Organic Thermoelectric Multilayers with High Stretchiness
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Substrates
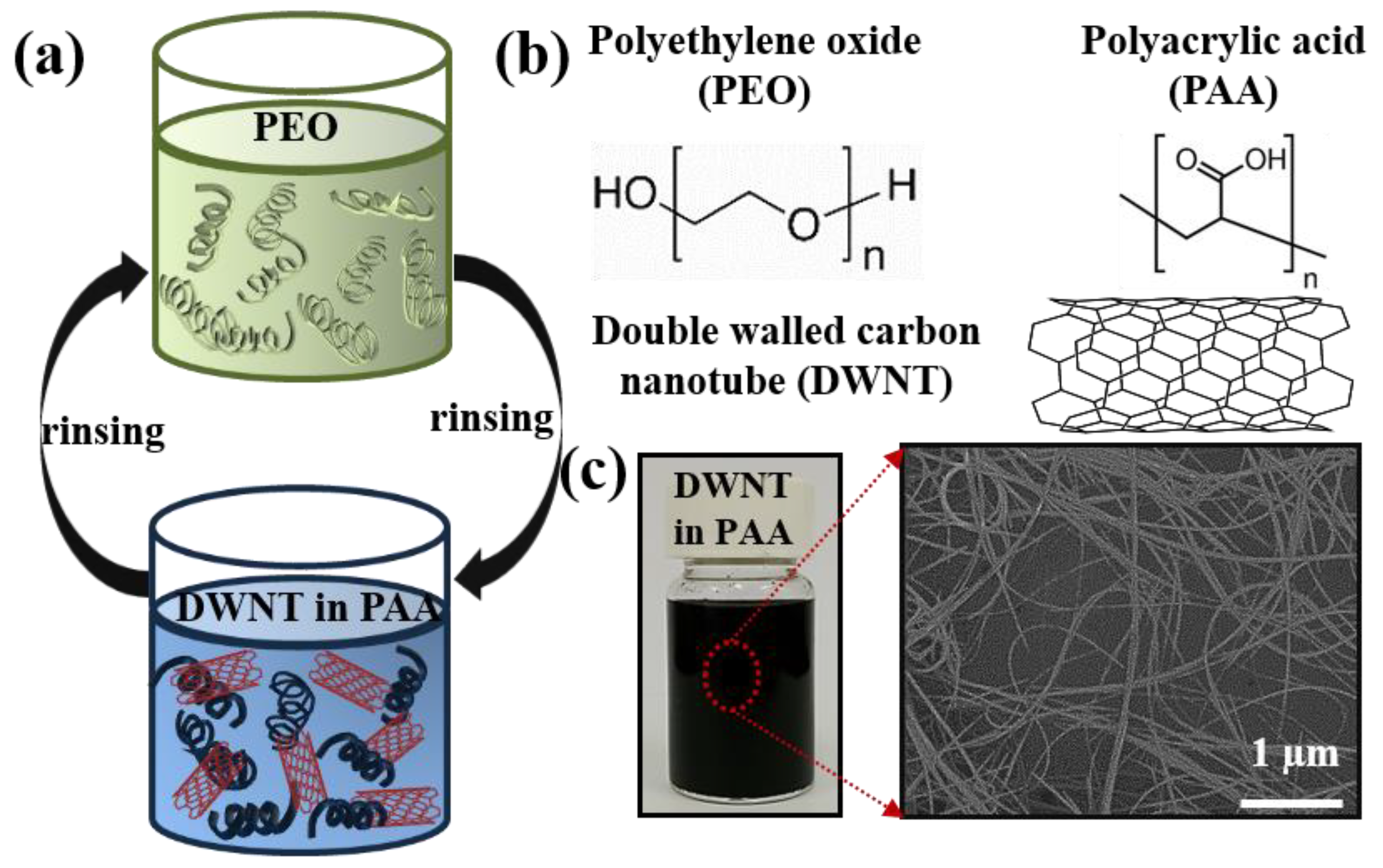
2.3. Multilayer Formation
2.4. Characterization of Thin Films
2.5. Thermoelectric Measurements
3. Results
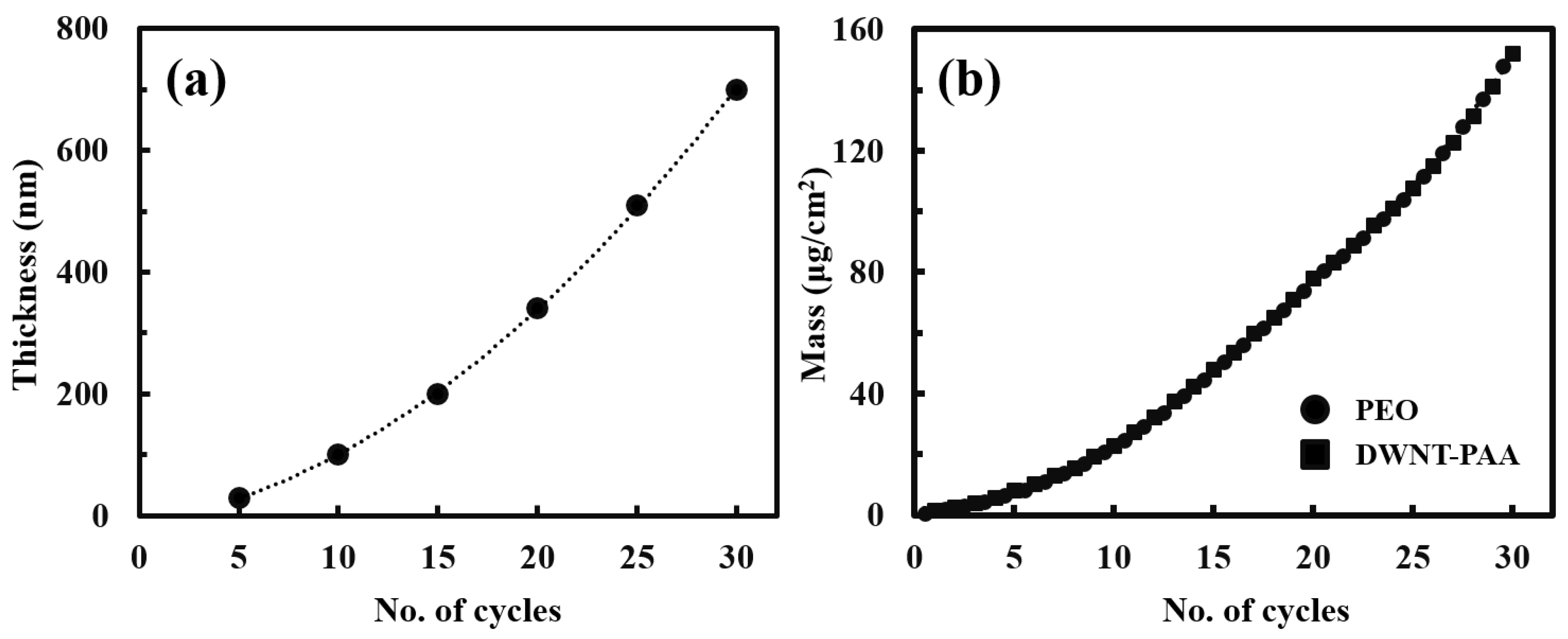
3.1. Growth Behavior
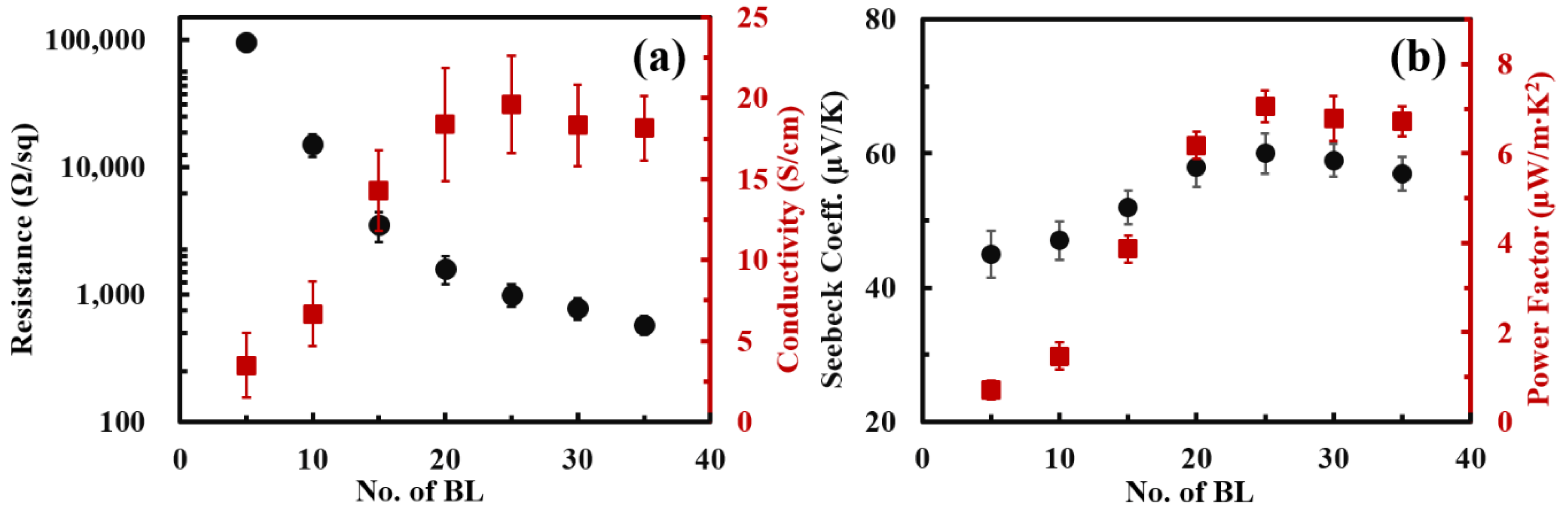
3.2. Thermoelectric Properties
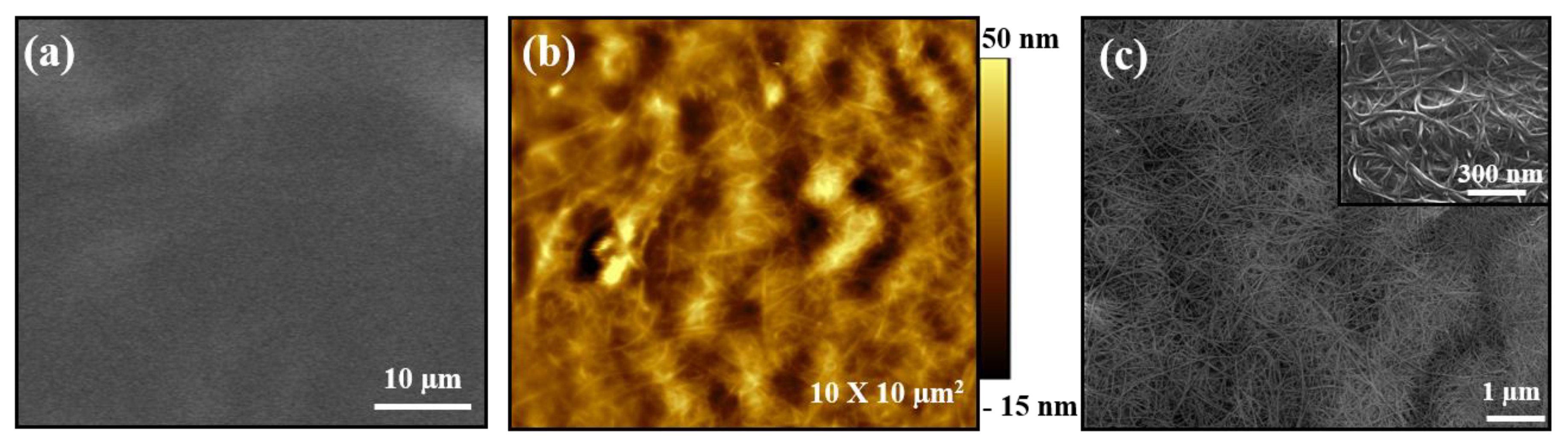
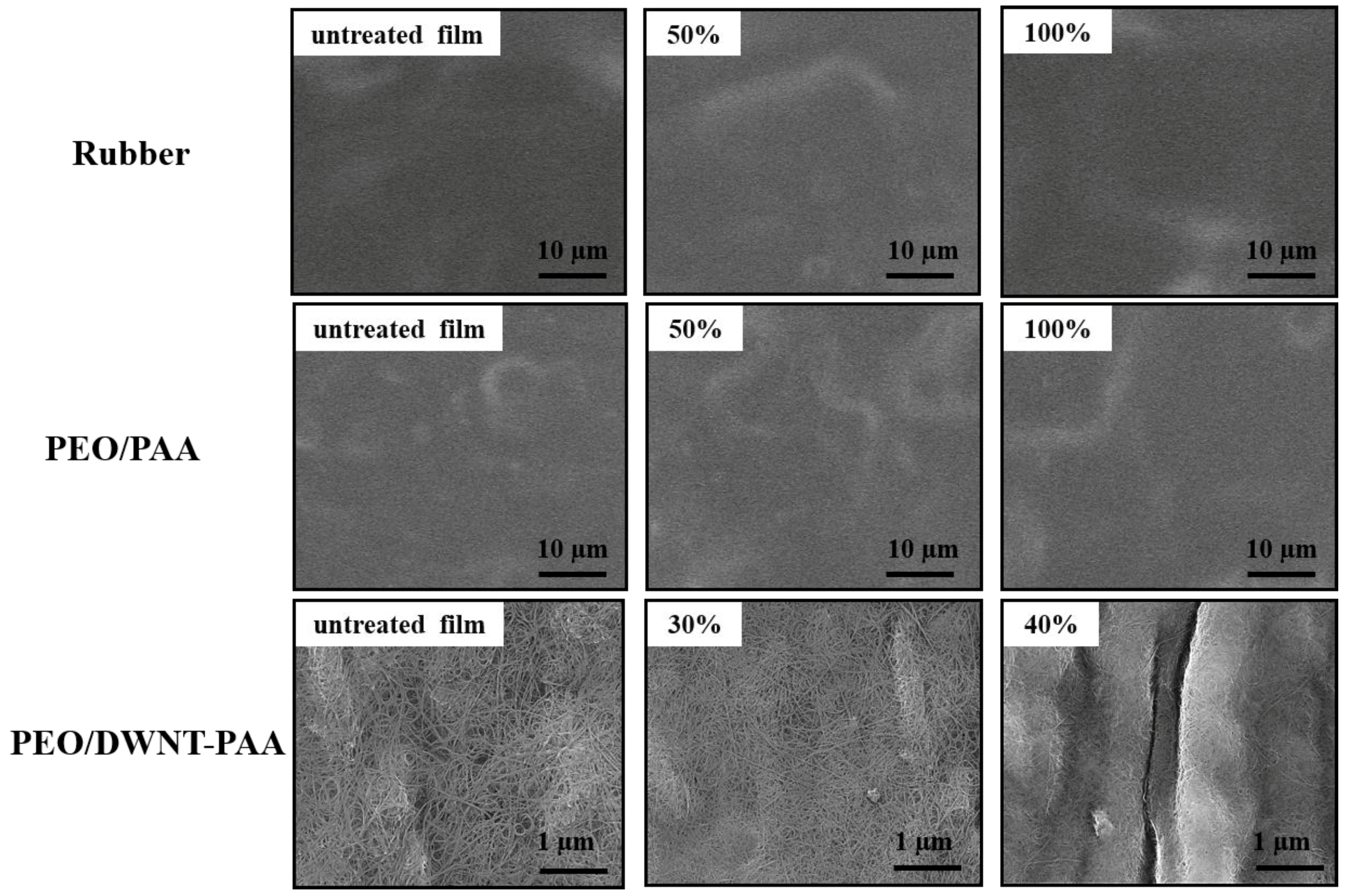
3.3. Multilayer Structure
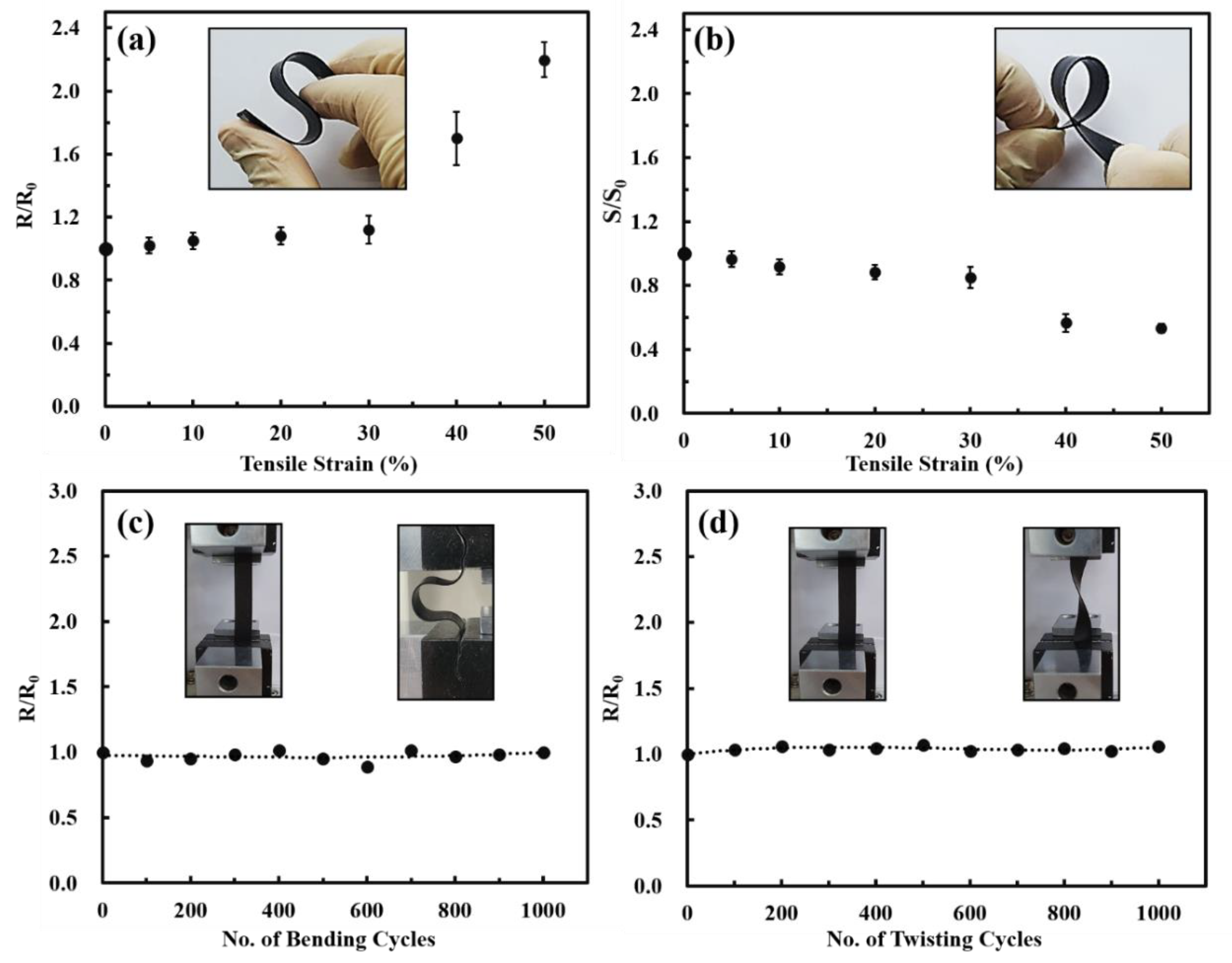
3.4. Influence of Strain on Thermoelectric Performance
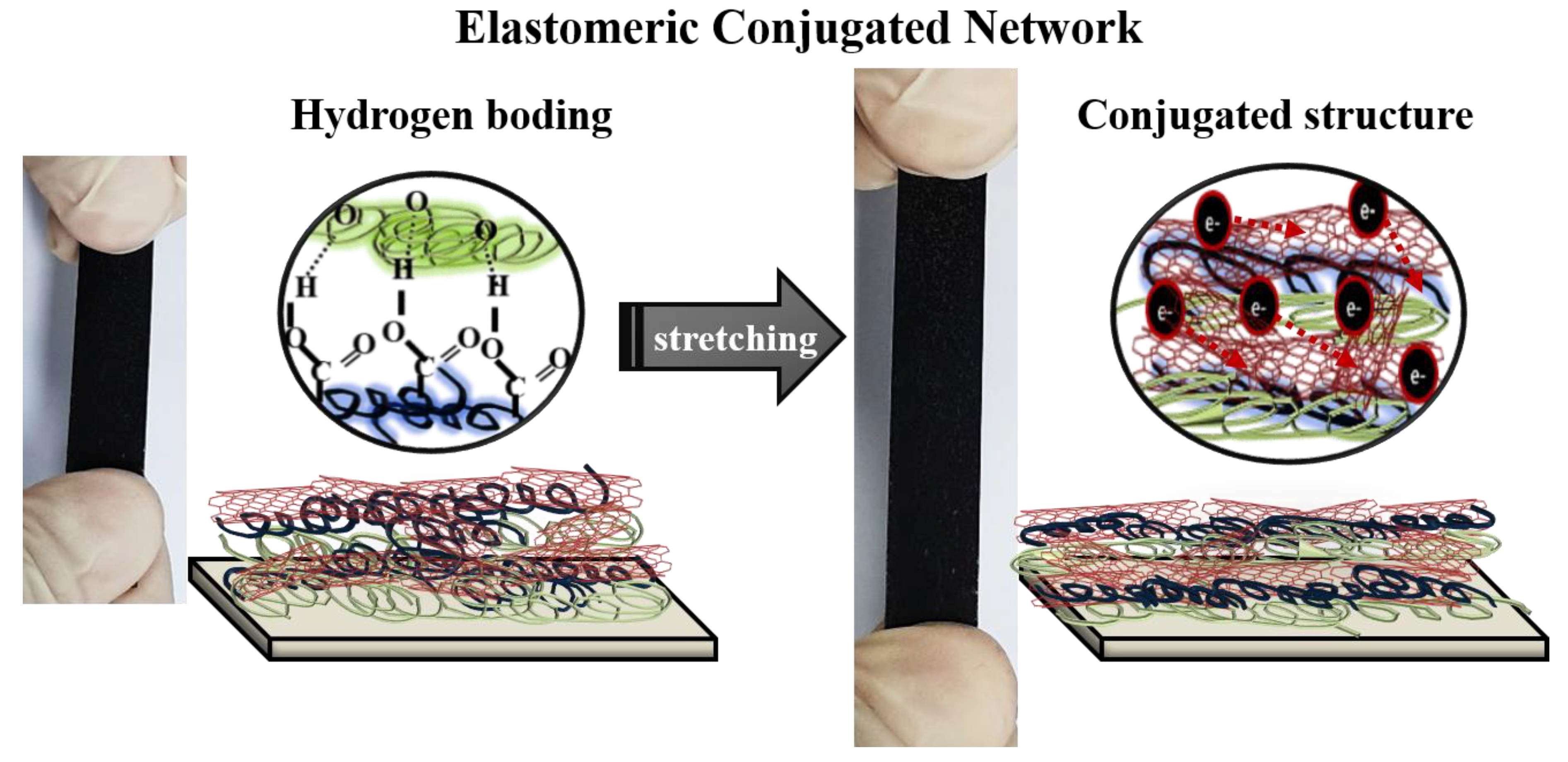
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nan, K.; Kang, S.D.; Li, K.; Yu, K.J.; Zhu, F.; Wang, J.; Dunn, A.C.; Zhou, C.; Xie, Z.; Agne, M.T.; et al. Compliant and stretchable thermoelectric coils for energy harvesting in miniature flexible devices. Sci. Adv. 2018, 4, 5849. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Nayeem, O.G.; Lee, S.; Matsuhisa, N.; Inoue, D.; Yokota, T.; Hashizume, D.; Someya, T. Highly durable nanofiber-reinforced elastic conductors for skin-tight electronic textiles. ACS Nano 2019, 13, 7905–7912. [Google Scholar] [CrossRef]
- Yu, L.; Yeo, J.C.; Soon, R.H.; Yeo, T.; Lee, H.H.; Lim, C.T. Highly stretchable, weavable, and washable piezoresistive microfiber sensors. ACS Appl. Mater. Interfaces 2018, 10, 12773–12780. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.H.; Xue, Q.W.; Pang, C.Y.; Guo, P.W.; Yao, W.J.; Zhu, H.P.; Wu, W. Printing the Ultra-Long Ag Nanowires Inks onto the Flexible Textile Substrate for Stretchable Electronics. Nanomaterials 2019, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wang, D.; Lu, J.; He, M.; Xu, C.; Li, Y.; Zhu, S. A review on organic polymer based thermoelectric materials. J. Polym. Environ. 2017, 25, 1208–1218. [Google Scholar] [CrossRef]
- Jin, H.; Li, J.; Iocozzia, J.; Zeng, X.; Wei, P.C.; Yang, C.; Li, N.; Liu, Z.; He, J.H.; Zhu, T. Hybrid Organic-Inorganic Thermoelectric Materials and Devices. Angew. Chem. 2019, 131, 15348–15370. [Google Scholar] [CrossRef]
