The Application of Indium Oxide@CPM-5-C-600 Composite Material Derived from MOF in Cathode Material of Lithium Sulfur Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Synthesis of CPM-5 Materials
2.3. Synthesis of CPM-5-C-600 Materials
2.4. Synthesis of CPM-5-C-600@S Materials
2.5. Materials Characterization
2.6. Cells Assembly and Electrochemical Measurements
3. Results and Discussions
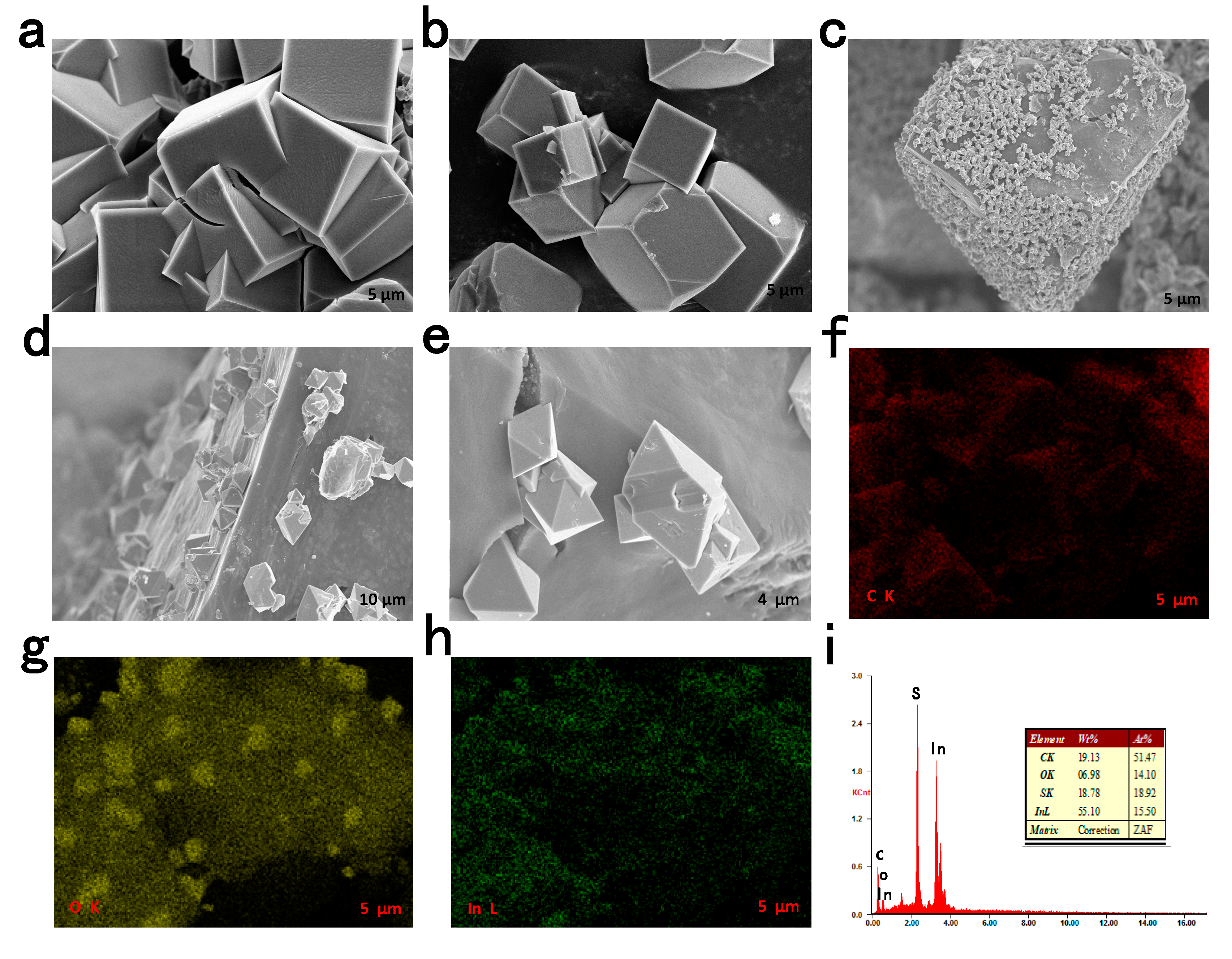
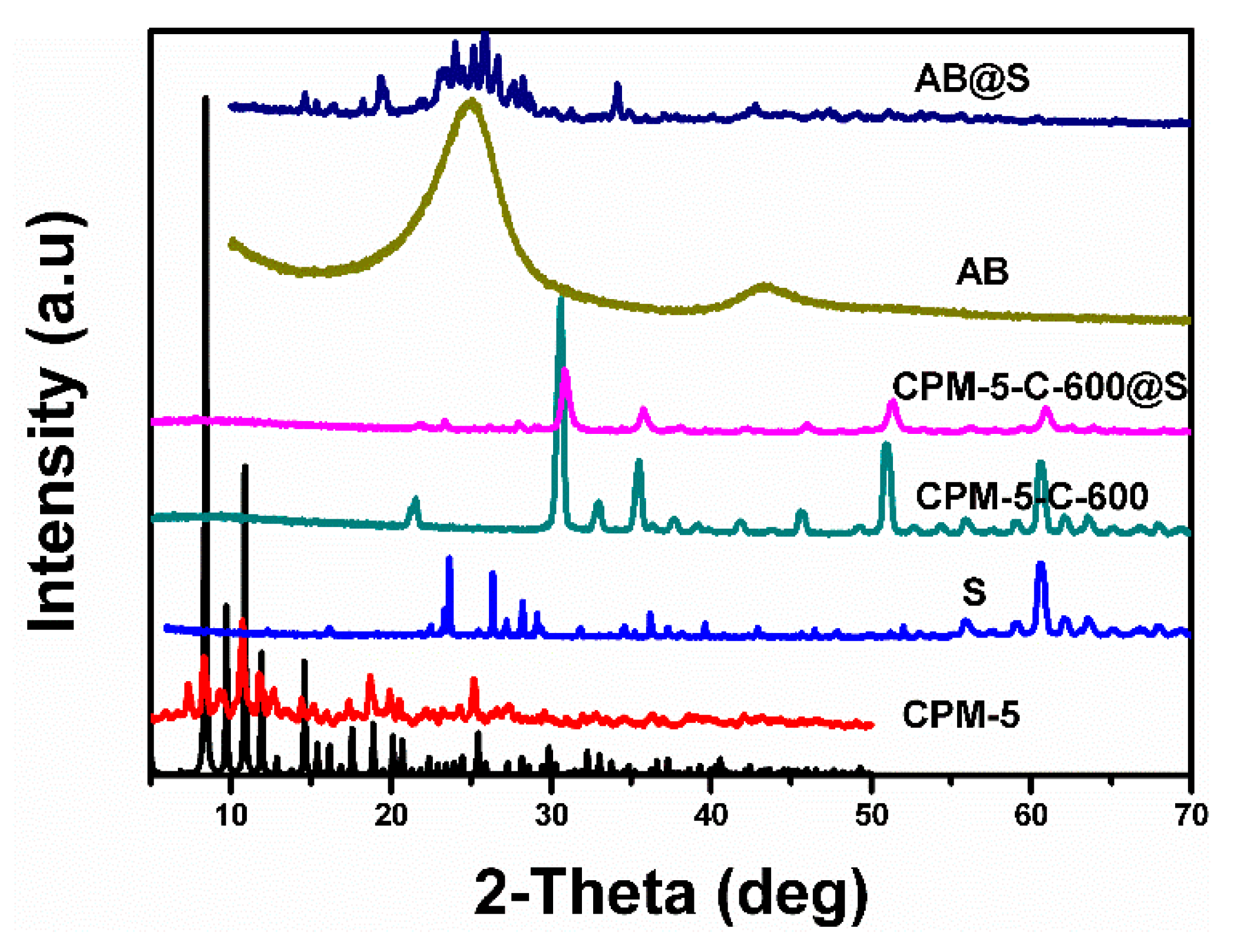
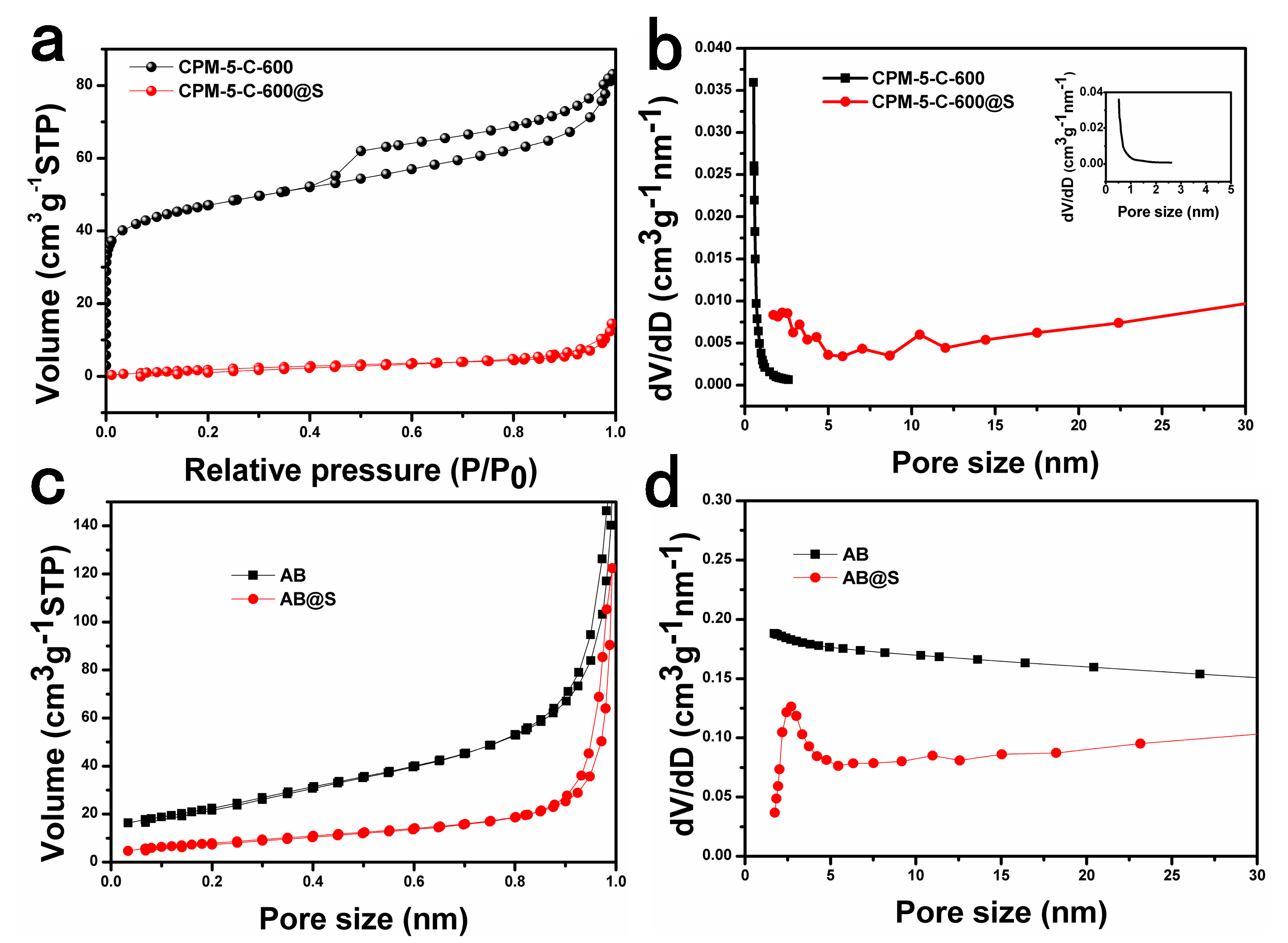
3.1. Characteristics
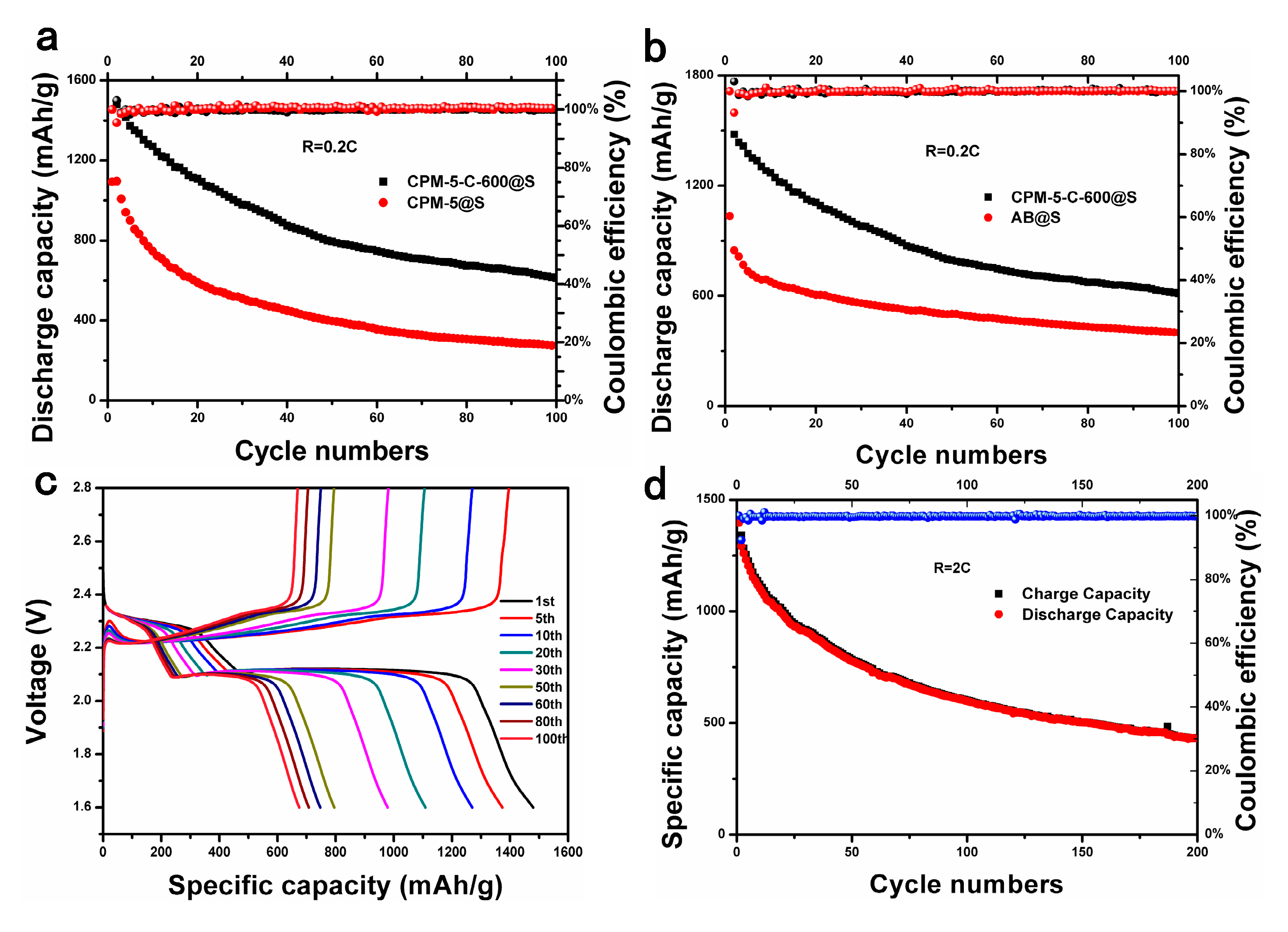
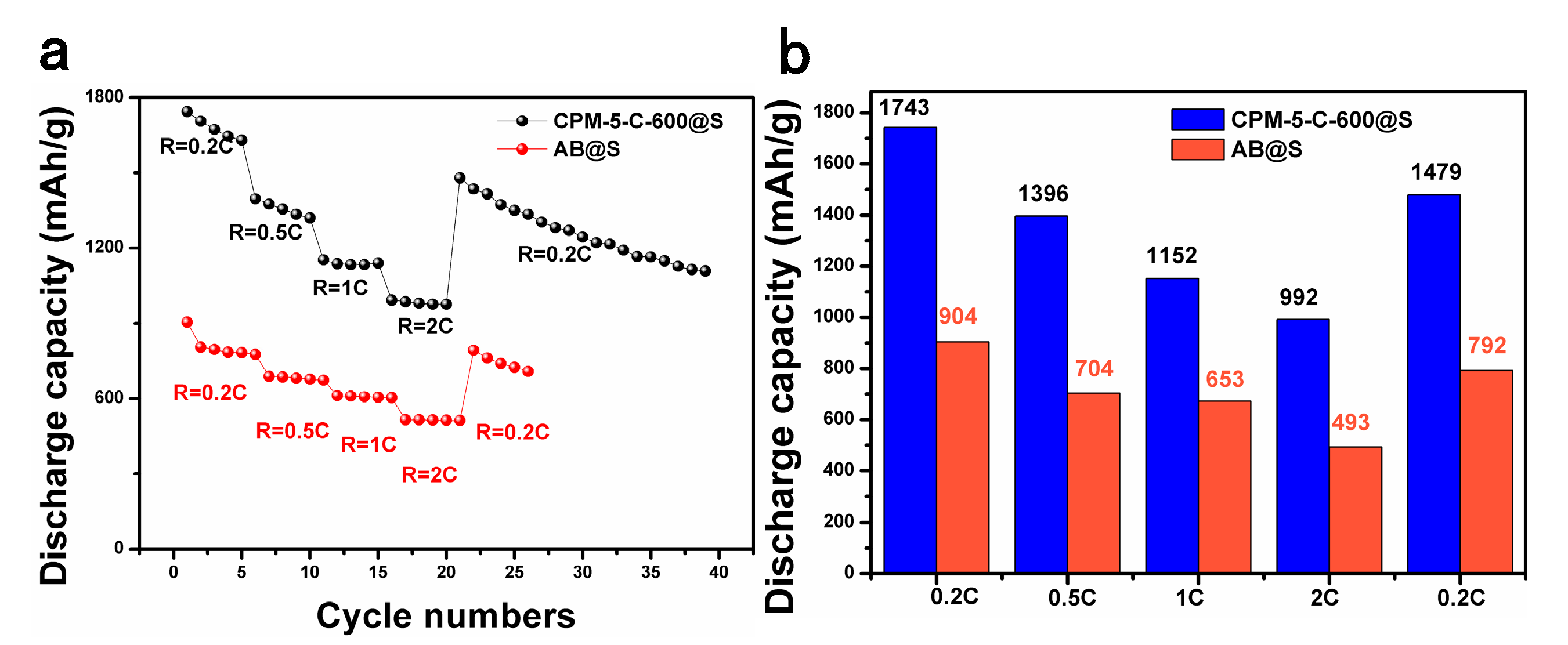
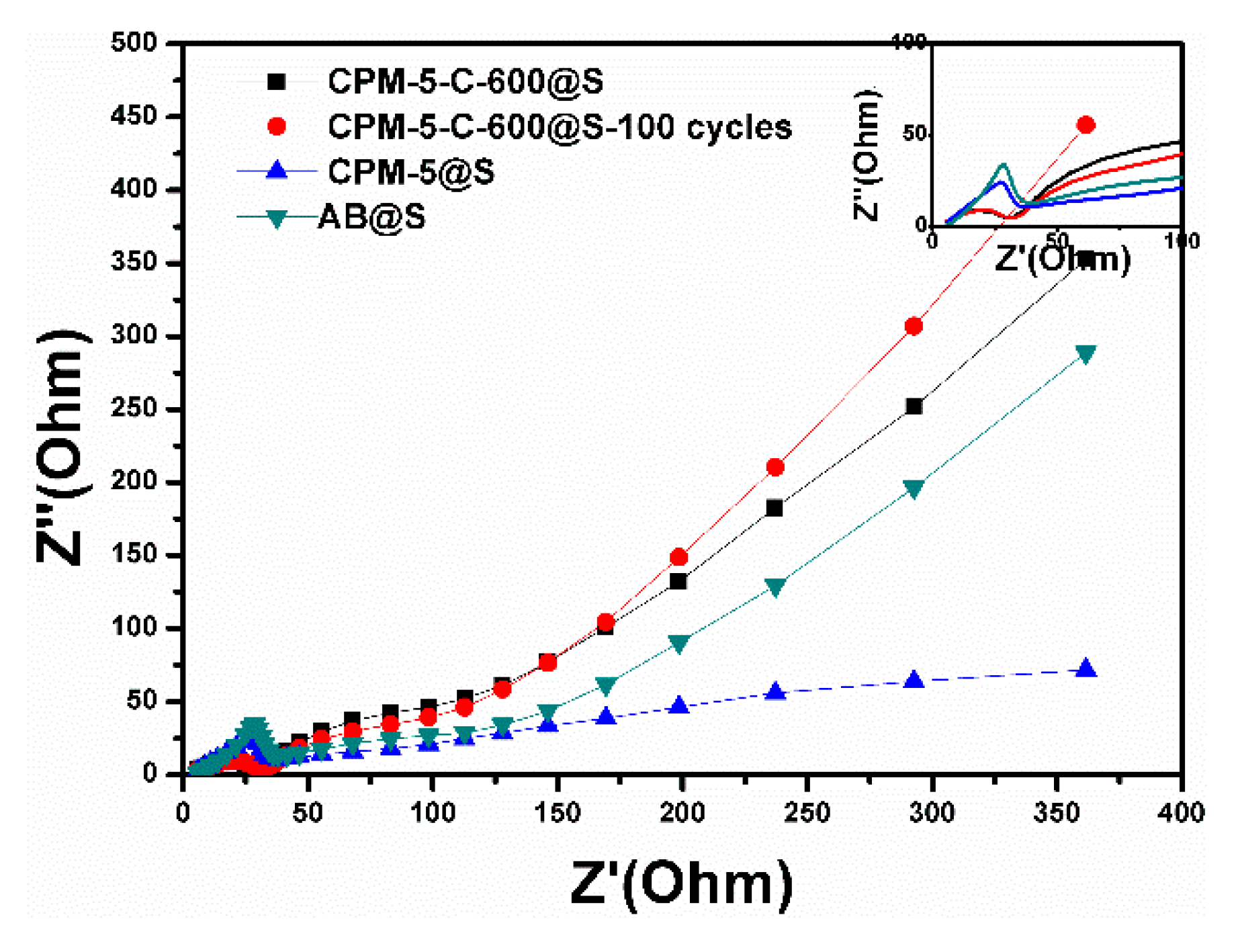
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Kaneti, Y.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal-Organic Framework-Derived One-Dimensional Porous or Hollow Carbon-Based Nanofibers for Energy Storage and Conversion. Mater. Horiz. 2018, 5, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, Y.; Yang, W.; Shen, W.; Xue, H.; Pang, H. MOF-Derived Metal Oxide Composites for Advanced Electrochemical Energy Storage. Small 2018, 14, 1704435. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, J.; Chen, Z.; Liu, K.; Zhou, G.; Sun, Y.; Song, M.-S.; Bao, Z.; Cui, Y. Stretchable Lithium-Ion Batteries Enabled by Device-Scaled Wavy Structure and Elastic-Sticky Separator. Adv. Energy Mater. 2017, 7, 1701076. