An Efficient Synthesis and Photoelectric Properties of Green Carbon Quantum Dots with High Fluorescent Quantum Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Green Carbon Quantum Dots (g-CQDs)
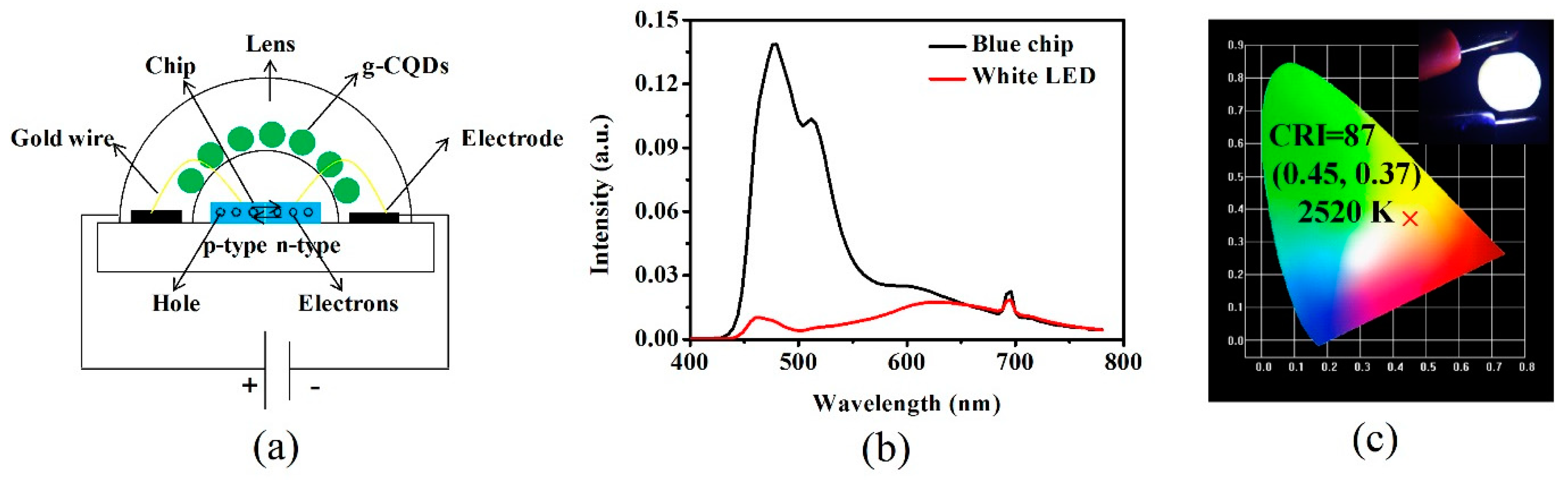
