CPP-GMR Performance of Electrochemically Synthesized Co/Cu Multilayered Nanowire Arrays with Extremely Large Aspect Ratio
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Structure of Anodized Aluminum Oxide Membrane Filters
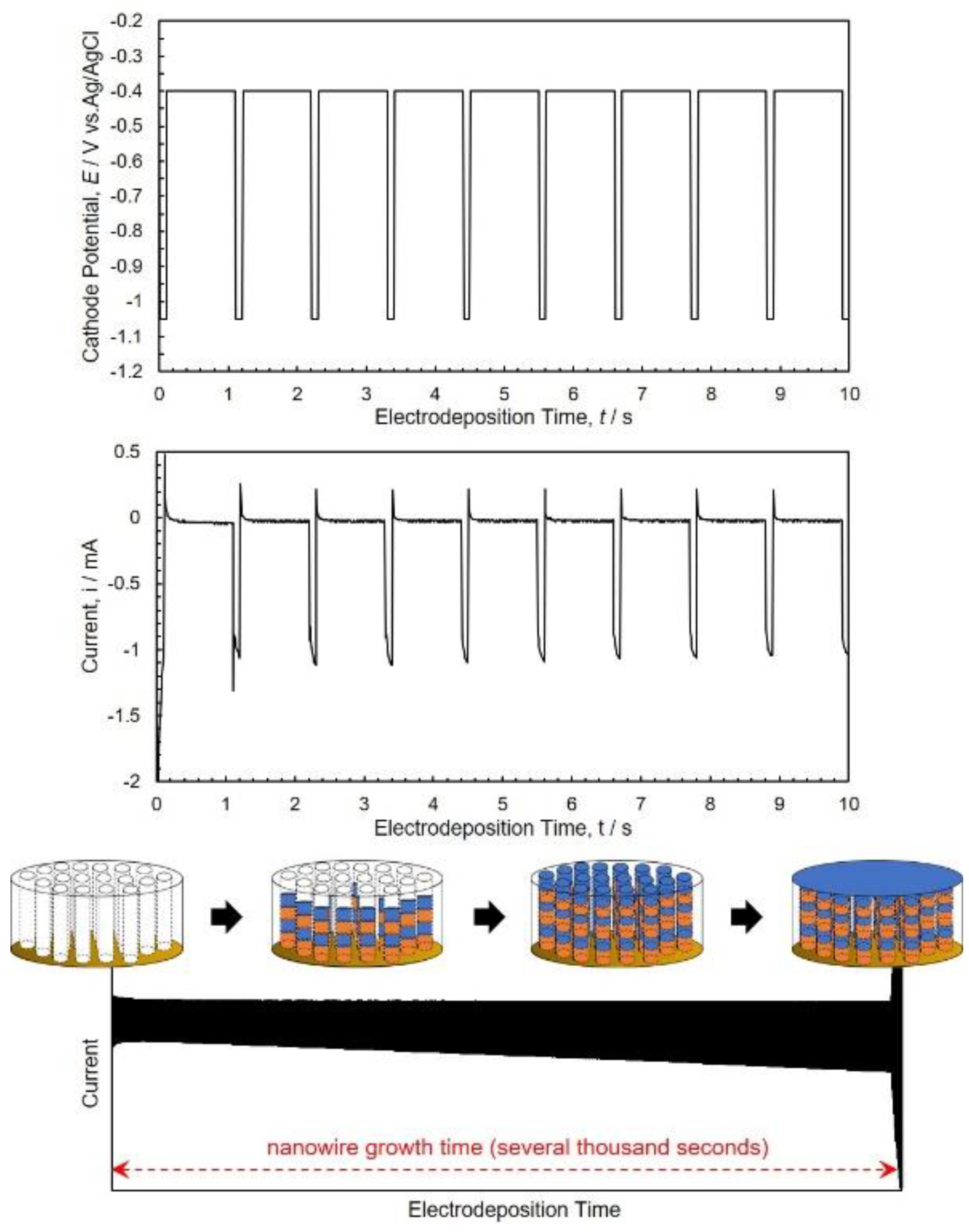
3.2. Electrodeposition Process of Co/Cu Multilayered Nanowires
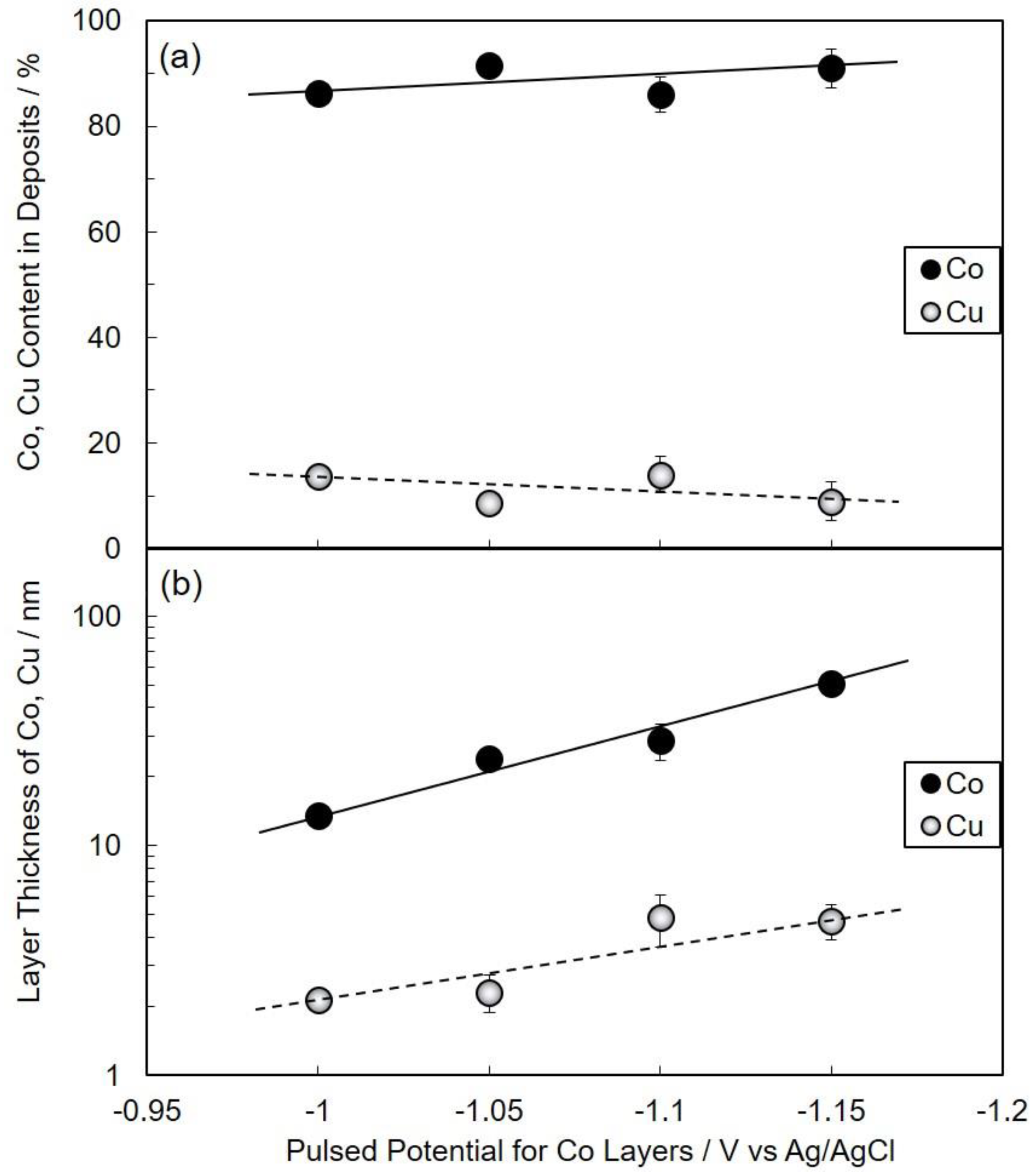
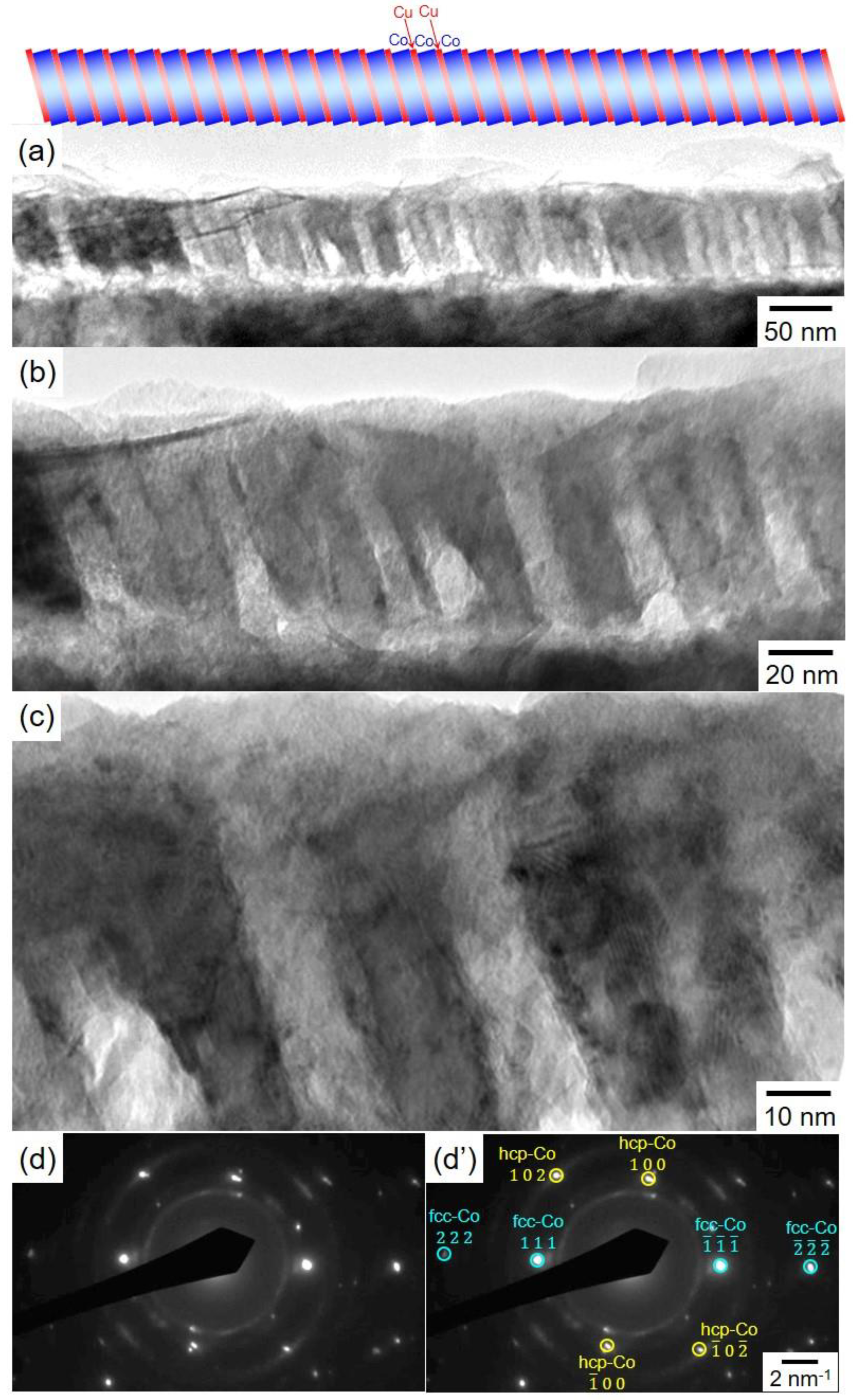
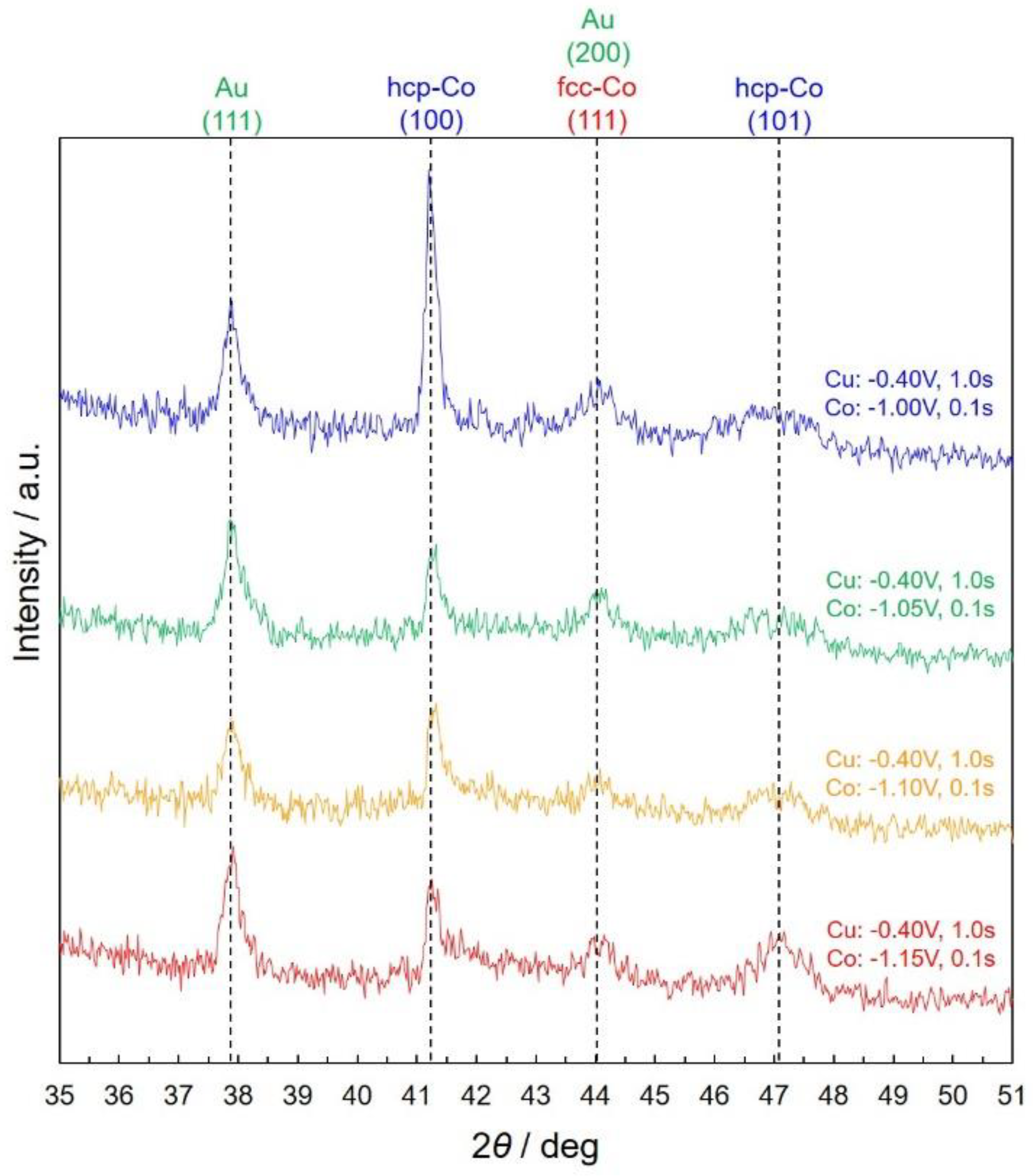
3.3. Structure of Co/Cu Multilayered Nanowires
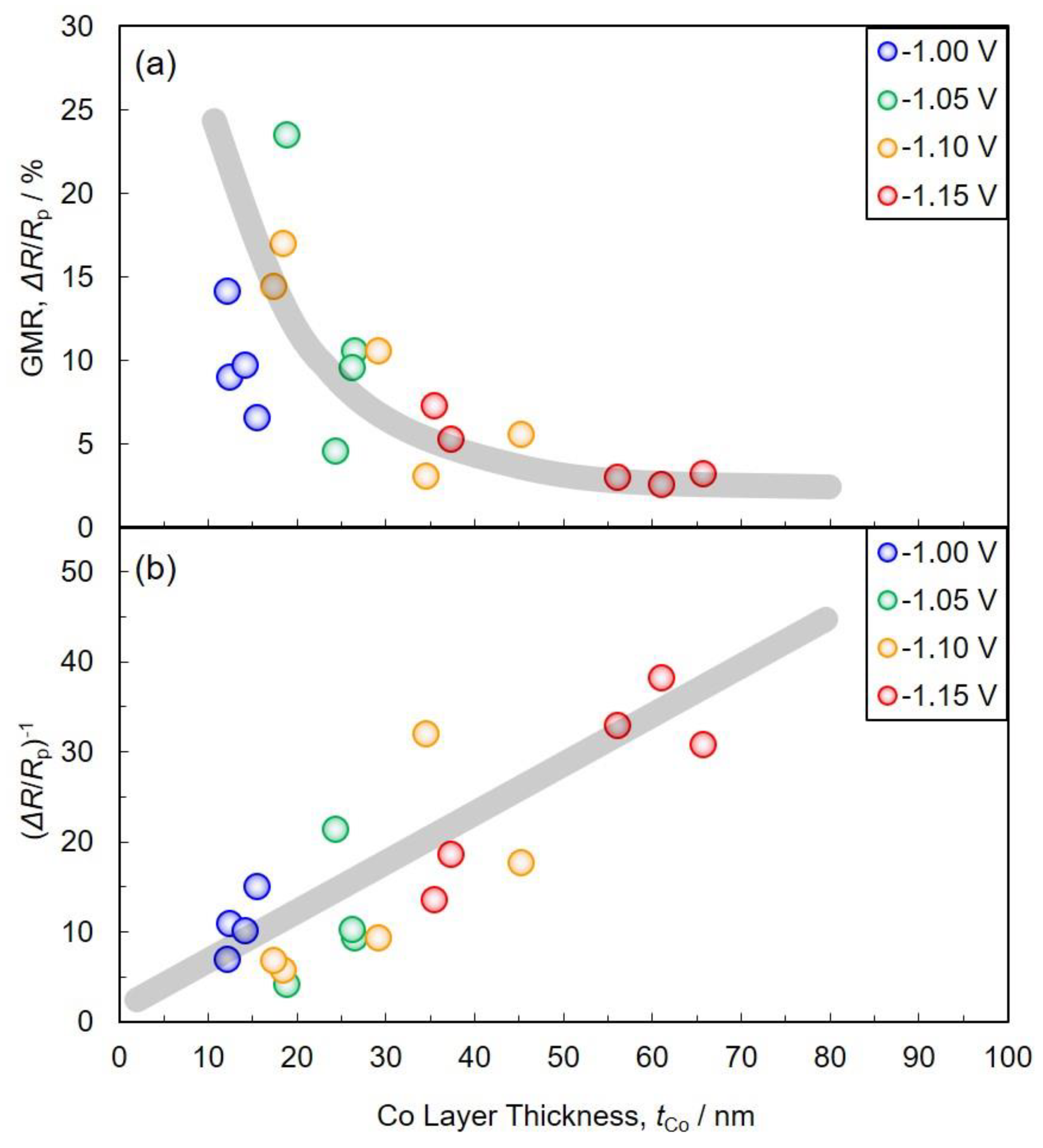
3.4. Magnetoresistance Properties of Co/Cu Multilayered Nanowires
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zach, M.P.; Ng, K.H.; Penner, R.M. Molybdenum nanowires by electrodeposition. Science 2000, 290, 2120–2123. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, H.; Shen, P.K.; Jiang, S.P. Highly ordered Pd nanowire arrays as effective electrocatalysts for ethanol oxidation in direct alcohol fuel cells. Adv. Mater. 2007, 19, 4256–4259. [Google Scholar] [CrossRef]