- Culebras, M.; Choi, K.; Cho, C. Recent Progress in Flexible Organic Thermoelectrics. Micromachines 2018, 9, 638. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-Nanotube-Based Thermoelectric Materials and Devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef]
- Siddique, A.R.M.; Mahmud, S.; Van Heyst, B. A review of the state of the science on wearable thermoelectric power generators (TEGs) and their existing challenges. Renew. Sustain. Energy Rev. 2017, 73, 730–744. [Google Scholar] [CrossRef]
- Du, B.; Liu, M.; Xu, J.; Hu, B.; Liu, B.; Su, T.; Wang, J. Thermodynamic, Structural and Thermoelectric Properties of AgSbTe2 Thick Films Developed by Melt Spinning. Nanomaterials 2018, 8, 474. [Google Scholar] [CrossRef]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Review of thermoelectric materials. In Introduction to Thermoelectricity; Springer: Berlin/Heidelberg, Germany, 2016; pp. 153–195. [Google Scholar]
- Wang, J.; Mu, X.; Sun, M. The Thermal, Electrical and Thermoelectric Properties of Graphene Nanomaterials. Nanomaterials 2019, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Koizumi, T.; Kojima, H.; Saito, T.; Nakamura, M. From materials to device design of a thermoelectric fabric for wearable energy harvesters. J. Mater. Chem. A 2017, 5, 12068–12072. [Google Scholar] [CrossRef]
- Cataldi, P.; Cassinelli, M.; Heredia-Guerrero, J.A.; Guzman-Puyol, S.; Naderizadeh, S.; Athanassiou, A.; Caironi, M. Green Biocomposites for Thermoelectric Wearable Applications. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Cho, C.; Wallace, K.L.; Tzeng, P.; Hsu, J.; Yu, C.; Grunlan, J.C. Outstanding Low Temperature Thermoelectric Power Factor from Completely Organic Thin Films Enabled by Multidimensional Conjugated Nanomaterials. Adv. Energy Mater. 2016, 6, 1502168. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, B. Carbon Nanotube-Based Organic Thermoelectric Materials for Energy Harvesting. Polymers 2018, 10, 1196. [Google Scholar] [CrossRef]
