Strong Biomimetic Immobilization of Pt-Particle Catalyst on ABS Substrate Using Polydopamine and Its Application for Contact-Lens Cleaning with H2O2
Abstract
:1. Introduction
2. Results and Discussion
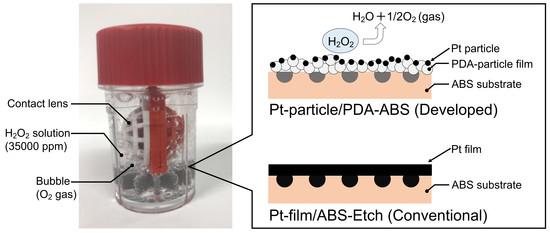
2.1. External Appearance
2.2. Confirmation of the Formation of a PDA Film by X-Ray Photoelectron Spectroscopy (XPS)
2.3. Observation of Pt Particles by SEM
2.4. Pt-Loading Weight Determined by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES)
2.5. Catalytic Activity for H2O2 Decomposition
2.6. Catalytic Durability during H2O2 Decomposition
2.7. Effect of Plasma Treatment on Catalytic Durability
3. Materials and Methods
3.1. PDA Coating and Immobilization of Pt Particles on ABS
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waite, J.H. Nature’s underwater adhesive specialist. Int. J. Adhes. Adhes. 1987, 7, 9–14. [Google Scholar] [CrossRef]
- Vreeland, V.; Waite, J.H.; Epstein, L. Polyphenols and oxidases in substratum adhesion by marine algae and mussels. J. Phycol. 1998, 34, 1–8. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H.; Tanzer, M.L. Polyphenolic substance of Mytilus edulis: Novel adhesive containing L-dopa and hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar] [CrossRef]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef]
- Waite, J.H. Adhesion a la Moule. Integr. Comp. Biol. 2002, 42, 1172–1180. [Google Scholar] [CrossRef]
- Korbel, J.O.; Urban, A.E.; Affourtit, J.P.; Godwin, B.; Grubert, F.; Simons, J.F.; Kim, P.M.; Palejev, D.; Carriero, N.J.; Du, L.; et al. Paired-end mapping reveals extensive structural variation in the human genome. Science 2007, 318, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Kawamura, A.; Kohri, M.; Morimoto, G.; Nannichi, Y.; Taniguchi, T.; Kishikawa, K. Color Biomimetic Photonic Materials with Iridescent and Non-Iridescent Structural Colors. Sci. Rep. 2016, 6, 33984. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Advanced titanium dioxide-polytetrafluorethylene (TiO2-PTFE) nanocomposite coatings on stainless steel surfaces with antibacterial and anti-corrosion properties. Appl. Surf. Sci. 2019, 490, 231–241. [Google Scholar] [CrossRef]
- Yamagishi, K.; Kirino, I.; Takahashi, I.; Amano, H.; Takeoka, S.; Morimoto, Y.; Fujie, T. Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy. Nat. Biomed. Eng. 2019, 3, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Muntz, A.; Subbaraman, L.N.; Sorbara, L.; Jones, L. Tear exchange and contact lenses: A review. J. Optom. 2015, 8, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.J.; Fisher, D. Contact Lenses 2018. Contact Lens Spectr. 2019, 34, 18–23. [Google Scholar]
- Paugh, J.R.; Brennan, N.A.; Efron, N. Ocular Response to Hydrogen Peroxide. Am. J. Optom. Physiol. Opt. 1988, 65, 91–98. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Radiolytic Synthesis of Pt-Particle/ABS Catalysts for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials 2017, 7, 235. [Google Scholar] [CrossRef] [Green Version]
- Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Improved Catalytic Durability of Pt-Particle/ABS for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials 2019, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Cahen, D.; Lester, J.E. Mixed and partial oxidation states. Photoelectron spectroscopic evidence. Chem. Phys. Lett. 1973, 18, 108–111. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Ntais, S.; Baranova, E.A. Particle size effect on catalytic activity of carbon-supported Pt nanoparticles for complete ethylene oxidation. Appl. Catal. A Gen. 2013, 464, 87–94. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Kageyama, S.; Seino, S.; Nakagawa, T.; Kugai, J.; Ueno, K.; Yamamoto, T.A. Mass production of highly loaded and highly dispersed PtRu/C catalysts for methanol oxidation using an electron-beam irradiation reduction method. J. Exp. Nanosci. 2016, 11, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Mizukoshi, Y.; Oshima, R.; Maeda, Y.; Nagata, Y. Preparation of platinum nanoparticles by sonochemical reduction of the Pt(II) ion. Langmuir 1999, 15, 2733–2737. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Yuya, M.; Ono, T.; Nakagawa, T.; Seino, S.; Okitsu, K.; Mizukoshi, Y.; Emura, S.; Yamamoto, T.A. Sonochemically synthesized core-shell structured Au-Pd nanoparticles supported on γ-Fe2O3 particles. J. Nanoparticle Res. 2006, 15, 875–880. [Google Scholar]
