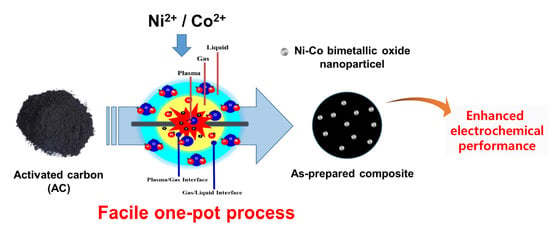
Facile Preparation of Ni-Co Bimetallic Oxide/Activated Carbon Composites Using the Plasma in Liquid Process for Supercapacitor Electrode Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials and Apparatus
2.2. Experimental Procedure
2.3. Characterization of NCOCC
3. Results and Discussion
3.1. Characterization of NCOCCs
3.2. Electrochemical Performance of NCOCCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Q.L.; Xue, K.H.; Shen, W.; Tao, F.F.; Yin, S.Y. Fabrication and electrochemical properties of carbon nanotube array electrode for supercapacitors. Electrochim. Acta 2004, 49, 4157–4161. [Google Scholar] [CrossRef]
- Feng, Z.H.; Xue, R.S.; Shao, X. Highly mesoporous carbonaceous material of activated carbon beads for electric double layer capacitor. Electrochim. Acta 2010, 55, 7334–7340. [Google Scholar] [CrossRef]
- Yan, L.J.; Niu, X.L.; Wang, W.C.; Li, X.B.; Sun, X.H.; Zheng, C.J.; Wang, J.W.; Sun, W. Electrochemical Sensor for Rutin Detection with Graphene Oxide and Multi-Walled Carbon Nanotube Nanocomposite Modified Electrode. Int. J. Electrochem. Sci. 2016, 11, 1738–1750. [Google Scholar]
- Peng, C.; Yan, X.B.; Wang, R.T.; Lang, J.W.; Ou, Y.J.; Xue, Q.J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Mai, Y.J.; Chen, R.; Wang, X.L.; Gu, C.D.; Zhao, X.B. Graphene sheet/porous NiO hybrid film for supercapacitor applications. Chem. Eur. J. 2011, 17, 10898–10905. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Li, Y.F.; Wang, R.D.; Cao, R. Synthesis and electrochemical capacitor performance of mesostructured nickel oxide/carbon composites by a co-casting method. J. Alloys Compd. 2009, 481, 100–105. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent Advances in Metal Oxide-based Electrode Architecture Design for Electrochemical Energy Storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef]
- Eskusson, J.; Rauwel, P.; Nerut, J.; Jänes, A. A Hybrid Capacitor Based on Fe3O4-Graphene Nanocomposite/Few-Layer Graphene in Different Aqueous Electrolytes. J. Electrochem. Soc. 2016, 163, A2768–A2775. [Google Scholar] [CrossRef]
- Justin, P.; Meher, S.K.; Rao, G.R. Tuning of Capacitance Behavior of NiO Using Anionic, Cationic, and Nonionic Surfactants by Hydrothermal Synthesis. J. Phys. Chem. C 2010, 114, 5203–5210. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Ultralayered Co3O4 for High-Performance Supercapacitor Applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Hu, C.C.; Chang, K.H.; Lin, M.C.; Wu, Y.T. Design and Tailoring of the Nanotubular Arrayed Architecture of Hydrous RuO2 for Next Generation Supercapacitors. Nano Lett. 2006, 6, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Long, J.W.; Sassin, M.B.; Fischer, A.E.; Rolison, D.R.; Mansour, A.N.; Johnson, V.S.; Stallworth, P.E.; Greenbaum, S.G. Multifunctional MnO2-Carbon Nanoarchitectures Exhibit Battery and Capacitor Characteristics in Alkaline Electrolytes. J. Phys. Chem. C 2009, 113, 17595–17598. [Google Scholar] [CrossRef]
- Qu, Q.T.; Shi, Y.; Li, L.L.; Guo, W.L.; Wu, Y.P.; Zhang, H.P.; Guan, S.Y.; Holze, R. V2O5∙0.6H2O nanoribbons as cathode material for asymmetric supercapacitor in K2SO4 solution. Electrochem. Commun. 2009, 11, 1325–1328. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Dhawale, D.S.; Salunkhe, R.R.; Jamdade, V.S.; Lokhande, C.D. Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J. Alloys Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Patake, V.D.; Joshi, S.S.; Lokhande, C.D.; Joo, O.S. Electrodeposited porous and amorphous copper oxide film for application in supercapacitor. Mater. Chem. Phys. 2009, 114, 6–9. [Google Scholar] [CrossRef]