- CaraDonna, A.; Badini, C.; Padovano, E.; Pietroluongo, M. Electrical and Thermal Conductivity of Epoxy-Carbon Filler Composites Processed by Calendaring. Materials 2019, 12, 1522. [Google Scholar] [CrossRef]
- Nan, C.W.; Liu, G.; Lin, Y.; Li, M. Interface effect on thermal conductivity of carbon nanotube composites. Appl. Phys. Lett. 2004, 85, 3549–3551. [Google Scholar] [CrossRef]
- Dul, S.; Pegoretti, A.; Fambri, L. Effects of the Nanofillers on Physical Properties of Acrylonitrile-Butadiene-Styrene Nanocomposites: Comparison of Graphene Nanoplatelets and Multiwall Carbon Nanotubes. Nanomaterials 2018, 8, 674. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, L.; Zhang, W.; Liufu, S.; Chen, X. Enhanced Thermoelectric Performance of Single-Walled Carbon Nanotubes/Polyaniline Hybrid Nanocomposites. ACS Nano 2010, 4, 2445–2451. [Google Scholar] [CrossRef]
- Liu, P.F.; Wang, J.L.; Meng, X.J.; Yang, J.; Dkhil, B.; Chu, J.H. Huge electrocaloric effect in Langmuir–Blodgett ferroelectric polymer thin films. New J. Phys. 2010, 12, 023035. [Google Scholar] [CrossRef]
- Mani, G.; Johnson, D.M.; Marton, D.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Agrawal, C.M. Drug delivery from gold and titanium surfaces using self-assembled monolayers. Biomaterials 2008, 29, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Rawtani, D.; Agrawal, Y.K. Emerging Strategies and Applications of Layer-by-Layer Self-Assembly. Nanobiomedicine 2014, 1. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F.; Björnmalm, A.M.H. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, F.; Cao, Y. Layer-by-Layer Assembly of Multilayer Thin Films for Organic Optoelectronic Devices. Small Methods 2017, 1, 1700264. [Google Scholar] [CrossRef]
- Cho, C.; Zacharia, N.S. Film Stability during Postassembly Morphological Changes in Polyelectrolyte Multilayers Due to Acid and Base Exposure. Langmuir 2011, 28, 841–848. [Google Scholar] [CrossRef]
- Sui, Z.; Salloum, D.; Schlenoff, J.B. Effect of Molecular Weight on the Construction of Polyelectrolyte Multilayers: Stripping versus Sticking. Langmuir 2003, 19, 2491–2495. [Google Scholar] [CrossRef]