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Chen, J.; Su, H.; Liu, F.; Yang, W. One-step synthesis of hierarchically porous carbons for high-performance electric double layer supercapacitors. J. Power Sources 2016, 315, 120–126. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J.; et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with alighed nanoporous host for lithium batteries. Nat. Nanotechnol. 2019, 14, 705–711. [Google Scholar] [CrossRef]
- Bustamante, E.; Fernández, J.; Zamaro, J. Influence of the solvent in the synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals at room temperature. J. Colloid Interface Sci. 2014, 424, 37–43. [Google Scholar] [CrossRef]
- Liu, K.; Kong, B.; Liu, W.; Sun, Y.; Song, M.-S.; Chen, J.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Stretchable Lithium Metal Anode with Improved Mechanical and Electrochemical Cycling Stability. Joule 2018, 2, 1857–1865. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Huang, H.; Zhang, H.; Chu, X.; Zhang, B.; Gu, B.; Zheng, X.; Wu, S.; He, W.; Yan, C.; et al. In Situ Direct Method To Massively Prepare Hydrophilic Porous Carbide-Derived Carbons for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2018, 1, 3544–3553. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W.; Zou, M.; Wang, Y.; Chen, Y.; Xu, L.; Wu, H.; Cao, A. 3D, Mutually Embedded MOF@Carbon Nanotube Hybrid Networks for High-Performance Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1800013. [Google Scholar] [CrossRef]