- Sarkar, D.; Khan, G.G.; Singh, A.K.; Mandal, K. High-Performance Pseudocapacitor Electrodes Based on α-Fe2O3/MnO2 Core-Shell Nanowire Heterostructure Arrays. J. Phys. Chem. C 2013, 117, 15523–15531. [Google Scholar] [CrossRef]
- Wang, G.; Huang, J.; Chen, S.; Gao, Y.; Cao, D. Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam. J. Power Sources 2011, 196, 5756–5760. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Seo, S.G.; Kim, S.J.; Kim, S.C.; Park, Y.K.; Jung, S.C. Preparation and Characterization of Copper Nanoparticles via the Liquid Phase Plasma Method. Curr. Nanosci. 2014, 10, 7–10. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.; Jeon, K.J.; Park, H.; Park, Y.K.; Jung, S.C. Characterization of Bimetallic Fe-Ru Oxide Nanoparticles Prepared by Liquid-Phase Plasma Method. Nanoscale Res. Lett. 2016, 11, 344. [Google Scholar] [CrossRef]
- Chung, K.H.; Jeong, S.; Kim, B.J.; Kim, J.S.; Park, Y.K.; Jung, S.C. Development of hydrogen production by liquid phase plasma process of water with Ni-TiO2/carbon nanotube photocatalysts. Int. J. Hydrogen Energy 2018, 43, 5873–5880. [Google Scholar] [CrossRef]
- Chung, K.H.; Jeong, S.; Kim, B.J.; An, K.H.; Park, Y.K.; Jung, S.C. Enhancement of photocatalytic hydrogen production by liquid phase plasma irradiation on metal-loaded TiO2/carbon nanofiber photocatalysts. Int. J. Hydrogen Energy 2018, 43, 11422–11429. [Google Scholar] [CrossRef]
- Jeong, S.; Chung, K.H.; Lee, H.; Park, H.; Jeon, K.J.; Park, Y.K.; Jung, S.C. Enhancement of Hydrogen Evolution from Water Photocatalysis Using Liquid Phase Plasma on Metal Oxide-Loaded Photocatalysts. ACS Sustain. Chem. Eng. 2017, 5, 3659–3666. [Google Scholar] [CrossRef]
- Ki, S.J.; Jeon, K.J.; Park, Y.K.; Jeong, S.; Lee, H.; Jung, S.C. Improving removal of 4-chlorophenol using a TiO2 photocatalytic system with microwave and ultraviolet radiation. Catal. Today 2017, 293, 15–22. [Google Scholar] [CrossRef]
- Lee, H.; Park, I.S.; Bang, H.J.; Park, Y.K.; Kim, H.; Ha, H.H.; Kim, B.J.; Jung, S.C. Fabrication of Gd-La codoped TiO2 composite via a liquid phase plasma method and its application as visible-light photocatalysts. Appl. Surf. Sci. 2019, 471, 893–899. [Google Scholar] [CrossRef]
- Lee, H.; Park, I.S.; Bang, H.J.; Park, Y.K.; Cho, E.B.; Kim, B.J.; Jung, S.C. Preparation of silicon oxide–carbon composite from benzene and trimethoxyphenylsilane by a liquid phase plasma method for supercapacitor applications. Appl. Surf. Sci. 2019, 481, 625–631. [Google Scholar] [CrossRef]
- Ki, S.J.; Jeon, K.J.; Park, Y.K.; Park, H.W.; Jeong, S.; Lee, H.; Jung, S.C. Assembling a supercapacitor electrode with dual metal oxides and activated carbon using a liquid phase plasma. J. Environ. Manag. 2017, 203, 880–887. [Google Scholar] [CrossRef]
- Mun, M.K.; Lee, W.O.; Park, J.W.; Kim, D.S.; Yeom, G.Y.; Kim, D.W. Nanoparticles Synthesis and Modification using Solution Plasma Process. Appl. Sci. Converg. Technol. 2017, 26, 164–173. [Google Scholar] [CrossRef]
- Ghosh, S.; Hawtof, R.; Rumbach, P.; Go, D.B.; Akolkar, R.; Sankaran, R.M. Quantitative Study of Electrochemical Reduction of Ag+ to Ag Nanoparticles in Aqueous Solutions by a Plasma Cathode. J. Electrochem. Soc. 2017, 164, D818–D824. [Google Scholar] [CrossRef]
