In Situ Construction of a MgSn(OH)6 Perovskite/SnO2 Type-II Heterojunction: A Highly Efficient Photocatalyst towards Photodegradation of Tetracycline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SnO2, MgSn(OH)6, and MgSn(OH)6/SnO2 Heterojunctions
2.2. Characterization
2.3. Photocatalytic Activity Measurements
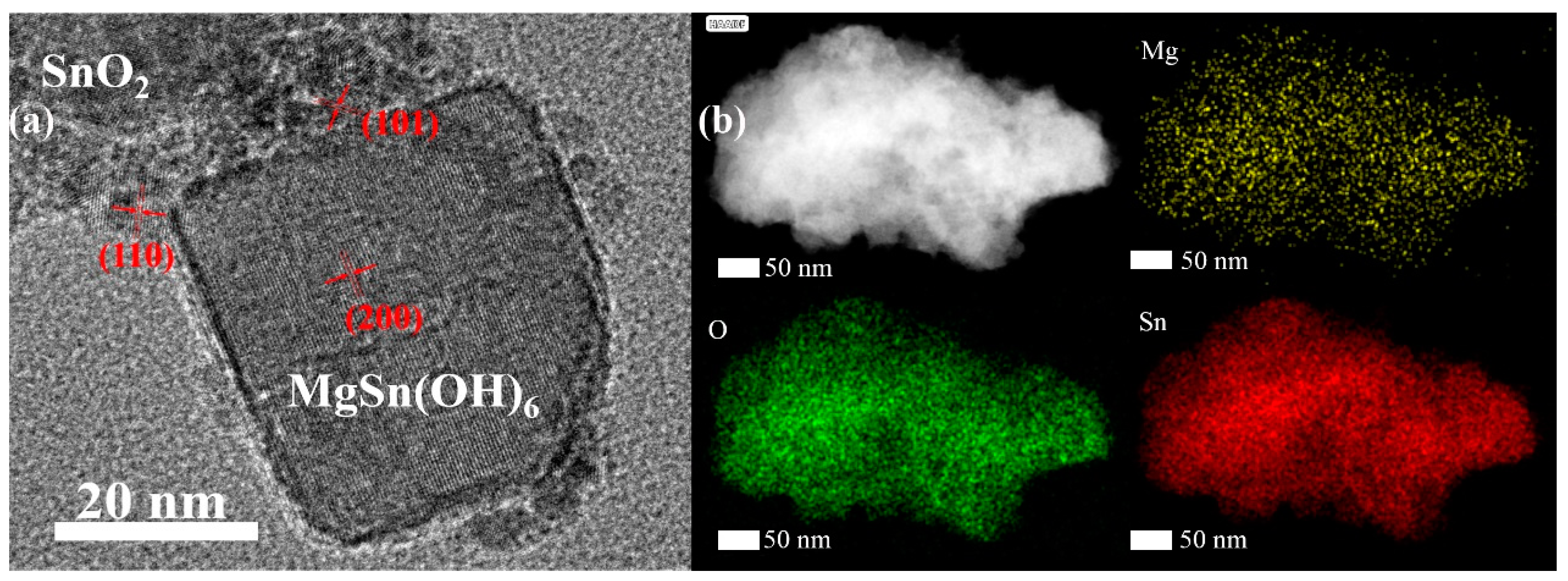
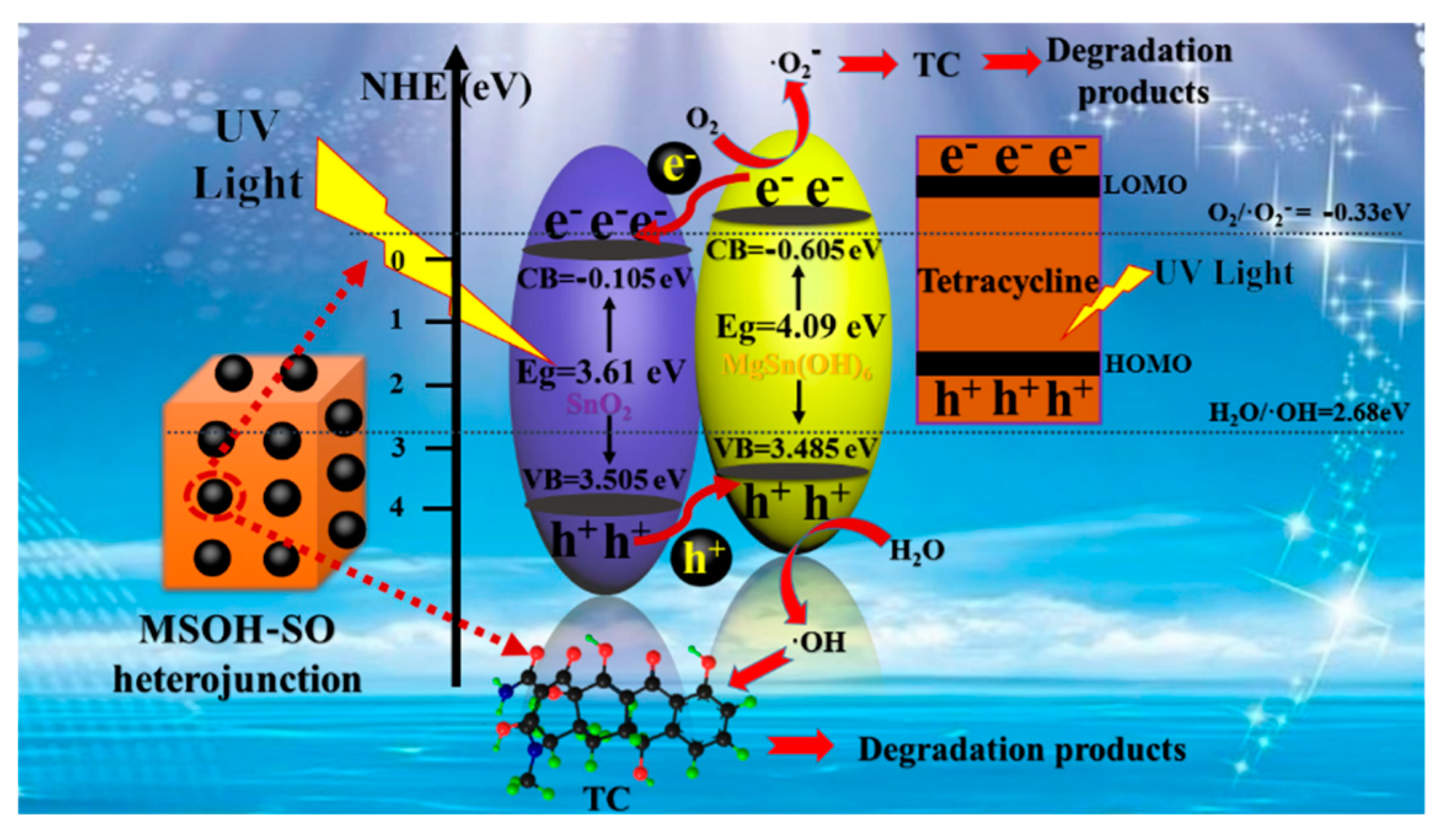
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bakkeren, E.; Huisman, J.S.; Fattinger, S.A.; Hausmann, A.; Furter, M.; Egli, A.; Slack, E.; Sellin, M.E.; Bonhoeffer, S.; Regoes, R.R.; et al. Salmonella Persisters Promote the Spread of Antibiotic Resistance Plasmids in the Gut. Nature 2019, 573, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Snitser, O.; Novich, G.; Katz, R.; Tal, O.; Parizade, M.; Chodick, G.; Koren, G.; Shalev, V.; Kishony, R. Personal clinical history predicts antibiotic resistance of urinary tract infections. Nat. Med. 2019, 25, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Reyes, S.G.; Tsuda, S.; Horinouchi, T.; Furusawa, C.; Cronin, L. Time Programmable Drug Dosing Allows the Manipulation, Suppression and Reversal of Antibiotic Drug Reversal of Antibiotic Drug Resistance in Vitro. Nat. Commun. 2017, 8, 15589–15599. [Google Scholar] [CrossRef] [PubMed]
- Amado, S.H.; Coque, T.M.; Baquero, F.; Martinez, J.L. Defining and Combating Antibiotic Resistance from One Health and Global Health Perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Blaney, L.; Cagnetta, G.; Huang, J.; Wang, B.; Wang, Y.J.; Deng, S.B.; Yu, G. Degradation of Ofloxacin by Perylene Diimide Supramolecular Nanofiber Sunlight Driven Photocatalyst. Environ. Sci. Technol. 2019, 53, 1564–1575. [Google Scholar] [CrossRef]
- Yan, J.F.; Peng, J.L.; Lai, L.; Ji, F.Z.; Zhang, Y.H.; Lai, B.; Chen, Q.X.; Yao, G.; Chen, X.; Song, L.P. Activation CuFe2O4 by Hydroxylamine for Oxidation of Antibiotic Sulfamethoxazole. Environ. Sci. Technol. 2018, 52, 14302–14310. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.D.; Wu, D.H.; Jing, Y.; Zhou, Z. MnPSe3 Monolayer: A Promising 2D Visible Light Photohydrolytic Catalyst with High Carrier Mobility. Adv. Sci. 2016, 3, 1600062–1600066. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Z.H.; Wu, D.H.; Zhang, X.; Zhao, X.D.; Zhou, Z. Computational Screening of 2D Materials and Rational Design of Heterojunctions for Water Splitting Photocatalysis. Small Methods 2018, 2, 1700359–1700367. [Google Scholar] [CrossRef]