- Mizukoshi, Y.; Tsuru, Y.; Tominaga, A.; Seino, S.; Masahashi, N.; Tanabe, S.; Yamamoto, T.A. Sonochemical immobilization of noble metal nanoparticles on the surface of maghemite: Mechanism and morphological control of the products. Ultrason. Sonochem. 2008, 15, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ziylan-Yavas, A.; Mizukoshi, Y.; Maeda, Y.; Ince, N.H. Supporting of pristine TiO2 with noble metals to enhance the oxidation and mineralization of paracetamol by sonolysis and sonophotolysis. Appl. Catal. B Environ. 2015, 172, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Toshima, N.; Wang, Y. Preparation and Catalysis of Novel Colloidal Dispersions of Copper/Noble Metal Bimetallic Clusters. Langmuir 1994, 10, 4574–4580. [Google Scholar] [CrossRef]
- Toshima, B.N.; Wang, Y. Polymer-Protected Cu/Pd Bimetallic Clusters. Adv. Mater. 1994, 100080, 245–247. [Google Scholar] [CrossRef]
- Daimon, H.; Kurobe, Y. Size reduction of PtRu catalyst particle deposited on carbon support by addition of non-metallic elements. Catal. Today 2006, 111, 182–187. [Google Scholar] [CrossRef]
- Liao, P.C. Activity and XPS Studies Catalysts Bimetallic. J. Catal. 1982, 316, 307–316. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A. Characterization and catalytic properties of nano-sized Ag metal catalyst on TiO2-SiO2 synthesized by photo-assisted deposition and impregnation methods. J. Alloys Compd. 2010, 501, 301–306. [Google Scholar] [CrossRef]
- Rahsepar, M.; Pakshir, M.; Piao, Y.; Kim, H. Preparation of highly active 40 wt.%pt on multiwalled carbonnanotube by improved impregnation method for fuel cell applications. Fuel Cells 2012, 12, 827–834. [Google Scholar] [CrossRef]
- Belloni, J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry: Application to catalysis. Catal. Today 2006, 113, 141–156. [Google Scholar] [CrossRef]
- Seino, S.; Kinoshita, T.; Nakagawa, T.; Kojima, T.; Taniguci, R.; Okuda, S.; Yamamoto, T.A. Radiation induced synthesis of gold/iron-oxide composite nanoparticles using high-energy electron beam. J. Nanoparticle Res. 2008, 10, 1071–1076. [Google Scholar] [CrossRef]












| Sample ID | Pt(0) | Pt(II) | Pt(IV) |
|---|---|---|---|
| Pt/ABS-untreated | 69.1 | 16.4 | 14.5 |
| Pt/ABS-PDA(24h) | 81.1 | 11.0 | 7.9 |
| Pt/ABS-plasma-PDA(24h) | 86.7 | 7.1 | 6.2 |
| Sample ID | Plasma pre-treatment | PDA coating | Pt-particle deposition |
|---|---|---|---|
| Glass-untreated | ― | ― | ― |
| Glass-PDA(1h) | ― | ◯ | ― |
| Glass-PDA(3h) | ― | ◯ | ― |
| Glass-PDA(24h) | ― | ◯ | ― |
| ABS-untreated | ― | ― | ― |
| ABS-PDA(1h) | ― | ◯ | ― |
| ABS-PDA(3h) | ― | ◯ | ― |
| ABS-PDA(24h) | ― | ◯ | ― |
| ABS-plasma | ◯ | ― | ― |
| ABS-plasma-PDA(24h) | ◯ | ◯ | ― |
| Pt/ABS-untreated | ― | ― | ◯ |
| Pt/ABS-PDA(1h) | ― | ◯ | ◯ |
| Pt/ABS-PDA(3h) | ― | ◯ | ◯ |
| Pt/ABS-PDA(24h) | ― | ◯ | ◯ |
| Pt/ABS-plasma-PDA(24h) | ◯ | ◯ | ◯ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohkubo, Y.; Aoki, T.; Kaibara, D.; Seino, S.; Mori, O.; Sasaki, R.; Endo, K.; Yamamura, K. Strong Biomimetic Immobilization of Pt-Particle Catalyst on ABS Substrate Using Polydopamine and Its Application for Contact-Lens Cleaning with H2O2. Nanomaterials 2020, 10, 114. https://doi.org/10.3390/nano10010114
Ohkubo Y, Aoki T, Kaibara D, Seino S, Mori O, Sasaki R, Endo K, Yamamura K. Strong Biomimetic Immobilization of Pt-Particle Catalyst on ABS Substrate Using Polydopamine and Its Application for Contact-Lens Cleaning with H2O2. Nanomaterials. 2020; 10(1):114. https://doi.org/10.3390/nano10010114
Chicago/Turabian StyleOhkubo, Yuji, Tomonori Aoki, Daisuke Kaibara, Satoshi Seino, Osamu Mori, Rie Sasaki, Katsuyoshi Endo, and Kazuya Yamamura. 2020. "Strong Biomimetic Immobilization of Pt-Particle Catalyst on ABS Substrate Using Polydopamine and Its Application for Contact-Lens Cleaning with H2O2" Nanomaterials 10, no. 1: 114. https://doi.org/10.3390/nano10010114
APA StyleOhkubo, Y., Aoki, T., Kaibara, D., Seino, S., Mori, O., Sasaki, R., Endo, K., & Yamamura, K. (2020). Strong Biomimetic Immobilization of Pt-Particle Catalyst on ABS Substrate Using Polydopamine and Its Application for Contact-Lens Cleaning with H2O2. Nanomaterials, 10(1), 114. https://doi.org/10.3390/nano10010114