- Ma, L.; Hendrickson, K.E.; Wei, S.; Archer, L.A. Nanomaterials: Science and applications in the lithium–sulfur battery. Nano Today 2015, 10, 315–338. [Google Scholar] [CrossRef] [Green Version]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, X.; Liu, Y.; Wang, W.; Shao, Z. Recent Progress in Metal–Organic Frameworks for Lithium–Sulfur Batteries. Polyhedron 2018, 155, 464–484. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.; Zu, C.; Su, Y. Rechargeable Lithium−Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Qin, T.; Guo, P.; Sun, K.; Su, H.; Tao, K.; He, D. A Novel Strategy for the Selection of Polysulfde Adsorbents toward High-Performance Lithium-Sulfur Batteries. Adv. Mater. 2019, 6, 1900393. [Google Scholar]
- Wu, Q.; Zhou, X.; Xu, J.; Cao, F.; Li, C. Carbon-based derivatives from metal-organic frameworks as cathode hosts for Li–S batteries. J. Energy Chem. 2019, 38, 94–113. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, S.; Zhang, L.; Deng, Y.; Xu, H.; Qin, X.; Chen, G. CoS-interposed and Ketjen black-embedded carbon nanofiber framework as a separator modulation for high performance Li-S batteries. Chem. Eng. J. 2019, 269, 77–86. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, Q.; An, Y.; Anasori, B.; Wang, H.; Xu, B. Ultra-microporous carbons encapsulate small sulfur molecules for high performance lithium-sulfur battery. Nano Energy 2017, 33, 402–409. [Google Scholar] [CrossRef]