- Sauer, G.; Brehm, G.; Schneider, S.; Nielsch, K.; Wehrspohn, R.B.; Choi, J.; Hofmeister, H.; Gösele, U. Highly ordered monocrystalline silver nanowire arrays. J. Appl. Phys. 2002, 91, 3243–3247. [Google Scholar] [CrossRef]
- Menke, E.J.; Thompson, M.A.; Xiang, C.; Yang, L.C.; Penner, R.M. Lithographically patterned nanowire electrodeposition. Nat. Mater. 2006, 5, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Ferré, R.; Ounadjela, K.; George, J.M.; Piraux, L.; Dubois, S. Magnetization processes in nickel and cobalt electrodeposited nanowires. Phys. Rev. B 1997, 56, 14066–14075. [Google Scholar] [CrossRef]
- Ohgai, T.; Enculescu, I.; Zet, C.; Westerberg, L.; Hjort, K.; Spohr, R.; Neumann, R. Magneto-sensitive nickel nanowires fabricated by electrodeposition into multi-and single-ion track templates. J. Appl. Electrochem. 2006, 36, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.R.; Yi, G.; Schwarzacher, W. Current perpendicular to plane giant magnetoresistance of multilayered nanowires electrodeposited in anodic aluminum oxide membranes. Appl. Phys. Lett. 2000, 76, 481–483. [Google Scholar] [CrossRef]