- Hailili, R.; Wang, Z.Q.; Gong, X.Q.; Wang, C.Y. Octahedral-shaped Perovskite CaCu3Ti4O12 with Dual Defects and Coexposed {(001), (111)} Facets for Visible Light Photocatalysis. Appl. Catal. B 2019, 254, 86–97. [Google Scholar] [CrossRef]
- Kong, J.J.; Yang, T.; Rui, Z.B.; Ji, H.B. Perovskite Based Photocatalysts for Organic Contaminants Removal: Current Status and Future Perspectives. Catal. Today 2019, 327, 47–63. [Google Scholar] [CrossRef]
- Huang, D.W.; Fu, X.L.; Long, J.L.; Jiang, X.L.; Chang, L.; Meng, S.G.; Chen, S.F. Hydrothermal Synthesis of MSn(OH)6 (M = Co, Cu, Fe, Mg, Mn and Zn) and Their Photocatalytic Activity for the Destruction of Gaseous Benzene. Chem. Eng. J. 2015, 269, 168–179. [Google Scholar] [CrossRef]
- Wang, M.; Cao, X.L.; Huang, Y.F.; Guo, C.S.; Huang, L.J.; Yin, S.; Sato, T. Solvent Free Mechanochemical Synthesis of Well Dispersed Single Crystalline Zinc Hydroxystannate and Their Photocatalytic Properties. CrystEngComm 2012, 14, 2950–2953. [Google Scholar] [CrossRef]
- Luo, Y.P.; Chen, J.; Liu, J.W.; Shao, Y.; Li, X.F.; Li, D.Z. Hydroxide SrSn(OH)6: A New Photocatalyst for Degradation of Benzene and Rhodamine B. Appl. Catal. B 2016, 182, 533–540. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Bhuvanesh, N.S.P.; Kim, Y.I.; Ohara, S.; Woodward, P.M. Hydrothermal Crystal Growth and Structure Determination of Double Hydroxides LiSb(OH)6, BaSn(OH)6 and SrSn(OH)6. Inorg. Chem. 2014, 53, 10570–10577. [Google Scholar] [CrossRef]
- Kramer, J.W.; Kelly, B.; Manivannan, V. Synthesis of MSn(OH)6 (where M = Mg, Ca, Zn, Mn or Cu) materials at room temperature. Cent. Eur. J. Chem. 2010, 8, 65–69. [Google Scholar] [CrossRef]
- Jena, H.; Kutty, K.V.G.; Kutty, T.R.N. Ionic Transport and Structural Investigations on MSn(OH)6 (M = Ba, Ca, Mg, Co, Zn, Fe and Mn) Hydroxide Perovskites Synthesized by Wet Sonochemical Methods. Mater. Chem. Phys. 2004, 88, 167–179. [Google Scholar] [CrossRef]