- Song, J.; Gordin, M.L.; Xu, T.; Chen, S.; Yu, Z.; Sohn, H.; Lu, J.; Ren, Y.; Duan, Y.; Wang, D. Strong Lithium Polysulfide Chemisorption on Electroactive Sites of Nitrogen-Doped Carbon Composites For High-Performance Lithium-Sulfur Battery Cathodes. Angew. Chem. Int. Ed. 2015, 54, 4325–4329. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal–organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef]
- Wu, H.; Gong, Q.; Olson, D.; Li, J. Commensurate Adsorption of Hydrocarbons and Alcohols in Microporous Metal Organic Frameworks. Am. Chem. Soc. 2012, 112, 836–868. [Google Scholar] [CrossRef]
- Zheng, S.; Bu, J.; Li, Y.; Wu, T.; Zuo, F.; Feng, P.; Bu, X. Pore space partition and charge separation in cage-within-cage indium-organic frameworks with high CO2 uptake. J. Am. Chem. Soc. 2010, 132, 17062–17064. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.; Cao, S.; Peng, X.; Ducati, C.; Kumar, R.V.; Cheetham, A.K. Carbon with hierarchical pores from carbonized metal–organic frameworks for lithium sulphur batteries. Chem. Commun. 2013, 49, 2192–2194. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.E.; Aversa, G.E.; Roy, A.; Falk, M.L.; Bedford, N.M.; Thoi, V.S. Promoting sulfur adsorption using surface Cu sites in metal-organic frameworks for lithium sulfur batteries. J. Mater. Chem. A 2018, 6, 4811–4821. [Google Scholar] [CrossRef]
