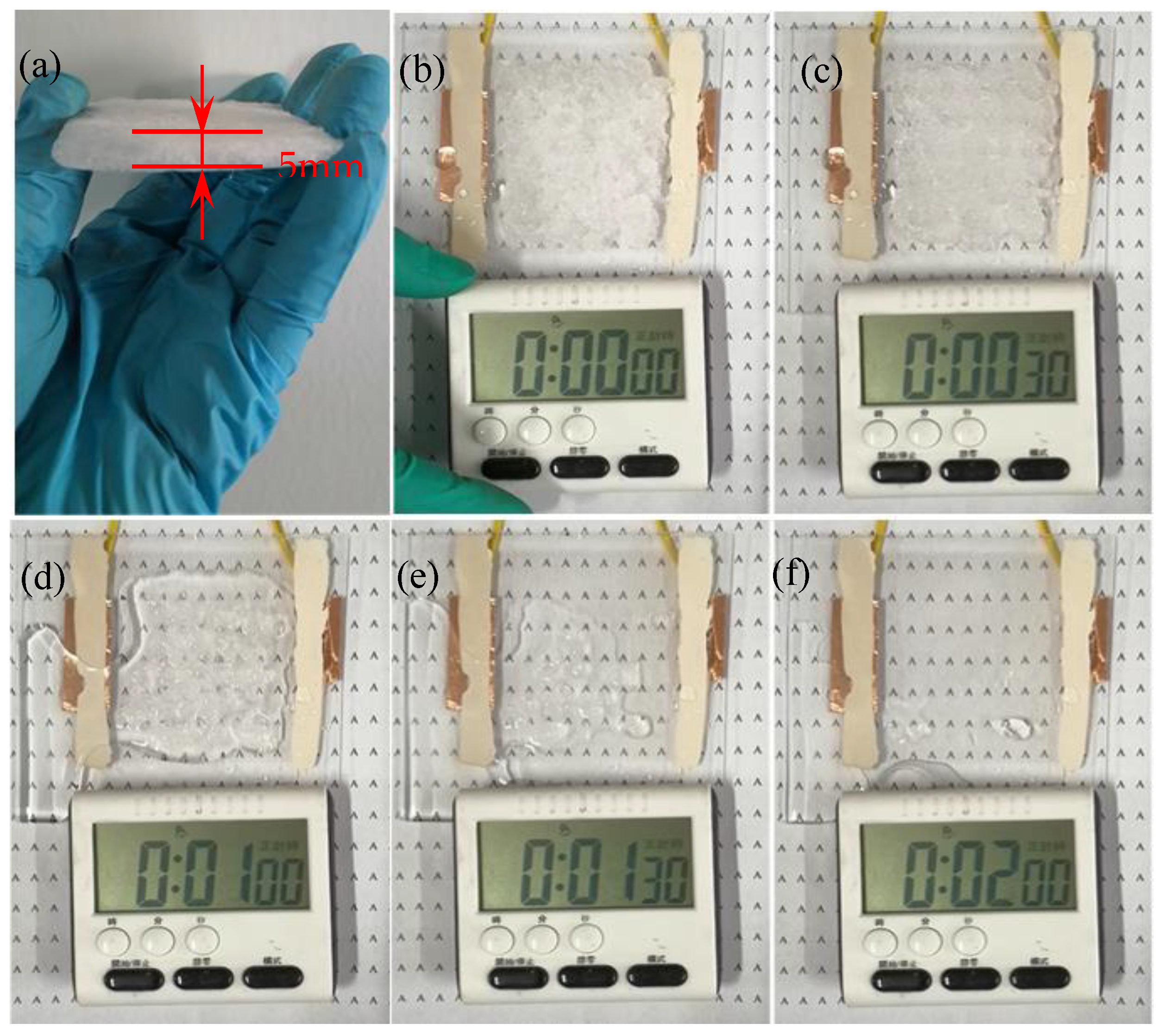
3.2.3. Practical Application Performance of the Printed Silver Mesh
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chu, X.; Tao, J.; Li, S.; Ji, S.; Ye, C. Sandwich-Structured Silver Nanowire Transparent Conductive Films with 3H Hardness and Robust Flexibility for Potential Applications in Curved Touch Screens. Nanomaterials 2019, 9, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, Y.S.; Bae, S.H.; Chen, H.; De Marco, N.; Yang, Y. Recent progress in materials and devices toward printable and flexible sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, K.; Kim, H. Highly Transparent Conductive Reduced Graphene Oxide/Silver Nanowires/Silver Grid Electrodes for Low-Voltage Electrochromic Smart Windows. ACS Appl. Mater. Interfaces 2018, 11, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Melzer, M.; Mönch, J.I.; Makarov, D.; Zabila, Y.; Cañón Bermúdez, G.S.; Karnaushenko, D.; Baunack, S.; Bahr, F.; Yan, C.; Schmidt, O.G.; et al. Wearable magnetic field sensors for flexible electronics. Adv. Mater. 2015, 27, 1274–1280. [Google Scholar] [CrossRef]
- Eh, A.L.S.; Tan, A.W.M.; Cheng, X.; Magdassi, S.; Lee, P.S. Recent advances in flexible electrochromic devices: Prerequisites, challenges, and prospects. Energy Technol. 2018, 6, 33–45. [Google Scholar] [CrossRef]
- Kim, Y.G.; Tak, Y.; Park, S.; Kim, H.; Kim, H. Structural engineering of metal-mesh structure applicable for transparent electrodes fabricated by self-formable cracked template. Nanomaterials 2017, 7, 214. [Google Scholar] [CrossRef] [Green Version]
- Park, M.U.; Shin, C.; Kim, H.; Kim, S.Y.; Choi, Y.J.; Chung, D.W. Improved Coating of PEDOT: PSS onto CVD Graphene by the Addition of PVA. Appl. Chem. Eng. 2018, 29, 734–739. [Google Scholar]
- Huang, Q.; Shen, W.; Song, W. Synthesis of colourless silver precursor ink for printing conductive patterns on silicon nitride substrates. Appl. Surf. Sci. 2012, 258, 7384–7388. [Google Scholar] [CrossRef]
- Kim, N.S.; Han, K.N. Future direction of direct writing. J. Appl. Phys. 2010, 108, 102801. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, H.; Zhang, X.; Liu, L.; Peng, P.; Zou, G.; Zhou, Y.N. Preparation of nanoparticle and nanowire mixed pastes and their low temperature sintering. J. Alloy. Compd. 2017, 690, 86–94. [Google Scholar] [CrossRef]