- Ohgai, T.; Hoffer, X.; Gravier, L.; Ansermet, J.P. Electrochemical surface modification of aluminium sheets for application to nano-electronic devices: Anodization aluminium and electrodeposition of cobalt-copper. J. Appl. Electrochem. 2004, 34, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; He, Y.; Zou, J.; Jiang, Y.; Xu, J.; Huang, B.; Liu, C.T.; Liaw, P.K. Synthesis of single-crystalline tungsten nanowires by nickel-catalyzed vapor-phase method at 850 °C. J. Cryst. Growth 2007, 306, 433–436. [Google Scholar] [CrossRef]
- Kim, D.J.; Seol, J.K.; Lee, M.R.; Hyung, J.H.; Kim, G.S.; Ohgai, T.; Lee, S.K. Ferromagnetic nickel silicide nanowires for isolating primary CD4+ T lymphocytes. Appl. Phys. Lett. 2012, 100, 163703. [Google Scholar] [CrossRef] [Green Version]
- Thurn-Albrecht, T.; Schotter, J.; Kastle, G.A.; Emley, N.; Shibauchi, T.; Krusin-Elbaum, L.; Guarini, K.; Black, C.T.; Tuominen, M.T.; Russell, T.P. Ultrahigh-density nanowire arrays grown in self-assembled diblock copolymer templates. Science 2000, 290, 2126–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsch, K.; Müller, F.; Li, A.P.; Gösele, U. Uniform nickel deposition into ordered alumina pores by pulsed electrodeposition. Adv. Mater. 2000, 12, 582–586. [Google Scholar] [CrossRef]