- Shi, M.; Yan, Y.; Wei, Y.; Zou, Y.; Deng, Q.; Wang, J.; Yang, R.; Xu, Y.; Han, T. Fabrication of ultrafine Gd2O3 nanoparticles/carbon aerogel composite as immobilization host for cathode for lithium-sulfur batteries. Int. J. Energy Res. 2019, 43, 7614–7626. [Google Scholar]
- Jiang, H.; Liu, X.; Wu, Y.; Shu, Y.; Deng, H. Metal-organic frameworks for high charge-discharge rates in lithium-sulfur batteries. Angew. Chem. Int. Ed. 2018, 57, 3916–3921. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Shen, Q. Modification of cobalt-containing MOF-derived mesoporous carbon as an effective sulfur-loading host for rechargeable lithium-sulfur batteries. J. Alloys Compd. 2019, 772, 843–851. [Google Scholar] [CrossRef]
- Yang, X.; Yan, N.; Zhou, W.; Zhang, H.; Li, X.; Zhang, H. Sulfur embedded in one-dimension French fries-like hierarchical porous carbon derived from metal organic framework for high performance lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 15314–15323. [Google Scholar] [CrossRef]
- Hu, M.; Reboul, J.; Furukawa, S.; Torad, N.L.; Ji, Q.; Srinivasu, P.; Ariga, K.; Kitagawa, S.; Yamauchi, Y. Direct carbonization of al-based porous coordination polymer for synthesis of nanoporous carbon. J. Am. Chem. Soc. 2010, 134, 2864–2867. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, Q.; Li, S.; Jiang, Y.; Hu, M. Mesoporous In2O3 materials prepared by solid-state thermolysis of indium-organic frameworks and their high HCHO-sensing performance. Inorg. Chem. Commun. 2016, 63, 48–52. [Google Scholar] [CrossRef]