- Dodoo, S.; Balzer, B.N.; Hugel, T.; Laschewsky, A.; Von Klitzing, R. Effect of Ionic Strength and Layer Number on Swelling of Polyelectrolyte Multilayers in Water Vapour. Soft Mater. 2013, 11, 157–164. [Google Scholar] [CrossRef]
- Jaffar, S.; Nam, K.T.; Khademhosseini, A.; Xing, J.; Langer, R.S.; Belcher, A.M. Layer-by-Layer Surface Modification and Patterned Electrostatic Deposition of Quantum Dots. Nano Lett. 2004, 4, 1421–1425. [Google Scholar] [CrossRef]
- Wu, W.; Niu, H.; Yang, D.; Wang, S.B.; Wang, J.; Lin, J.; Hu, C. Controlled Layer-By-Layer Deposition of Carbon Nanotubes on Electrodes for Microbial Fuel Cells. Energies 2019, 12, 363. [Google Scholar] [CrossRef]
- Sharma, R.; Zhang, I.; Abbassi, L.; Rej, R.; Maysinger, D.; Roy, R. A fast track strategy toward highly functionalized dendrimers with different structural layers: An “onion peel approach”. Polym. Chem. 2015, 6, 1436–1444. [Google Scholar] [CrossRef]
- Deng, F.; Sun, J.; Dou, R.; Deng, W.; Liu, Y.; Yang, C.; Dang, Z. Mechanism of enhancing pyrene-degradation ability of bacteria by layer-by-layer assembly bio-microcapsules materials. Ecotoxicol. Environ. Saf. 2019, 181, 525–533. [Google Scholar] [CrossRef]
- Selin, V.; Aliakseyeu, A.; Ankner, J.F.; Sukhishvili, S.A. Effect of a Competitive Solvent on Binding Enthalpy and Chain Intermixing in Hydrogen-Bonded Layer-by-Layer Films. Macromolecules 2019, 52, 4432–4440. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Han, L.; Zhong, Y. Layer-by-layer films assembled from natural polymers for sustained release of neurotrophin. Biomed. Mater. 2015, 10, 55006. [Google Scholar] [CrossRef]
- Ren, W.; Wu, R.; Guo, P.; Zhu, J.; Li, H.; Xu, S.; Wang, J. Preparation and characterization of covalently bonded PVA/Laponite/HAPI nanocomposite multilayer freestanding films by layer-by-layer assembly. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 545–551. [Google Scholar] [CrossRef]