2.3. Preparation of White Light Emitting Diodes (White LEDs)
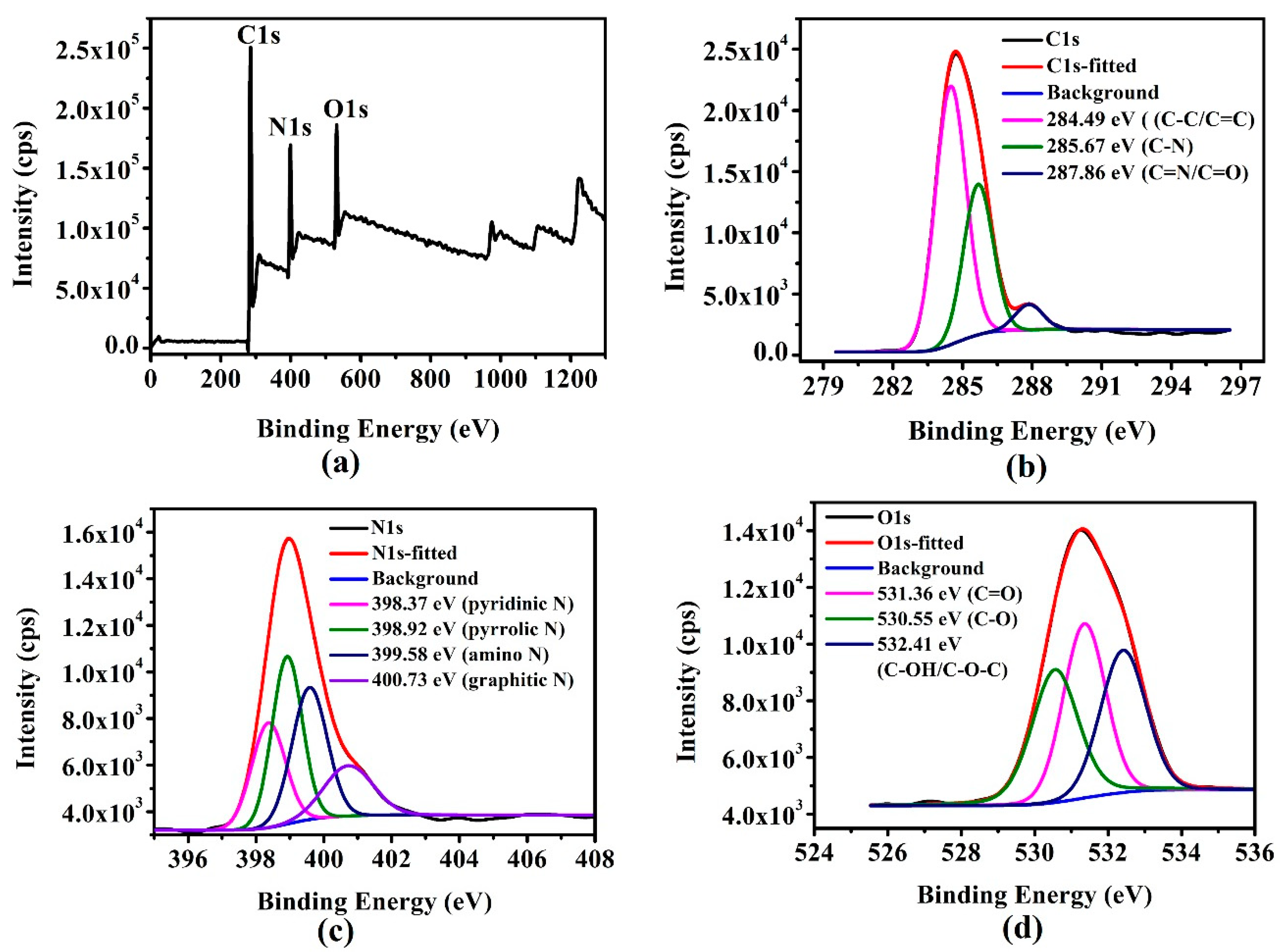
2.4. Characterisation
2.5. Calculation of Quantum Yield (QY)
2.6. Calculation of Product Yield (PY)
3. Results and Discussion