- Xu, G.; Ding, B.; Shen, L.; Nie, P.; Han, J.; Zhang, X. Sulfur embedded in metal organic framework-derived hierarchically porous carbon nanoplates for high performance lithium–sulfur battery. J. Mater. Chem. A 2013, 1, 4490–4496. [Google Scholar] [CrossRef]
- Fan, L.; Wu, H.; Wu, X.; Wang, M.; Cheng, J.; Zhang, N.; Feng, Y.; Sun, K. Fe-MOF derived jujube pit like Fe3O4/C composite as sulfur host for lithium-sulfur battery. Electrochim. Acta 2019, 295, 444–451. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.; Yuan, L.; Zeng, R.; Xiang, J.; Chen, W.; Yuan, K.; Chen, J.; Huang, Y.; Xie, J. Facile synthesis of Li2S@C composites as cathode for Li–S batteries. J. Energy Chem. 2019, 37, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Geng, P.; Cao, S.; Guo, X.; Ding, J.; Zhang, S.; Zheng, M.; Pang, H. Polypyrrole indexing hollow metal-organic framework composites for lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 19465–19470. [Google Scholar] [CrossRef]
- Wu, H.; Wei, S.; Zhang, L.; Xu, R.; Hng, H.; Lou, X. Embedding Sulfur in MOF-Derived Microporous Carbon Polyhedrons for Lithium–Sulfur Batteries. Chem. A Eur. J. 2013, 19, 10804–10808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, W.; Wu, Y.; Wang, Y.; Zou, M.; Cao, A. Dense monolithic MOF and carbon nanotube hybrid with enhanced volumetric and areal capacities for lithium-sulfur battery. J. Mater. Chem. A 2019, 7, 9195–9201. [Google Scholar] [CrossRef]
