Preparation of Hollow Flower-Like Microspherical β-Bi2O3/BiOCl Heterojunction and High Photocatalytic Property for Tetracycline Hydrochloride Degradation
Abstract
:1. Introduction
2. Materials and Experimental
2.1. Materials
2.2. Synthesis of β-Bi2O3/BiOCl
2.2.1. Preparation of β-Bi2O3
2.2.2. Preparation of β-Bi2O3/BiOCl
2.3. Sample Characterization
2.4. Photocatalytic Performance Tests
2.5. Recycling Performance of the Sample
3. Results and Discussion
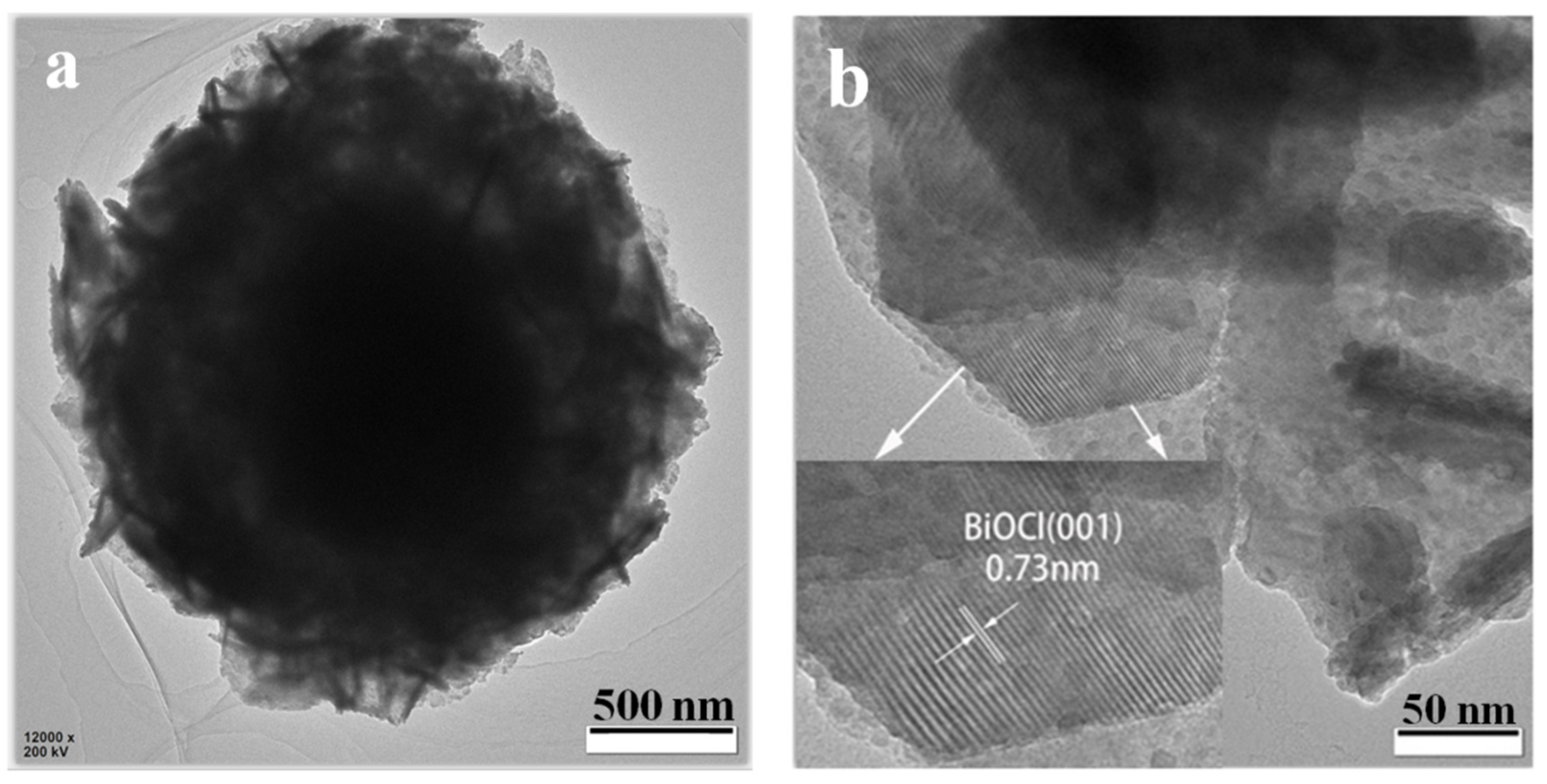
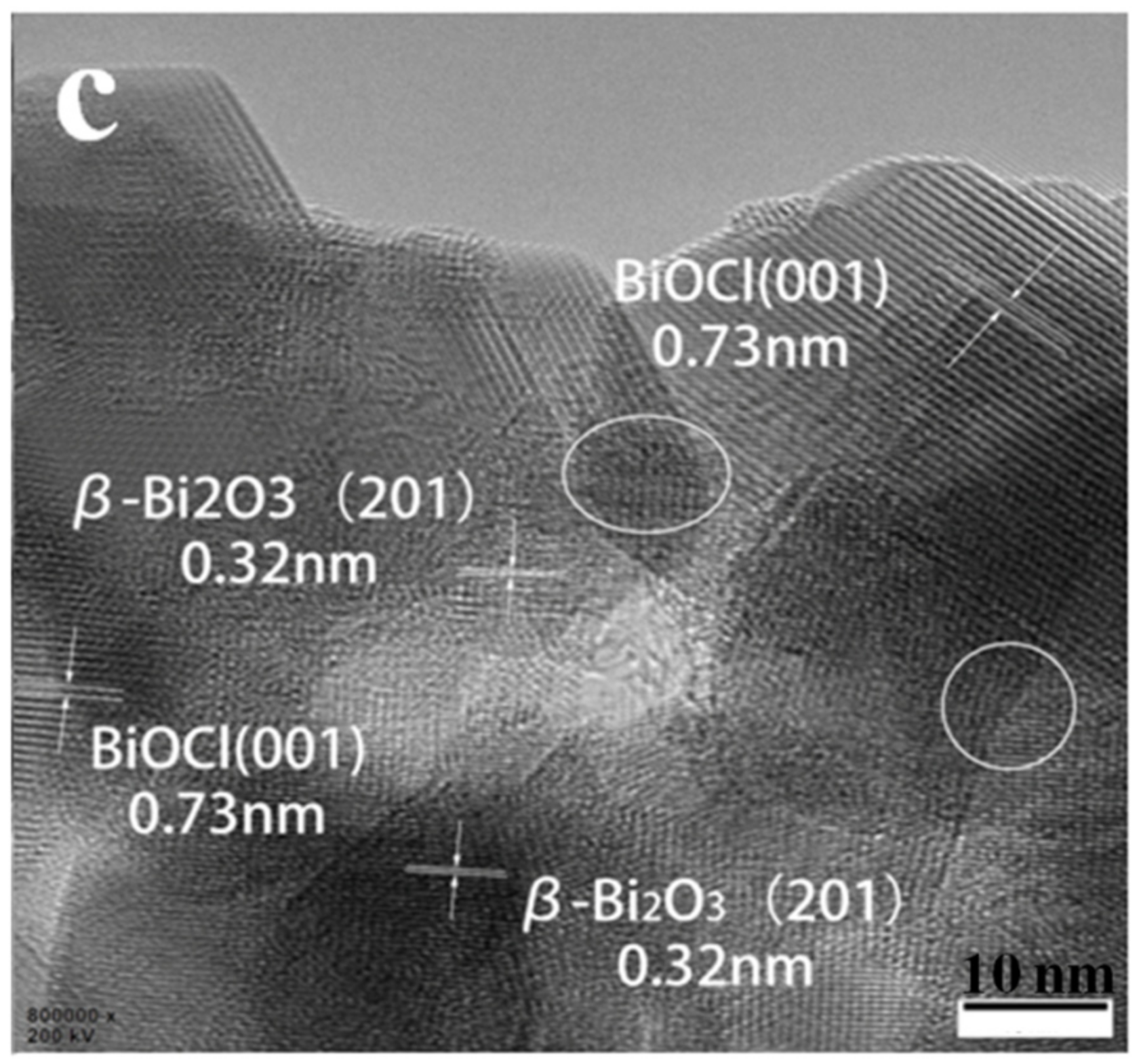
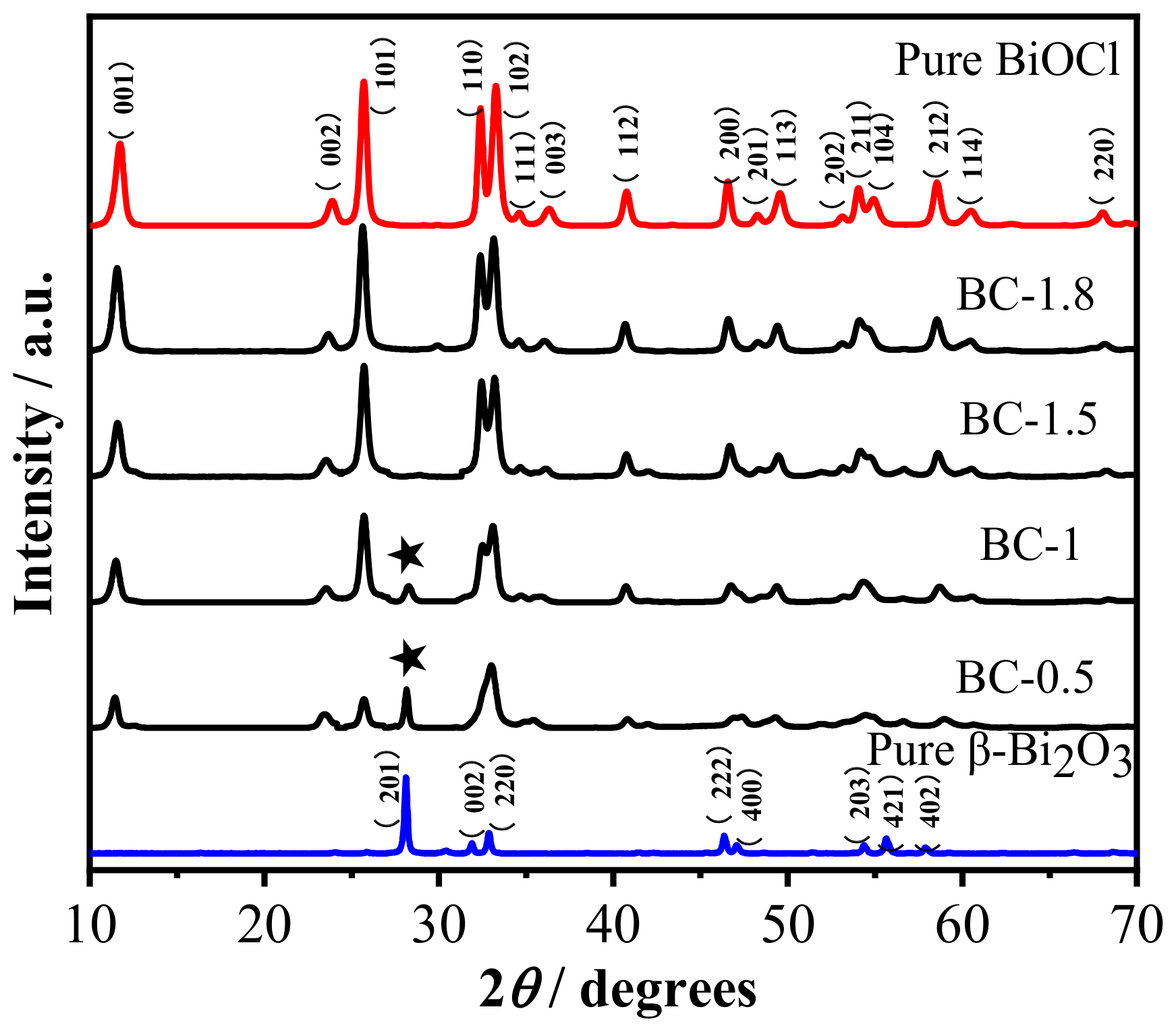
3.1. Phase Composition and Microstructure Analysis of the Samples
3.2. XPS Analysis of the Samples
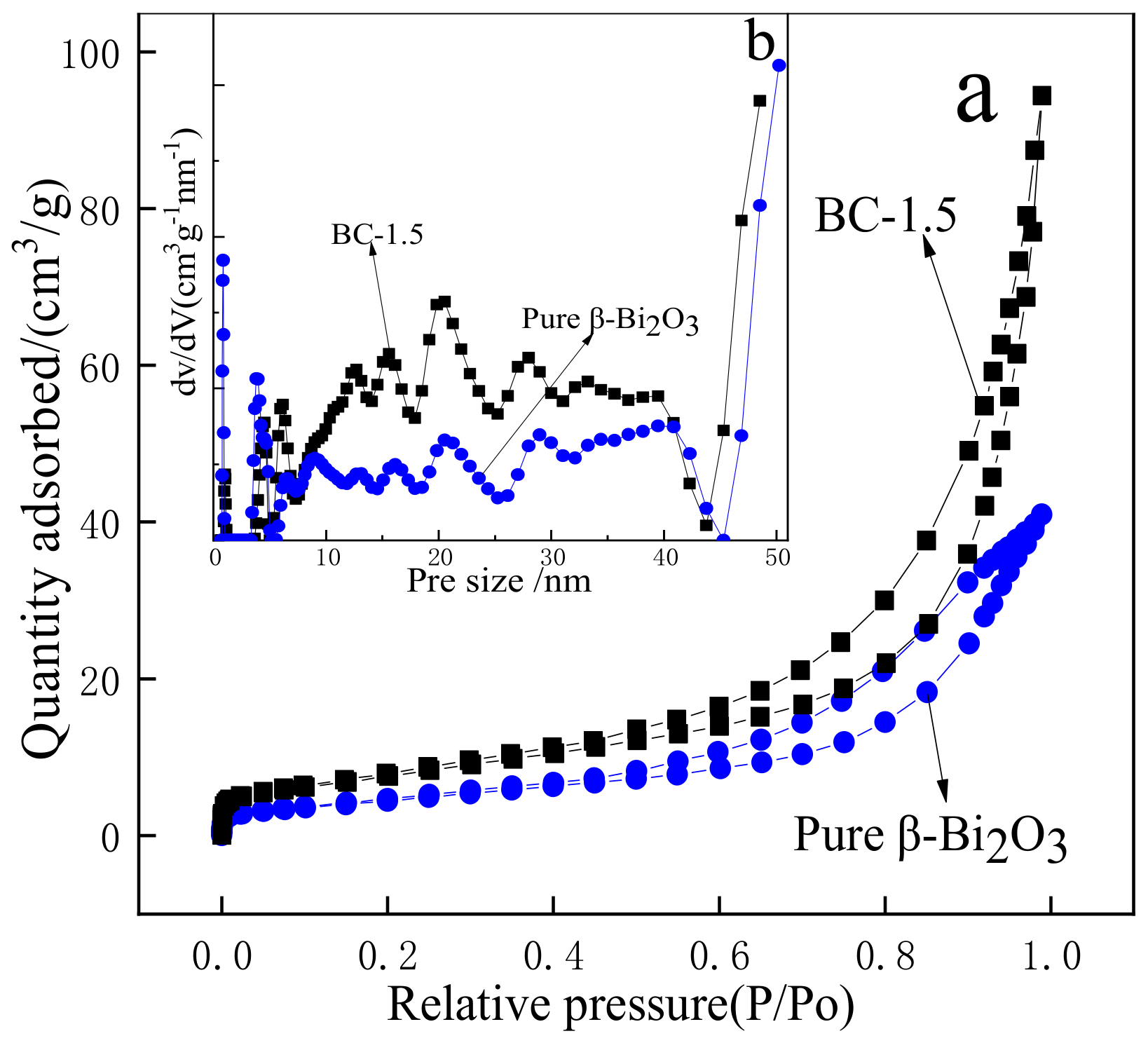
3.3. BET Analysis of the Samples
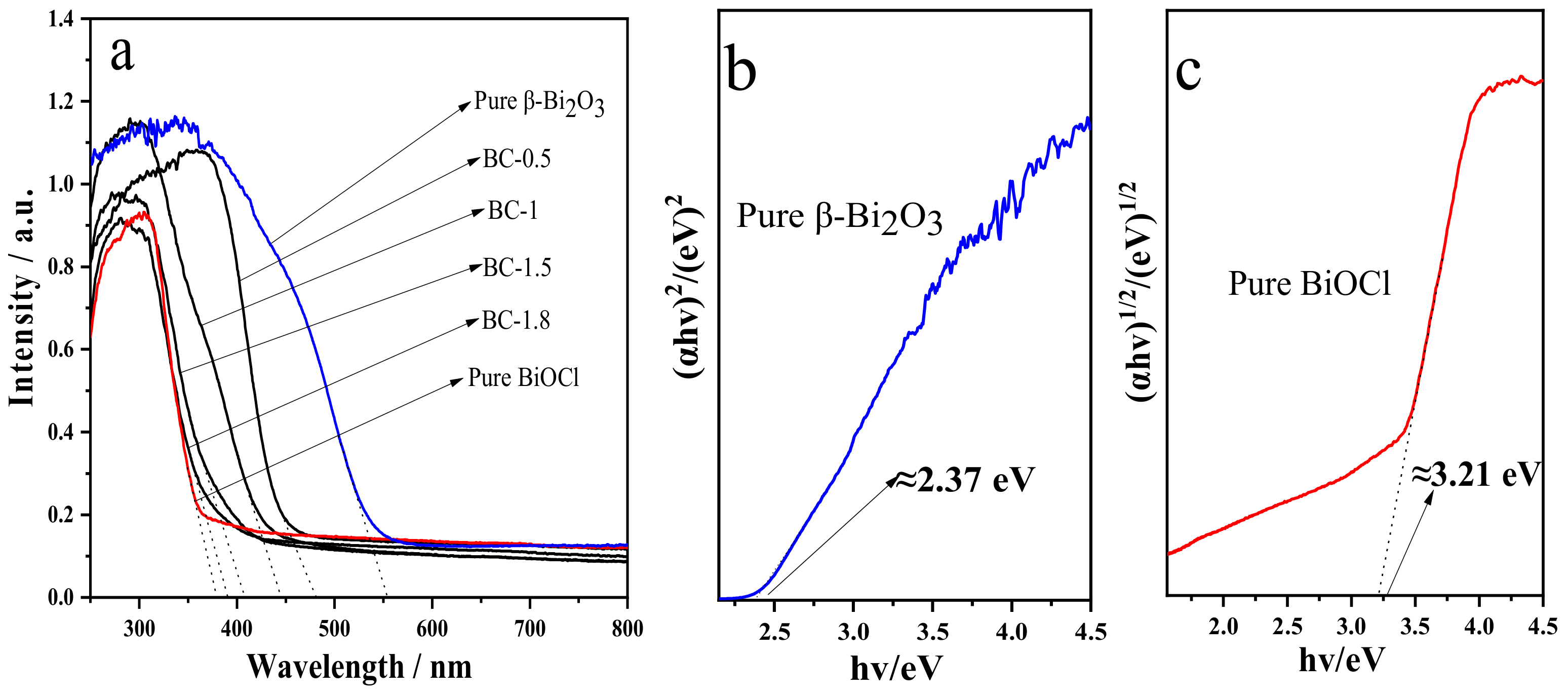
3.4. UV-Vis Absorption Spectrum Analysis of the Samples
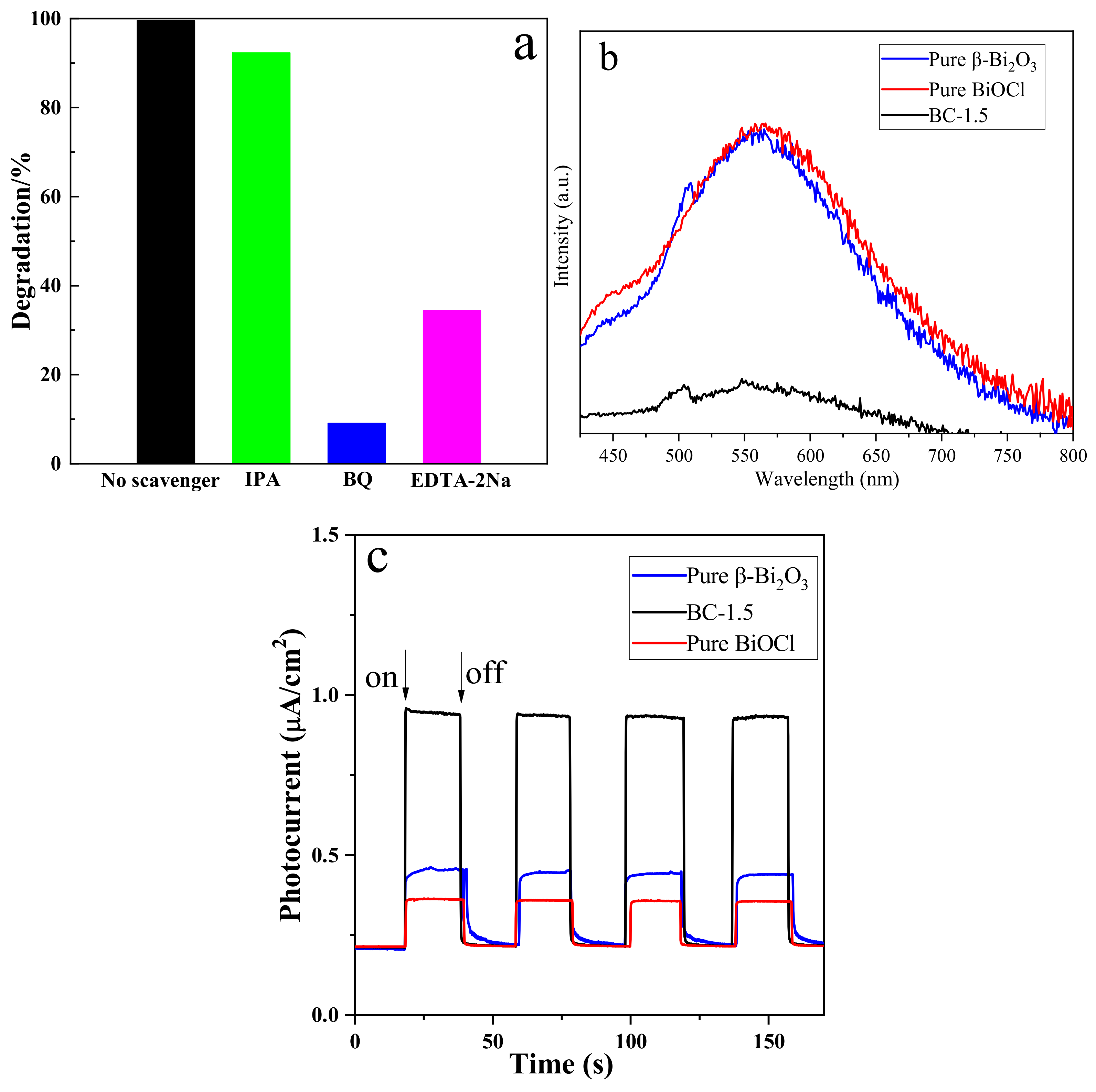
3.5. Analysis of Photocatalytic Properties
3.6. Photodegradation Mechanism of the Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, S.; Li, D.; Hapgood, K.; Zhang, X.; Wang, H. Ni(OH)2 decorated rutile TiO2 for efficient removal of tetracycline from wastewater. Appl. Catal. B Environ. 2016, 198, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Bautitz, I.R.; Nogueira, R.F.P. Degradation of tetracycline by photo-Fenton process—Solar irradiation and matrix effects. J. Photochem. Photobiol. A Chem. 2012, 187, 33–39. [Google Scholar] [CrossRef]