- Pidcock, G.C.; in het Panhuis, M. Extrusion printing of flexible electrically conducting carbon nanotube networks. Adv. Funct. Mater. 2012, 22, 4790–4800. [Google Scholar] [CrossRef]
- Koerner, H.; Liu, W.; Alexander, M.; Mirau, P.; Dowty, H.; Vaia, R.A. Deformation–morphology correlations in electrically conductive carbon nanotube—Thermoplastic polyurethane nanocomposites. Polymer 2005, 46, 4405–4420. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. A review of production methods of carbon nanotube and graphene thin films for electrothermal applications. Nanoscale 2014, 6, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Chizari, K.; Arjmand, M.; Liu, Z.; Sundararaj, U.; Therriault, D. Three-dimensional printing of highly conductive polymer nanocomposites for EMI shielding applications. Mater. Today Commun. 2017, 11, 112–118. [Google Scholar] [CrossRef]
- Richner, P.; Kress, S.J.; Norris, D.J.; Poulikakos, D. Charge effects and nanoparticle pattern formation in electrohydrodynamic NanoDrip printing of colloids. Nanoscale 2016, 8, 6028–6034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.; Rohner, P.; Thureja, D.; Schmid, M.; Galliker, P.; Poulikakos, D. Electrohydrodynamic nanodrip printing of high aspect ratio metal grid transparent electrodes. Adv. Funct. Mater. 2016, 26, 833–840. [Google Scholar] [CrossRef]
- Wang, Y.; Du, D.; Yang, X.; Zhang, X.; Zhao, Y. Optoelectronic and Electrothermal Properties of Transparent Conductive Silver Nanowires Films. Nanomaterials 2019, 9, 904. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Rahman, K.; Kim, D.S.; Choi, K.H. Direct printing of copper conductive micro-tracks by multi-nozzle electrohydrodynamic inkjet printing process. J. Mater. Process. Technol. 2012, 212, 700–706. [Google Scholar] [CrossRef]
- Huang, F.; Li, W.; Xiong, Q.; Li, X.; Yan, T.; Yu, W.; Zhang, H.; Liu, J. Preparation of Flake Silver Powders with High Diameter-to-Thickness Ratio. Guijinshu 2012, 33, 30–35. [Google Scholar]
- Yang, J.; Lu, L.; Wang, H.; Shi, W.; Zhang, H. Glycyl glycine templating synthesis of single-crystal silver nanoplates. Cryst. Growth Des. 2006, 6, 2155–2158. [Google Scholar] [CrossRef]