- Baeissa, E.S. Novel Pd/CaSn(OH)6 Nanocomposite Prepared by Modified Sonochemical Method for Photocatalytic Degradation of Methylene Blue Dye. J. Alloys Compd. 2014, 590, 303–308. [Google Scholar] [CrossRef]
- Liu, T.Y.; Ma, X.L.; Yang, L.F.; Li, H.; Li, H.H.; Lee, S.W.; Wang, Y.H. Highly Enhanced Photocatalytic Activity of CaSn(OH)6 through Tuning CaSn(OH)6/SnO2 Heterostructural Interaction and Optimizing Fe3+ Doping Concentration. Appl. Catal. B 2017, 217, 256–264. [Google Scholar] [CrossRef]
- Dong, S.Y.; Cui, L.F.; Zhao, Y.L.; Wu, Y.W.; Xia, L.J.; Su, X.F.; Zhang, C.Y.; Wang, D.; Guo, W.; Sun, J.H. Crystal Structure and Photocatalytic Properties of Perovskite MSn(OH)6 (M = Cu and Zn) Composites with d10-d10 configuration. Appl. Surf. Sci. 2019, 463, 659–667. [Google Scholar] [CrossRef]
- Gao, Y.; Ye, L.; Cao, S.Y.; Chen, H.; Yao, Y.N.; Jiang, J.; Sun, L.C. Perovskite Hydroxide CoSn(OH)6 Nanocubes for Efficient Photoreduction of CO2 to CO. ACS Sustain. Chem. Eng. 2018, 6, 781–786. [Google Scholar] [CrossRef]
- Fu, X.L.; Leung, D.Y.C.; Wang, X.X.; Xue, W.W.; Fu, X.Z. Photocatalytic Reforming of Ethanol to H2 and CH4 over ZnSn(OH)6 nanocubes. J. Hydrog. Energy 2011, 36, 1524–1530. [Google Scholar] [CrossRef]
- Li, M.Y.; Tang, Y.B.; Shi, W.L.; Chen, F.Y.; Shi, Y.; Gu, H.C. Design of Visible Light Response Core-Shell Fe2O3/CuBi2O4 Heterojunctions with Enhanced Photocatalytic Activity Towards the Degradation of Tetracycline: Z-scheme Photocatalytic Mechanism Insight. Inorg. Chem. Front. 2018, 5, 3148–3154. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materails for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhang, L.S.; Chen, Z.G.; Hu, J.Q.; Li, S.J.; Wang, Z.H.; Liu, J.S.; Wang, X.C. Semiconductor Heterojunction Photocatalysis: Design, Construction and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.G.; Jaroniec, M. All Solid State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 6, 4920–4935. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4) Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yang, Q.M.; Wang, Z.M.; Wang, G.Y.; Zhang, B.; Zhang, Q.; Yang, D.F. Rapid Fabrication of SnO2 Nanoparticle Photocatalyst: Computational Understanding and Photocatalytic Degradaton of Organic Dye. Inorg. Chem. Front. 2018, 5, 3005–3014. [Google Scholar] [CrossRef]