- Ohgai, T.; Washio, R.; Tanaka, Y. Anisotropic magnetization behavior of electrodeposited nanocrystalline Ni-Mo alloy thin films and nanowires array. J. Electrochem. Soc. 2012, 159, H800–H804. [Google Scholar] [CrossRef] [Green Version]
- Pignard, S.; Goglio, G.; Radulescu, A.; Piraux, L.; Dubois, S.; Declémy, A.; Duvail, J.L. Study of the magnetization reversal in individual nickel nanowires. J. Appl. Phys. 2000, 87, 824–829. [Google Scholar] [CrossRef]
- Ohgai, T.; Mizumoto, M.; Nomura, S.; Kagawa, A. Electrochemical fabrication of metallic nanowires and metal oxide nanopores. Mater. Manuf. Process. 2007, 22, 440–443. [Google Scholar] [CrossRef] [Green Version]
- Sellmyer, D.J.; Zheng, M.; Skomski, R. Magnetism of Fe, Co and Ni nanowires in self-assembled arrays. J. Phys. Condens. Matter. 2001, 13, R433–R460. [Google Scholar] [CrossRef]
- Zeng, H.; Skomski, R.; Menon, L.; Liu, Y.; Bandyopadhyay, S.; Sellmyer, D.J. Structure and magnetic properties of ferromagnetic nanowires in self-assembled arrays. Phys. Rev. B 2002, 65, 1344261–1344268. [Google Scholar] [CrossRef] [Green Version]