- Hui, W.; Hong, Y.; Jin, P.; Fan, L.; Li, D. Photodegradation of tetracycline antibiotics in aqueous solution by UV/ZnO. Desalin. Water Treat. 2016, 57, 19981–19987. [Google Scholar]
- Zhang, M.; He, L.Y.; Liu, Y.S.; Zhao, J.L.; Liu, W.R.; Zhang, J.N.; Chen, J.; He, L.K.; Zhang, Q.Q.; Ying, G.G. Fate of veterinary antibiotics during animal manure composting. Sci. Total. Environ. 2019, 650, 1363–1370. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Lahr, J.; Nibbeling, C.; Jansen, L.J.M.; Bongers, I.E.A.; Wipfler, E.L.; Schans, M.G.M.V.D. The persistence of a broad range of antibiotics during calve, pig and broiler manure storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef]
- Guo, T.; Lou, C.; Zhai, W.; Tang, X.; Hashmi, M.Z.; Murtaza, R.; Li, Y.; Liu, X.; Xu, J. Increased occurrence of heavy metals, antibiotics and resistance genes in surface soil after long-term application of manure. Sci. Total. Environ. 2018, 635, 995–1003. [Google Scholar] [CrossRef]
- Kay, P.; Blackwell, P.A.; Boxall, A. Transport of veterinary antibiotics in overland flow following the application of slurry to arable land. Chemosphere 2005, 59, 951–959. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef]
- Choi, K.J.; Kim, S.G.; Kim, C.W.; Kim, S.H. Determination of antibiotic compounds in water by on-line SPE-LC/MSD. Chemosphere 2007, 66, 977–984. [Google Scholar] [CrossRef]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Peñuela, G.; Mansilla, H.D. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal. Today 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Yan, W.; Hui, Z.; Lu, C. Ultrasound enhanced catalytic ozonation of tetracycline in a rectangular air-lift reactor. Catal. Today 2011, 175, 283–292. [Google Scholar]
- Michael, I.; Rizzo, L.; Mcardell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, M.; Motlagh, H.R.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorfi, S. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar] [CrossRef]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.; Fernández, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and inactivation of tetracycline by TiO2 photocatalysis. J. Photochem. Photobiol. A Chem. 2008, 184, 141–146. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Green and low cost tetracycline degradation processes by nanometric and immobilized TiO2 systems. Catal. Today 2016, 281, 38–44. [Google Scholar] [CrossRef]
- Wang, X.; Jia, J.; Wang, Y. Combination of Photocatalysis with Hydrodynamic Cavitation for Degradation of Tetracycline. Chem. Eng. J. 2017, 315, 274–282. [Google Scholar] [CrossRef]
- Di, L.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799. [Google Scholar]
- Zhu, X.D.; Wang, Y.J.; Sun, R.J.; Zhou, D.M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef]
- Gondal, M.A.; Chang, X.F.; Yamani, Z.H. UV-light induced photocatalytic decolorization of Rhodamine 6G molecules over BiOCl from aqueous solution. Chem. Eng. J. 2010, 165, 250–257. [Google Scholar] [CrossRef]