- Selvan, R.K.; Perelshtein, I.; Perkas, N.; Gedanken, A. Synthesis of Hexagonal Shaped SnO2 Nanocrystals and SnO2@C Nanocomposites for Electrochemical Redox Supercapacitors. J. Phys. Chem. C 2008, 112, 1825–1830. [Google Scholar] [CrossRef]
- Sandesh, S.; Kristachar, P.K.R.; Manjunathan, P.; Halgeri, A.B.; Shanbhag, G.V. Synthesis of Biodiesel and Acetins by Transesterification Reactions using Novel CaSn(OH)6 Heterogeneous Base Catalyst. Appl. Catal. A Gen. 2016, 523, 1–11. [Google Scholar] [CrossRef]
- Thi, V.H.T.; Lee, B.K. Great Improvement on Tetracycline Removal using ZnO Rod-activated Carbon Fiber Composite Prepared with a Facile Microwave method. J. Haz. Mater. 2017, 324, 329–339. [Google Scholar]
- Xie, Z.J.; Feng, Y.P.; Wang, F.L.; Chen, D.N.; Zhang, Q.X.; Zeng, Y.Q.; Lv, W.Y.; Liu, G.G. Construction of Carbon Dots Modified MoO3/g-C3N4 Z-scheme Photocatalyst with Enhanced Visible Light Photocatalytic Activity for the Degradation of Tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Hailili, R.; Wang, Z.Q.; Li, Y.X.; Wang, Y.H.; Sharma, V.K.; Gong, X.Q.; Wang, C.Y. Oxygen Vacancies Induced Visible Light Photocatalytic Activities of CaCu3Ti4O12 with Controllable Morphologies for Antibiotic Degradation. Appl. Catal. B Environ. 2018, 221, 422–432. [Google Scholar] [CrossRef]
- Babu, B.; Harish, V.V.N.; Koutavarapu, R.; Shim, J.; Yoo, K. Enhanced Visible Light Active Photocatalytic Performance Using CdS Nanorods Decorated with Colloidal SnO2 Quantum Dots: Optimization of Core Shell Nanostructure. J. Ind. Eng. Chem. 2019, 76, 476–487. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.T.; Zeng, G.M.; Huang, D.L.; Xiao, R.; Zhang, C.; Zhou, C.Y.; Xiong, W.P.; Wang, W.J.; Cheng, M.; et al. Ti3C2 Mxene/porous g-C3N4 Interfacial Schottky Junction for Boosting Spatial Charge Separation in Photocatalytic H2O2 Production. Appl. Catal. B Environ. 2019, 258, 117956–117966. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat Band Potential of a Semiconductor Using the Mott Schottky Equation. J. Chem. Edu. 2007, 84, 685–688. [Google Scholar] [CrossRef]