- Guo, S.; Fang, W.; Li, Y.; Yang, Y.; Wang, C.; Meng, X.; Chao, Y.; Yang, H. Synthesis and formation mechanism of micron-size silver flakes with high radius-thickness ratio: Application to silver paste. J. Mater. Sci. Mater. Electron. 2017, 28, 16267–16273. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Jiang, Z.; Yu, D. Low temperature sintering nano-silver conductive ink printed on cotton fabric as printed electronics. Prog. Org. Coat. 2016, 101, 604–611. [Google Scholar] [CrossRef]
- Jiu, J.; Zhang, H.; Nagao, S.; Sugahara, T.; Kagami, N.; Suzuki, Y.; Akai, Y.; Suganuma, K. Die-attaching silver paste based on a novel solvent for high-power semiconductor devices. J. Mater. Sci. 2016, 51, 3422–3430. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, Z.; Zhang, P. Preparation and characterization of low temperature curing conductive silver paste for screen printing. In Proceedings of the AIP Conference Proceedings AIP Publishing, Tianjin, China, 26 July 2018; Volume 1995, p. 020020. [Google Scholar]
- Lei, Q.; He, J.; Zhang, B.; Chang, J.; Li, D. Microscale electrohydrodynamic printing of conductive silver features based on in situ reactive inks. J. Mater. Chem. 2018, 6, 213–218. [Google Scholar] [CrossRef]
- Hyun, W.J.; Secor, E.B.; Hersam, M.C.; Frisbie, C.D.; Francis, L.F. High-resolution patterning of graphene by screen printing with a silicon stencil for highly flexible printed electronics. Adv. Mater. 2015, 27, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tong, K.; Pei, Q. A water-based silver-nanowire screen-print ink for the fabrication of stretchable conductors and wearable thin-film transistors. Adv. Mater. 2016, 28, 5986–5996. [Google Scholar] [CrossRef]
- Nir, M.M.; Zamir, D.; Haymov, I.; Ben-Asher, L.; Cohen, O.; Faulkner, B.; De La Vega, F. Electrically conductive inks for inkjet printing. Chem. Inkjet Inks 2010, 225–254. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Multi-nozzle electrohydrodynamic inkjet printing of silver colloidal solution for the fabrication of electrically functional microstructures. Appl. Phys. 2011, 104. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, J.; Byun, D. Invisible metal-grid transparent electrode prepared by electrohydrodynamic (EHD) jet printing. J. Phys. Appl. Phys. 2013, 46, 155103. [Google Scholar] [CrossRef]