- Zhang, K.L.; Liu, C.M.; Huang, F.Q.; Zheng, C.; Wang, W.D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Li, J.Z.; Zhong, J.B.; Zeng, J.; Feng, F.M.; He, J.J. Improved photocatalytic activity of dysprosium-doped Bi 2O3 prepared by sol–gel method. Mater. Sci. Semicond. Process. 2013, 16, 379–384. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zhang, Z.J.; Zhang, W.D. Facile preparation of heterostructured Bi2O3/Bi2MoO6 hollow microspheres with enhanced visible-light-driven photocatalytic and antimicrobial activity. Mater. Res. Bull. 2013, 48, 1420–1427. [Google Scholar] [CrossRef]
- Jha, R.K.; Pasricha, R.; Ravi, V. Synthesis of bismuth oxide nanoparticles using bismuth nitrate and urea. Ceram. Int. 2005, 31, 495–497. [Google Scholar] [CrossRef]
- He, W.; Qin, W.; Wu, X.; Ding, X.; Chen, L.; Jiang, Z. The photocatalytic properties of bismuth oxide films prepared through the sol–gel method. Thin Solid Films 2007, 515, 5362–5365. [Google Scholar]
- Maruthamuthu, P.; Gurunathan, K.; Subramanian, E.; Sastri, M.V.C. Visible light-induced hydrogen production from water with Pt/Bi2O3/RuO2 in presence of electron relay and photosensitizer. Int. J. Hydrog. Energy 1994, 19, 889–893. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Yang, J.; Chen, Z.; Zhang, W.; Lin, Z.; Liu, S. Sonochemical synthesis of nanocrystallite Bi2O3 as a visible-light-driven photocatalyst. Appl. Catal. A Gen. 2006, 308, 105–110. [Google Scholar] [CrossRef]