- Neetzel, C.; Ohgai, T.; Yanai, T.; Nakano, M.; Fukunaga, H. Uniaxial magnetization performance of textured Fe nanowire arrays electrodeposited by a pulsed potential deposition technique. Nanoscale Res. Lett. 2017, 12, 598. [Google Scholar] [CrossRef]
- Cox, B.; Davis, D.; Crews, N. Creating magnetic field sensors from GMR nanowire networks. Sens. Actuat. A Phys. 2013, 203, 335–340. [Google Scholar] [CrossRef]
- Davis, D.; Zamanpour, M.; Moldovan, M.; Young, D.; Podlaha, E.J. Electrodeposited, GMR CoNiFeCu nanowires and nanotubes from electrolytes maintained at different temperatures. J. Electrochem. Soc. 2010, 157, D317–D322. [Google Scholar] [CrossRef]
- Tang, X.T.; Wang, G.C.; Shima, M. Layer thickness dependence of CPP giant magnetoresistance in individual CoNi/Cu multilayer nanowires grown by electrodeposition. Phys. Rev. B 2007, 75, 134404. [Google Scholar] [CrossRef] [Green Version]
- Wegrowe, J.E.; Gilbert, S.E.; Scarani, V.; Kelly, D.; Doudin, B.; Ansermet, J.P. Anisotropic magnetoresistance as a probe of magnetization reversal in individual nono-sized nickel wires. IEEE Trans. Magn. 1998, 34, 903–905. [Google Scholar] [CrossRef]