- Koo, W.T.; Choi, S.J.; Kim, N.H.; Jang, J.S.; Kim, I.D. Catalyst-decorated hollow WO 3 nanotubes using layer-by-layer self-assembly on polymeric nanofiber templates and their application in exhaled breath sensor. Sens. Actuators B Chem. 2016, 223, 301–310. [Google Scholar] [CrossRef]
- Krieger, G.; Tieke, B. Coordinative Layer-by-Layer Assembly of Thin Films Based on Metal Ion Complexes of Ligand-Substituted Polystyrene Copolymers and Their Use as Separation Membranes. Macromol. Chem. Phys. 2017, 218, 1700052. [Google Scholar] [CrossRef]
- Marmisollé, W.A.; Azzaroni, O. Recent developments in the layer-by-layer assembly of polyaniline and carbon nanomaterials for energy storage and sensing applications. From synthetic aspects to structural and functional characterization. Nanoscale 2016, 8, 9890–9918. [Google Scholar] [CrossRef]
- Gibbons, E.N.; Winder, C.; Barron, E.; Fernandes, D.; Krysmann, M.J.; Kelarakis, A.; Parry, A.V.S.; Yeates, S.G. Layer by Layer Antimicrobial Coatings Based on Nafion, Lysozyme, and Chitosan. Nanomaterials 2019, 9, 1563. [Google Scholar] [CrossRef]
- Liang, L.; Gao, C.; Chen, G.; Guo, C.Y. Large-area, stretchable, super flexible and mechanically stable thermoelectric films of polymer/carbon nanotube composites. J. Mater. Chem. C 2016, 4, 526–532. [Google Scholar] [CrossRef]
- Jo, J.; Oh, I.; Jin, M.J.; Park, J.; Son, J.S.; An, K.S.; Yoo, J.W. Highly stretchable organic thermoelectrics with an enhanced power factor due to extended localization length. Org. Electron. 2017, 50, 367–375. [Google Scholar] [CrossRef]
- Taroni, P.J.; Santagiuliana, G.; Wan, K.; Calado, P.; Qiu, M.; Zhang, H.; Pugno, N.M.; Palma, M.; Stingelin-Stutzman, N.; Heeney, M. Toward Stretchable Self-Powered Sensors Based on the Thermoelectric Response of PEDOT: PSS/Polyurethane Blends. Adv. Funct. Mater. 2018, 28, 1704285. [Google Scholar] [CrossRef]