- Su, Y.; Manthiram, A. Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Su, D.; Zhang, W.; Guo, X.; Wang, G. 3D Metal Carbide@Mesoporous Carbon Hybrid Architecture as a New Polysulfide Reservoir for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 8746–8756. [Google Scholar] [CrossRef]
- Xu, G.; Zou, Y.; Huang, B. Metal-organic framework-74-Ni/carbon nanotube composite as sulfur host for high performance lithium-sulfur batteries. J. Electroanal. Chem. 2018, 830, 43–49. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, Z.; Qu, Y.; Zhou, C.; Wang, X.; Li, J. Confine sulfur in mesoporous metal–organic framework @ reduced graphene oxide for lithium sulfur battery. J. Alloys Compd. 2014, 582, 334–340. [Google Scholar] [CrossRef]
- Xu, S.; Liang, X.; Wu, X.; Zhao, S.; Chen, J.; Wang, K.; Chen, J. Multistage discharge constructing hererostructure with enhanced solid-solution behavior for long-life lithium-oxygen batteries. Nat. Commun. 2019, 10, 5810. [Google Scholar] [CrossRef] [Green Version]



| Samples | Initial Capacity | Cycling Stability | Rate Capacity | Reference |
|---|---|---|---|---|
| CPM-5-600-C@S | 1500 mAh g−1 | 794 mAh g−1 at 0.2 C after 50 cycles 650 mAh g−1 after 100 cycles | 992 mAh g−1 at 2 C | this work |
| CoS/KB | 1500 mAh g−1 | 600 mAh g−1 at 0.5 C after 200 cycles | 700 mAh g-1 at 2 C | [16] |