- Neetzel, C.; Kamimura, H.; Hayashida, M.; Ohgai, T. Uniaxial magnetization reversal process in electrodeposited high-density iron nanowire arrays with ultra-large aspect ratio. Results Phys. 2019, 15, 102653. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Yuan, J.H.; He, F.Y.; Sun, D.C.; Xia, X.H. A simple method for preparation of through-hole porous anodic alumina membrane. Chem. Mater. 2004, 16, 1841–1844. [Google Scholar] [CrossRef]
- Ohgai, T.; Tanaka, Y.; Fujimaru, T. Soft magnetic properties of Ni–Cr and Co–Cr alloy thin films electrodeposited from aqueous solutions containing trivalent chromium ions and glycine. J. Appl. Electrochem. 2012, 42, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Bockris, J.O.M.; Kita, H. Analysis of galvanostatic transients and application to the iron electrode reaction. J. Electrochem. Soc. 1961, 108, 676–685. [Google Scholar] [CrossRef]
- Proenca, M.P.; Merazzo, K.J.; Vivas, L.G.; Leitao, D.C.; Sousa, C.T.; Ventura, J.; Araujo, J.P.; Vazquez, M. Co nanostructures in ordered templates: Comparative FORC analysis. Nanotechnology 2013, 24, 475703. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, T.; Ishida, K. The Co−Cu (Cobalt-Copper) system. Bull. Alloy Phase Diagr. 1984, 5, 161–165. [Google Scholar] [CrossRef]
- Shamaila, S.; Sharif, R.; Riaz, S.; Khaleeq-ur-Rahman, M.; Han, X.F. Fabrication and magnetic characterization of CoxPt1−x nanowire arrays. Appl. Phys. A 2008, 92, 687–691. [Google Scholar] [CrossRef]
- Al Subhi, A.; Sbiaa, R.; Ranjbar, M.; Akerman, J. Magnetization reversal of antiferromagnetically coupled (Co/Ni) and (Co/Pt) multilayers. J. Magn. Magn. Mater. 2019, 479, 27–31. [Google Scholar] [CrossRef]
- Valet, T.; Fert, A. Theory of the perpendicular magnetoresistance in magnetic multilayers. Phys. Rev. B 1993, 48, 7099. [Google Scholar] [CrossRef] [PubMed]



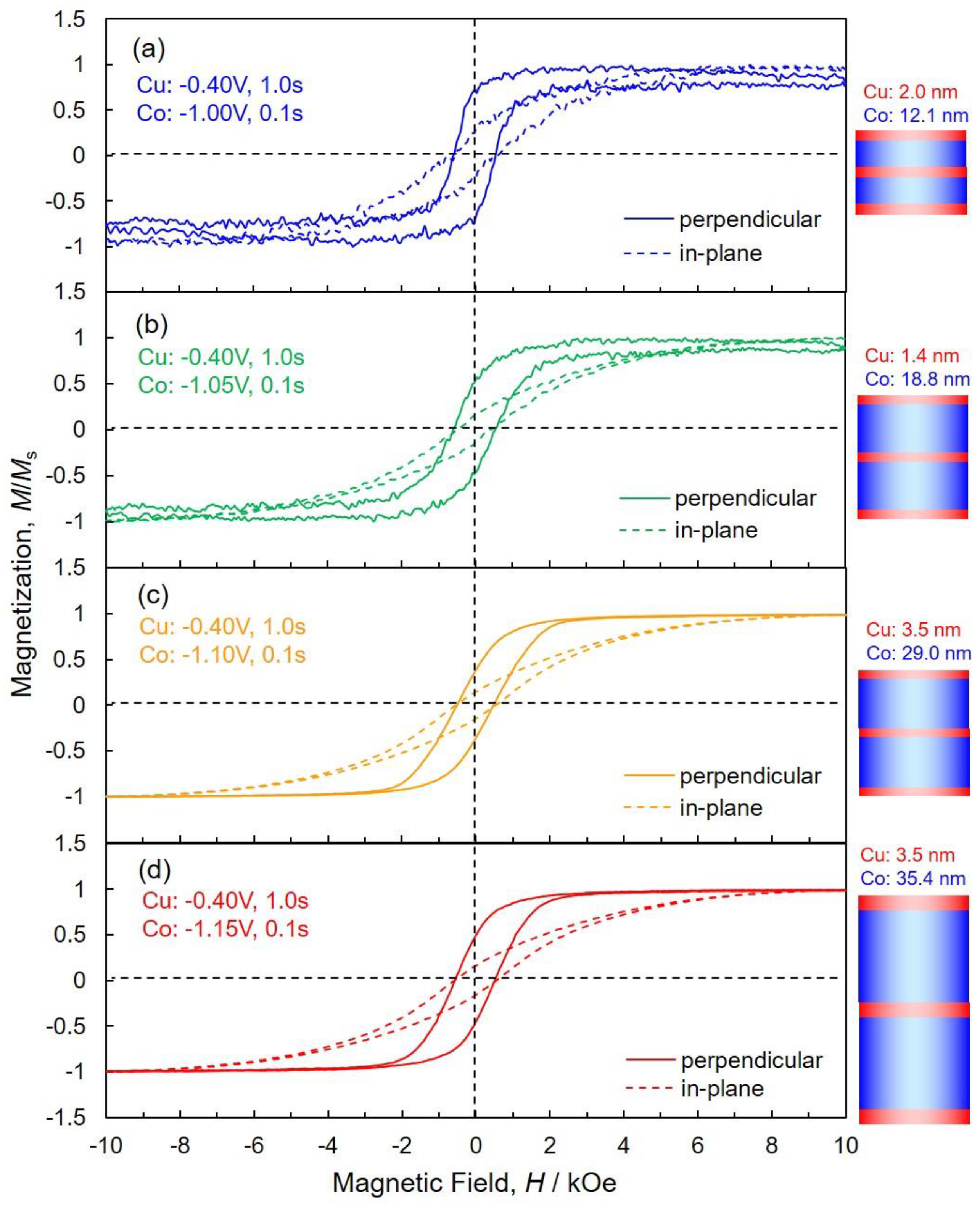

| ECo/V | tCo/nm | Hc (in-Plane)/kOe | Hc (Perpen.)/kOe | Mr/Ms (in-Plane) | Mr/Ms (Perpen.) |
|---|---|---|---|---|---|
| −1.00 | 11.8 | 0.56 | 0.62 | 0.27 | 0.69 |
| −1.05 | 19.3 | 0.48 | 0.59 | 0.17 | 0.55 |