- Tong, D.G.; Wu, P.; Su, P.K.; Wang, D.Q.; Tian, H.Y. Preparation of zinc oxide nanospheres by solution plasma process and their optical property, photocatalytic and antibacterial activities. Mater. Lett. 2012, 70, 94–97. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Iraji zad, A.; Ahadian, M.M.; Mahdavi Ardakani, S.A. Synthesis and photocatalytic activity of WO3 nanoparticles prepared by the arc discharge method in deionized water. J. Nanotechnol. 2008, 19, 195709. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Maiti, S.; Mahanty, S.; Baran, A. Large-scale synthesis of porous NiCo2O4 and rGO-NiCo2O4 hollow-spheres with superior electrochemical performance as a faradaic electrode. J. Mater. Chem. A 2017, 5, 16854–16864. [Google Scholar] [CrossRef]
- Pu, J.; Wang, J.; Jin, X.; Cui, F.; Sheng, E.; Wang, Z. Porous hexagonal NiCo2O4 nanoplates as electrode materials for supercapacitors. Electrochim. Acta 2013, 106, 226–234. [Google Scholar] [CrossRef]
- Kong, W.; Lu, C.; Zhang, W.; Pu, J.; Wang, Z. Homogeneous core–shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar] [CrossRef]
- Young, C.; Salunkhe, R.R.; Alshehri, S.M.; Ahamad, T.; Huang, Z.; Heinze, J.; Yamauchi, Y. High energy density supercapacitors composed of nickel cobalt oxide nanosheets on nanoporous carbon nanoarchitectures. J. Mater. Chem. A 2017, 5, 11834–11839. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Lin, J.; Zhang, X.; Xiong, S. Polymer-assisted synthesis of a 3D hierarchical porous network-like spinel NiCo2O4 framework towards high-performance electrochemical capacitors. J. Mater. Chem. A 2013, 1, 11145–11151. [Google Scholar] [CrossRef]
- Chang, S.K.; Zainal, Z.; Tan, K.B.; Yusof, N.A.; Yusoff, W.M.D.W.; Prabaharan, S.R.S. Nickel-cobalt oxide/activated carbon composite electrodes for electrochemical capacitors. Curr. Appl. Phys. 2012, 12, 1421–1428. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Cui, K.; Sun, F.; Xu, Y.; Kuang, Y. Preparation and capacitive properties of cobalt–nickel oxides/carbon nanotube composites. Electrochim. Acta 2007, 52, 2959–2965. [Google Scholar] [CrossRef]







| Samples | Ni:Co Concentration [mM] | Carbon | Oxygen | Nickel | Cobalt | ||||
|---|---|---|---|---|---|---|---|---|---|
| Wt % | At % | Wt % | At % | Wt % | At % | Wt % | At % | ||
| Bare AC | 0:0 | 96.98 | 97.71 | 3.02 | 2.29 | 0.00 | 0.00 | 0.00 | 0.00 |
| NCOCC-19 | 1:9 | 95.03 | 96.87 | 3.75 | 2.87 | 0.18 | 0.04 | 1.04 | 0.22 |
| NCOCC-55 | 5:5 | 94.48 | 96.63 | 3.97 | 3.05 | 0.83 | 0.17 | 0.72 | 0.15 |
| NCOCC-91 | 9:1 | 94.82 | 96.79 | 3.81 | 2.93 | 1.24 | 0.26 | 0.12 | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Park, I.-S.; Park, Y.-K.; An, K.-H.; Kim, B.-J.; Jung, S.-C. Facile Preparation of Ni-Co Bimetallic Oxide/Activated Carbon Composites Using the Plasma in Liquid Process for Supercapacitor Electrode Applications. Nanomaterials 2020, 10, 61. https://doi.org/10.3390/nano10010061
Lee H, Park I-S, Park Y-K, An K-H, Kim B-J, Jung S-C. Facile Preparation of Ni-Co Bimetallic Oxide/Activated Carbon Composites Using the Plasma in Liquid Process for Supercapacitor Electrode Applications. Nanomaterials. 2020; 10(1):61. https://doi.org/10.3390/nano10010061
Chicago/Turabian StyleLee, Heon, In-Soo Park, Young-Kwon Park, Kay-Hyeok An, Byung-Joo Kim, and Sang-Chul Jung. 2020. "Facile Preparation of Ni-Co Bimetallic Oxide/Activated Carbon Composites Using the Plasma in Liquid Process for Supercapacitor Electrode Applications" Nanomaterials 10, no. 1: 61. https://doi.org/10.3390/nano10010061
APA StyleLee, H., Park, I.-S., Park, Y.-K., An, K.-H., Kim, B.-J., & Jung, S.-C. (2020). Facile Preparation of Ni-Co Bimetallic Oxide/Activated Carbon Composites Using the Plasma in Liquid Process for Supercapacitor Electrode Applications. Nanomaterials, 10(1), 61. https://doi.org/10.3390/nano10010061