| MOF-74/CNT | 1065 mAh g−1 | 610 mAh g−1 at 0.5 C after 100 cycles | 667 mAh g−1 at 2 C | [38] |
| Fe3O4/C/S | 1050 mAh g−1 | 750 mAh g−1 at 0.2 C after 50 cycles | 640 mAh g−1 at 1 C | [31] |
| HPCN-S(MOF-5) | 1177 mAh g−1 | 730 mAh g −1 at 0.5 C after 50 cycles | / | [30] |
| C from ZnFumarate | 1500 mAh g−1 | 650 mAh g−1 at 400 mA g−1 after 40 cycles | / | [22] |
| MIL-101@rGO/S | 980 mAh g−1 | 650 mAh g−1 at 335 mA g−1 after 50 cycles | 980 mAh g−1 at 0.2 C | [39] |
| S/Gd2(Gd2O3)-CA | 1210 mAh g−1 | 555 mAh g−1 at 0.1 C after 50 cycles | 420 mAh g−1 at 1 C | [24] |
| S/FLHPC | 1206 mAh g−1 | 856 mAh g−1 at 0.2 C after 100 cycles | 763 mAh g−1 at 2 C | [27] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Wang, X.; Yao, J.; Zhang, M.; Wang, J. The Application of Indium Oxide@CPM-5-C-600 Composite Material Derived from MOF in Cathode Material of Lithium Sulfur Batteries. Nanomaterials 2020, 10, 177. https://doi.org/10.3390/nano10010177
Han G, Wang X, Yao J, Zhang M, Wang J. The Application of Indium Oxide@CPM-5-C-600 Composite Material Derived from MOF in Cathode Material of Lithium Sulfur Batteries. Nanomaterials. 2020; 10(1):177. https://doi.org/10.3390/nano10010177
Chicago/Turabian StyleHan, Guodong, Xin Wang, Jia Yao, Mi Zhang, and Juan Wang. 2020. "The Application of Indium Oxide@CPM-5-C-600 Composite Material Derived from MOF in Cathode Material of Lithium Sulfur Batteries" Nanomaterials 10, no. 1: 177. https://doi.org/10.3390/nano10010177