- Wan, K.; Taroni, P.J.; Liu, Z.; Liu, Y.; Tu, Y.; Santagiuliana, G.; Hsia, I.C.; Zhang, H.; Fenwick, O.; Krause, S. Flexible and Stretchable Self-Powered Multi-Sensors Based on the N-Type Thermoelectric Response of Polyurethane/Nax (Ni-ett) n Composites. Adv. Electron. Mater. 2019, 5, 1900582. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Y.; Xu, F.; Duan, Y.; Yin, Z. Energy Harvesters for Wearable and Stretchable Electronics: From Flexibility to Stretchability. Adv. Mater. 2016, 28, 9881–9919. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Wadsworth, L.C. Mechanism of corona treatment on polyolefin films. Polym. Eng. Sci. 1998, 38, 965–970. [Google Scholar] [CrossRef]
- Delongchamp, D.M.; Hammond, P.T. Highly Ion Conductive Poly(ethylene oxide)-Based Solid Polymer Electrolytes from Hydrogen Bonding Layer-by-Layer Assembly. Langmuir 2004, 20, 5403–5411. [Google Scholar] [CrossRef]
- Xiang, F.; Ward, S.M.; Givens, T.M.; Grunlan, J.C. Structural tailoring of hydrogen-bonded poly(acrylic acid)/poly(ethylene oxide) multilayer thin films for reduced gas permeability. Soft Matter 2015, 11, 1001–1007. [Google Scholar] [CrossRef]
- Smith, K.L.; Winslow, A.E.; Petersen, D.E. Association Reactions for Poly(alkylene Oxides) and Polymeric Poly(carboxylic Acids). Ind. Eng. Chem. 1959, 51, 1361–1364. [Google Scholar] [CrossRef]
- Choi, J.; Rubner, M.F. Influence of the Degree of Ionization on Weak Polyelectrolyte Multilayer Assembly. Macromolecules 2005, 38, 116–124. [Google Scholar] [CrossRef]
- Cho, C.; Xiang, F.; Wallace, K.L.; Grunlan, J.C. Combined Ionic and Hydrogen Bonding in Polymer Multilayer Thin Film for High Gas Barrier and Stretchiness. Macromolecules 2015, 48, 5723–5729. [Google Scholar] [CrossRef]
- Cho, C.; Song, Y.; Allen, R.; Grunlan, J.C.; Wallace, K.L. Stretchable electrically conductive and high gas barrier nanocomposites. J. Mater. Chem. C 2018, 6, 2095–2104. [Google Scholar] [CrossRef]
- Sung, C.; Vidyasagar, A.; Hearn, K.; Lutkenhaus, J.L. Effect of Thickness on the Thermal Properties of Hydrogen-Bonded LbL Assemblies. Langmuir 2012, 28, 8100–8109. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, S.S.; Rubner, M.F. pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Cramer, A.D.; Dong, W.F.; Benbow, N.L.; Webber, J.L.; Krasowska, M.; Beattie, D.A.; Ferri, J.K. The influence of polyanion molecular weight on polyelectrolyte multilayers at surfaces: Elasticity and susceptibility to saloplasticity of strongly dissociated synthetic polymers at fluid–fluid interfaces. Phys. Chem. Chem. Phys. 2017, 19, 23781–23789. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Besseling, N.A.M. Formation of polyelectrolyte multilayers: Ionic strengths and growth regimes. Soft Matter 2016, 12, 1032–1040. [Google Scholar] [CrossRef]
- Nakashima, T.; Zhu, J.; Qin, M.; Ho, S.; Kotov, N.A. Polyelectrolyte and carbon nanotube multilayers made from ionic liquid solutions. Nanoscale 2010, 2, 2084–2090. [Google Scholar] [CrossRef]
- Cho, C.; Culebras, M.; Wallace, K.L.; Song, Y.; Holder, K.; Hsu, J.H.; Yu, C.; Grunlan, J.C. Stable n-type thermoelectric multilayer thin films with high power factor from carbonaceous nanofillers. Nano Energy 2016, 28, 426–432. [Google Scholar] [CrossRef]
- Cho, C.; Stevens, B.; Hsu, J.H.; Bureau, R.; Hagen, D.A.; Regev, O.; Yu, C.; Grunlan, J.C. Completely Organic Multilayer Thin Film with Thermoelectric Power Factor Rivaling Inorganic Tellurides. Adv. Mater. 2015, 27, 2996–3001. [Google Scholar] [CrossRef]
- Cho, C.; Bittner, N.; Choi, W.; Hsu, J.H.; Yu, C.; Grunlan, J.C. Thermally Enhanced n-Type Thermoelectric Behavior in Completely Organic Graphene Oxide-Based Thin Films. Adv. Electron. Mater. 2018, 5, 1800465. [Google Scholar] [CrossRef]
- Moriarty, G.P.; De, S.; King, P.J.; Khan, U.; Via, M.; King, J.A.; Coleman, J.N.; Grunlan, J.C. Thermoelectric behavior of organic thin film nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 119–123. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Choi, K.; Grunlan, J.C.; Yu, C. Improved Thermoelectric Behavior of Nanotube-Filled Polymer Composites with Poly(3,4-ethylenedioxythiophene) Poly(styrenesulfonate). ACS Nano 2009, 4, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; LaLonde, A.D.; Wang, H.; Snyder, G.J. Low effective mass leading to high thermoelectric performance. Energy Environ. Sci. 2012, 5, 7963–7969. [Google Scholar] [CrossRef]