- Walsh, A.; Watson, G.W.; Payne, D.J.; Edgell, R.G.; Guo, J.; Glans, P.A.; Learmonth, T.; Smith, K.E. Electronic structure of the α and δ phases of Bi2O3: A combined ab initio and x-ray spectroscopy study. Phys. Rev. B 2006, 73, 235104. [Google Scholar] [CrossRef]
- Xu, Z.; Hirogaki, K.; Wang, S.; Tabata, I.; Hisada, K.; Wang, T.; Hori, T. UV-induced formation of activated Bi2O3 nanoflake: An enhanced visible light driven photocatalyst by platinum loading. RSC Adv. 2011, 2, 103–106. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, S.; Cai, C.; Bo, Z. β-and α-Bi2O3 nanoparticles synthesized via microwave-assisted method and their photocatalytic activity towards the degradation of rhodamine B. Mater. Lett. 2011, 65, 988–990. [Google Scholar] [CrossRef]
- Kijima, N.; Matano, K.; Saito, M.; Oikawa, T.; Konishi, T.; Yasuda, H.; Sato, T.; Yoshimura, Y. Oxidative catalytic cracking of n-butane to lower alkenes over layered BiOCl catalyst. Appl. Catal. A Gen. 2001, 206, 237–244. [Google Scholar] [CrossRef]
- Jun, G.; Wen-Hua, H.; Yi-Nong, L.; Jun-Jie, Z.; Hong-Yuan, C. One-dimensional BiPO4 nanorods and two-dimensional BiOCl lamellae: Fast low-temperature sonochemical synthesis, characterization, and growth mechanism. Cheminform 2006, 37, 8503–8509. [Google Scholar]