| −1.10 | 20.2 | 0.57 | 0.53 | 0.15 | 0.39 |
| −1.15 | 36.9 | 0.57 | 0.58 | 0.16 | 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamimura, H.; Hayashida, M.; Ohgai, T. CPP-GMR Performance of Electrochemically Synthesized Co/Cu Multilayered Nanowire Arrays with Extremely Large Aspect Ratio. Nanomaterials 2020, 10, 5. https://doi.org/10.3390/nano10010005
Kamimura H, Hayashida M, Ohgai T. CPP-GMR Performance of Electrochemically Synthesized Co/Cu Multilayered Nanowire Arrays with Extremely Large Aspect Ratio. Nanomaterials. 2020; 10(1):5. https://doi.org/10.3390/nano10010005
Chicago/Turabian StyleKamimura, Himeyo, Masamitsu Hayashida, and Takeshi Ohgai. 2020. "CPP-GMR Performance of Electrochemically Synthesized Co/Cu Multilayered Nanowire Arrays with Extremely Large Aspect Ratio" Nanomaterials 10, no. 1: 5. https://doi.org/10.3390/nano10010005
APA StyleKamimura, H., Hayashida, M., & Ohgai, T. (2020). CPP-GMR Performance of Electrochemically Synthesized Co/Cu Multilayered Nanowire Arrays with Extremely Large Aspect Ratio. Nanomaterials, 10(1), 5. https://doi.org/10.3390/nano10010005