- An, B.W.; Kim, K.; Lee, H.; Kim, S.Y.; Shim, Y.; Lee, D.Y.; Song, J.Y.; Park, J.U. High-resolution printing of 3D structures using an electrohydrodynamic inkjet with multiple functional inks. Adv. Mater. 2015, 27, 4322–4328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, Q.; Li, H.; Liu, M.; Li, Z.; Yang, K.; Zhao, J.; Qian, L.; Peng, Z.; Yang, J.; et al. Fabrication of High-Performance Silver Mesh for Transparent Glass Heaters via Electric-Field-Driven Microscale 3D Printing and UV-Assisted Microtransfer. Adv. Mater. 2019, 31, 1902479. [Google Scholar] [CrossRef]
- Peng, Z.; Gou, N.; Wei, Z.; Zhao, J.; Wang, F.; Yang, J.; Li, Y.; Lan, H. Fabrication of a Large-Area, Fused Polymer Micromold Based on Electric-Field-Driven (EFD) μ-3D Printing. Polymers 2019, 11, 1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Xu, Q.; Li, H.; Yang, K.; Hu, Y.; Li, Z.; Wang, F.; Peng, Z.; Lan, H. Fabricating transparent electrodes by combined electric-field-driven fusion direct printing and the liquid bridge transfer method. J. Phys. Appl. Phys. 2019, 52, 245103. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Z.; Hu, Y.; Li, H.; Yang, J.; Lan, H. Facile fabrication of defogging microlens arrays using electric field-driven jet printing. Opt. Laser Technol. 2019, 123, 105943. [Google Scholar] [CrossRef]
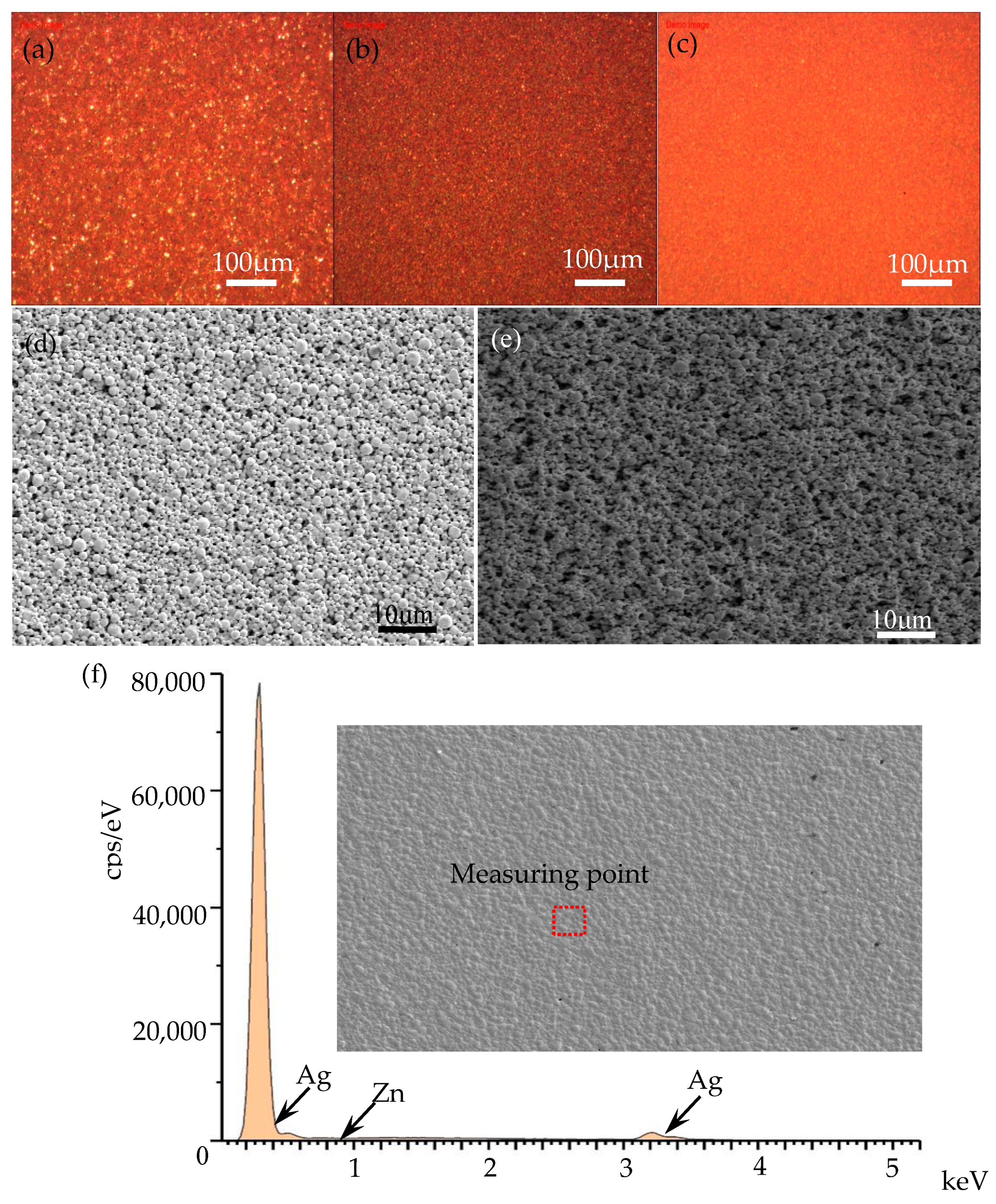


| Element | wt% | wt% Sigma | Atomic Percentage/% |
|---|---|---|---|
| Zn | 0.22 | 0.19 | 0.36 |
| Ag | 99.78 | 0.19 | 99.64 |
| Total | 100 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhu, X.; Li, Z.; Yang, J.; Lan, H. Preparation of Nano Silver Paste and Applications in Transparent Electrodes via Electric-Field Driven Micro-Scale 3D Printing. Nanomaterials 2020, 10, 107. https://doi.org/10.3390/nano10010107
Li H, Zhu X, Li Z, Yang J, Lan H. Preparation of Nano Silver Paste and Applications in Transparent Electrodes via Electric-Field Driven Micro-Scale 3D Printing. Nanomaterials. 2020; 10(1):107. https://doi.org/10.3390/nano10010107
Chicago/Turabian StyleLi, Hongke, Xiaoyang Zhu, Zhenghao Li, Jianjun Yang, and Hongbo Lan. 2020. "Preparation of Nano Silver Paste and Applications in Transparent Electrodes via Electric-Field Driven Micro-Scale 3D Printing" Nanomaterials 10, no. 1: 107. https://doi.org/10.3390/nano10010107