- Chakraborty, A.K.; Rawal, S.B.; Song, Y.H.; Chai, S.Y.; Wan, I.L. Enhancement of visible-light photocatalytic efficiency of BiOCl/Bi2O3 by surface modification with WO3. Appl. Catal. A Gen. 2011, 407, 217–223. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, G.; Song, S.; Fan, W.; Zhang, H. Synthesis, characterization and assembly of BiOCl nanostructure and their photocatalytic properties. CrystEngComm 2009, 11, 1857–1862. [Google Scholar] [CrossRef]
- Wang, C.; Shao, C.; Liu, Y.; Zhang, L. Photocatalytic properties BiOCl and BiO nanofibers prepared by electrospinning. Scr. Mater. 2008, 59, 332–335. [Google Scholar] [CrossRef]
- Priya, B.; Raizada, P.; Singh, N.; Thakur, P.; Singh, P. Adsorptional photocatalytic mineralization of oxytetracycline and ampicillin antibiotics using Bi2O3/BiOCl supported on graphene sand composite and chitosan. J. Colloid Interface Sci. 2016, 479, 271–283. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, Z.; Cheng, Y.; Qiu, L.; Gao, C.; Zhou, J. Template-free fabrication of α- and β-Bi2O3 hollow spheres and their visible light photocatalytic activity for water purification. J. Alloy. Compd. 2014, 605, 102–108. [Google Scholar] [CrossRef]
- Tang, X.; Ma, C.; Liu, N.; Liu, C.; Liu, S. Visible light β-Bi2O3/BiOCl heterojunction photocatalyst with highly enhanced photocatalytic activity. Chem. Phys. Lett. 2018, 709, 82–87. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, F.; Shen, Y.; Xie, A.; Song, X. Facile fabrication and optical property of β-Bi2O3 with novel porous nanoring and nanoplate superstructures. J. Nanopart. Res. 2011, 13, 4575–4582. [Google Scholar] [CrossRef]