- Boltersdorf, J.; Zoellner, B.; Fancher, C.M.; Jones, J.L.; Maggard, P.A. Single and Double Site Substitutions in Mixed Metal Oxides: Adjusting the Band Edges Toward the Water Redox Couples. J. Phys. Chem. C 2016, 120, 19175–19188. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.Q.; Zhang, H.Y.; Duan, X.G.; Liang, P.; Li, X.Y.; Tade, M.O.; Liu, S.M.; Wang, S.B. Facile Assembly of Bi2O3/Bi2S3/MoS2 n-p Heterojunction with Layered n-Bi2O3 and p-MoS2 for Enhanced Photocatalytic Water Oxidation and Pollutant Degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Lin, R.; Wan, J.W.; Xiong, Y.; Wu, K.L.; Cheong, W.C.; Zhou, G.; Wang, D.S.; Peng, Q.; Chen, C.; Li, Y.D. Quantitative Study of Charge Carrier Dynamics in Well-Defined WO3 Nanowires and Nanosheets: Insight into the Crystal Facet Effect in Photocatalysis. J. Am. Chem. Soc. 2018, 140, 9078–9082. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Guan, Z.G.; Yang, J.J.; Li, Q.Y. Band Positions and Photoelectrochemical Properties of Solution Processed Silver Substituted Cu2ZnSnS4 Photocathode. ACS Appl. Energy Mater. 2019, 2, 2779–2785. [Google Scholar] [CrossRef]
- Lin, Y.M.; Li, D.Z.; Hu, J.H.; Xiao, G.C.; Wang, J.X.; Li, W.J.; Fu, X.Z. Highly Efficient Photocatalytic Degradation of Organic Pollutants by PANI-Modified TiO2 Composite. J. Phys. Chem. C 2012, 116, 5764–5772. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tian, X.; Wang, Y.; Yang, Q.; Diao, Y.; Zhang, B.; Yang, D. In Situ Construction of a MgSn(OH)6 Perovskite/SnO2 Type-II Heterojunction: A Highly Efficient Photocatalyst towards Photodegradation of Tetracycline. Nanomaterials 2020, 10, 53. https://doi.org/10.3390/nano10010053
Li Y, Tian X, Wang Y, Yang Q, Diao Y, Zhang B, Yang D. In Situ Construction of a MgSn(OH)6 Perovskite/SnO2 Type-II Heterojunction: A Highly Efficient Photocatalyst towards Photodegradation of Tetracycline. Nanomaterials. 2020; 10(1):53. https://doi.org/10.3390/nano10010053
Chicago/Turabian StyleLi, Yuanyuan, Xiaofang Tian, Yaoqiong Wang, Qimei Yang, Yue Diao, Bin Zhang, and Dingfeng Yang. 2020. "In Situ Construction of a MgSn(OH)6 Perovskite/SnO2 Type-II Heterojunction: A Highly Efficient Photocatalyst towards Photodegradation of Tetracycline" Nanomaterials 10, no. 1: 53. https://doi.org/10.3390/nano10010053