- Holder, K.M.; Priolo, M.A.; Secrist, K.E.; Greenlee, S.M.; Nolte, A.J.; Grunlan, J.C. Humidity-Responsive Gas Barrier of Hydrogen-Bonded Polymer–Clay Multilayer Thin Films. J. Phys. Chem. C 2012, 116, 19851–19856. [Google Scholar] [CrossRef]
- Lutkenhaus, J.L.; Hrabak, K.D.; McEnnis, K.; Hammond, P.T. Elastomeric Flexible Free-Standing Hydrogen-Bonded Nanoscale Assemblies. J. Am. Chem. Soc. 2005, 127, 17228–17234. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical Properties of Monolayer Graphene Oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef]
- Xiang, F.; Ward, S.M.; Givens, T.M.; Grunlan, J.C. Super Stretchy Polymer Multilayer Thin Film with High Gas Barrier. ACS Macro Lett. 2014, 3, 1055–1058. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, B.S.; Chen, S.; Shao-Horn, Y.; Hammond, P.T. Layer-by-layer assembly of all carbon nanotube ultrathin films for electrochemical applications. J. Am. Chem. Soc. 2008, 131, 671–679. [Google Scholar] [CrossRef]
- Qin, S.; Song, Y.; Floto, M.E.; Grunlan, J.C. Combined High Stretchability and Gas Barrier in Hydrogen-Bonded Multilayer Nanobrick Wall Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 7903–7907. [Google Scholar] [CrossRef]
- Erbaş, A.; Horinek, D.; Netz, R.R. Viscous Friction of Hydrogen-Bonded Matter. J. Am. Chem. Soc. 2011, 134, 623–630. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, C.; Son, J. Organic Thermoelectric Multilayers with High Stretchiness. Nanomaterials 2020, 10, 41. https://doi.org/10.3390/nano10010041
Cho C, Son J. Organic Thermoelectric Multilayers with High Stretchiness. Nanomaterials. 2020; 10(1):41. https://doi.org/10.3390/nano10010041
Chicago/Turabian StyleCho, Chungyeon, and Jihun Son. 2020. "Organic Thermoelectric Multilayers with High Stretchiness" Nanomaterials 10, no. 1: 41. https://doi.org/10.3390/nano10010041
APA StyleCho, C., & Son, J. (2020). Organic Thermoelectric Multilayers with High Stretchiness. Nanomaterials, 10(1), 41. https://doi.org/10.3390/nano10010041