- Wu, Y.C.; Chaing, Y.C.; Huang, C.Y.; Wang, S.F.; Yang, H.Y. Morphology-controllable Bi2O3 crystals through an aqueous precipitation method and their photocatalytic performance. Dyes Pigment. 2013, 98, 25–30. [Google Scholar] [CrossRef]
- Han, S.; Jia, L.; Yang, K.; Lin, J. Fabrication of a β-Bi2O3/BiOI heterojunction and its efficient photocatalysis for organic dye removal. Chin. J. Catal. 2015, 36, 2119–2126. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Yu, S.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Chao, Z.; Zhang, X.; Wang, Y.; Xie, S.; Tong, Y. Facile electrochemical synthesis of CeO2 hierarchical nanorods and nanowires with excellent photocatalytic activities. New J. Chem. 2014, 38, 2581–2586. [Google Scholar]
- Xiao, X.; Hu, R.; Liu, C.; Xing, C.; Qian, C.; Zuo, X.; Nan, J.; Wang, L. Facile large-scale synthesis of β-Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation. Appl. Catal. B Environ. 2013, 140–141, 433–443. [Google Scholar] [CrossRef]
- Shan, L.; Wang, G.; Li, D.; San, X.; Liu, L.; Dong, L.; Wu, Z. Band alignment and enhanced photocatalytic activation of alpha/beta-Bi2O3 heterojunctions via in situ phase transformation. Dalton Trans. 2015, 44, 7835–7843. [Google Scholar] [CrossRef]
- Wang, N.; Pan, Y.; Lu, T.; Li, X.; Wu, S.; Wu, J. A new ribbon-ignition method for fabricating p-CuO/n-CeO2 heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2017, 403, 699–706. [Google Scholar] [CrossRef]
- Qu, L.; Lang, J.; Wang, S.; Chai, Z.; Su, Y.; Wang, X. Nanospherical composite of WO3 wrapped NaTaO3: Improved photodegradation of tetracycline under visible light irradiation. Appl. Surf. Sci. 2016, 388, 412–419. [Google Scholar] [CrossRef]
- Cao, X.; Tao, J.; Xiao, X.; Nan, J. Hydrothermal-assisted synthesis of the multi-element-doped TiO2 micro/nanostructures and their photocatalytic reactivity for the degradation of tetracycline hydrochloride under the visible light irradiation. J. Photochem. Photobiol. A Chem. 2018, 364, 202–207. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, S.; An, Z.; Zhang, W.; An, Z.; Yuan, M.; Chen, D. Preparation of Hollow Flower-Like Microspherical β-Bi2O3/BiOCl Heterojunction and High Photocatalytic Property for Tetracycline Hydrochloride Degradation. Nanomaterials 2020, 10, 57. https://doi.org/10.3390/nano10010057
Kong S, An Z, Zhang W, An Z, Yuan M, Chen D. Preparation of Hollow Flower-Like Microspherical β-Bi2O3/BiOCl Heterojunction and High Photocatalytic Property for Tetracycline Hydrochloride Degradation. Nanomaterials. 2020; 10(1):57. https://doi.org/10.3390/nano10010057
Chicago/Turabian StyleKong, Shulin, Zhaohui An, Wenwen Zhang, Zhihao An, Ming Yuan, and Donghui Chen. 2020. "Preparation of Hollow Flower-Like Microspherical β-Bi2O3/BiOCl Heterojunction and High Photocatalytic Property for Tetracycline Hydrochloride Degradation" Nanomaterials 10, no. 1: 57. https://doi.org/10.3390/nano10010057