3.1. Influence of Reaction Conditions on PY and QY
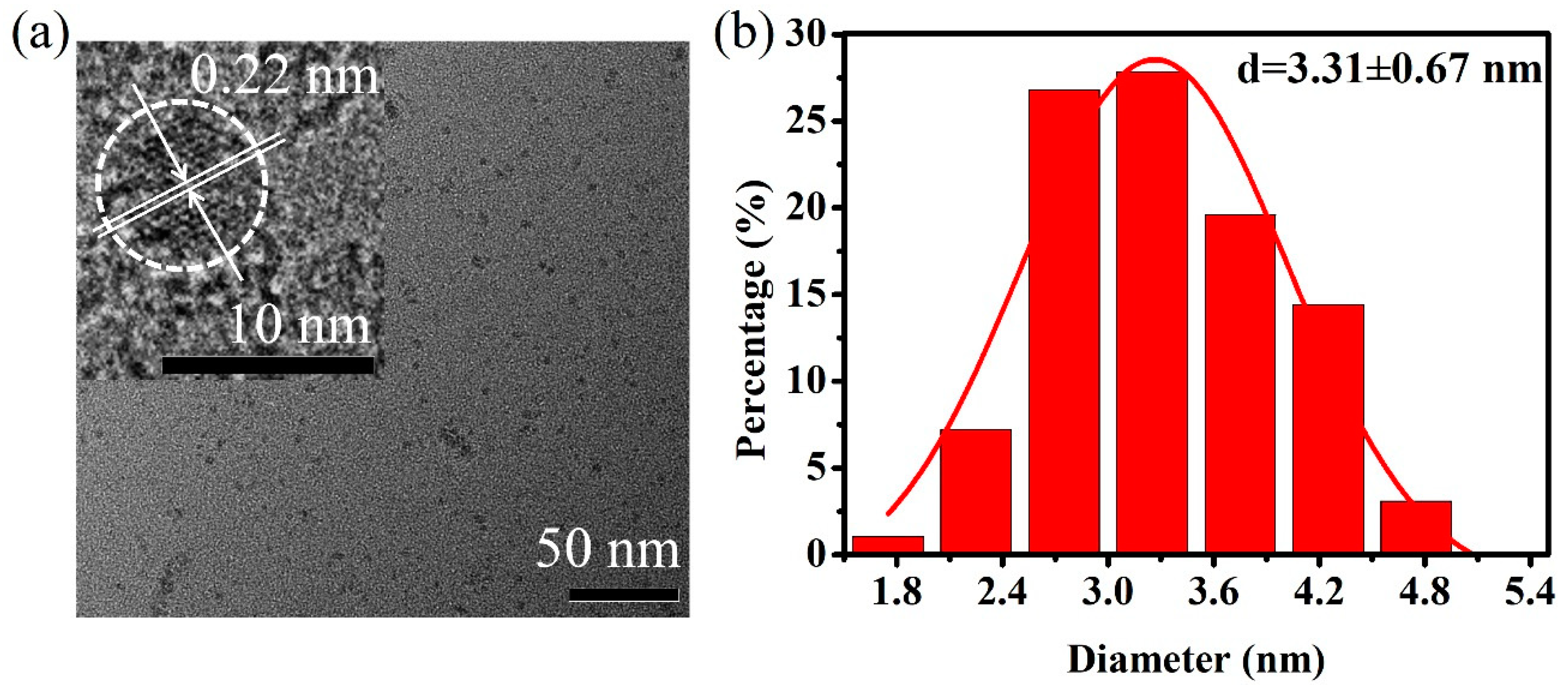
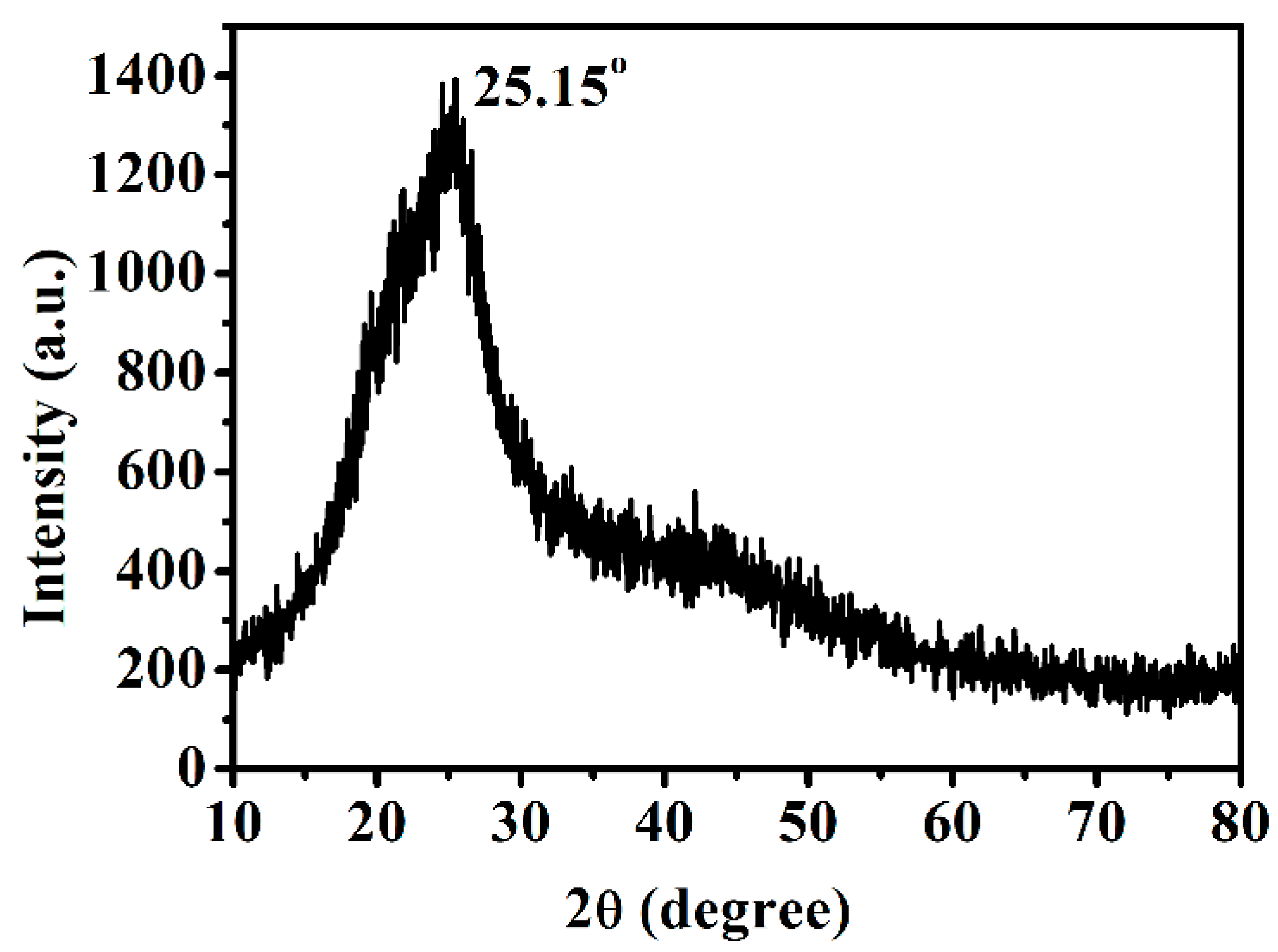
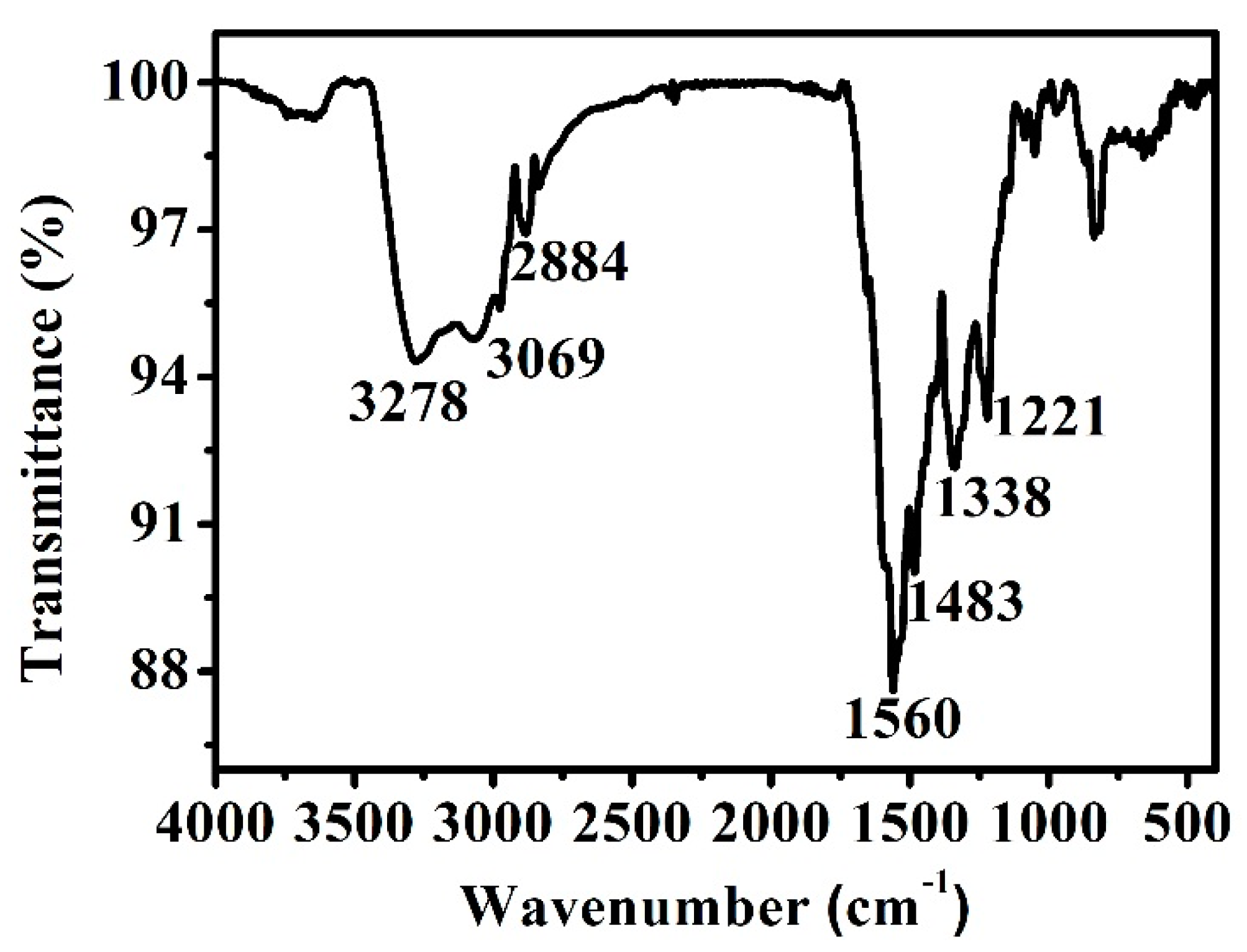
3.2. Morphology and Structures
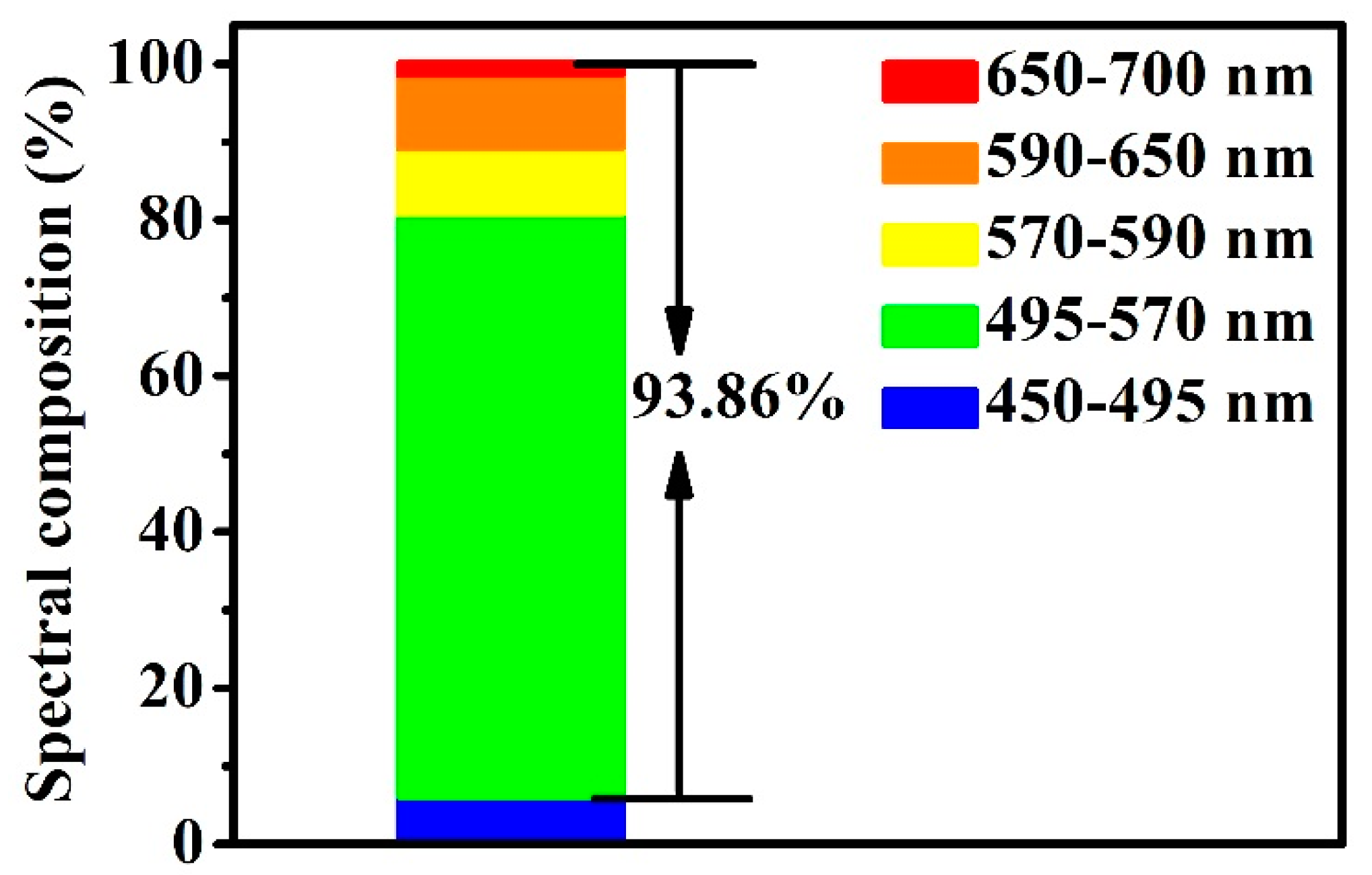
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, Y.L.; He, J.L.; Yu, Z.H.; Zhang, H.R.; Liu, Y.L.; Hu, G.Q.; Zheng, M.T.; Dong, H.W.; Zhuang, J.L.; Lei, B.F. Double carbon dot assembled mesoporous aluminas: Solid-state dual-emission photoluminescence and multifunctional applications. J. Mater. Chem. C 2018, 6, 2495–2501. [Google Scholar] [CrossRef]
- Zheng, J.X.; Wang, Y.L.; Zhang, F.; Yang, Y.Z.; Liu, X.G.; Guo, K.P.; Wang, H.; Xu, B.S. Microwave-assisted hydrothermal synthesis of solid-state carbon dots with intensive emission for white light-emitting devices. J. Mater. Chem. C 2017, 5, 8105–8111. [Google Scholar] [CrossRef]
- Yuan, B.; Xie, Z.; Chen, P.; Zhou, S.Y. Highly efficient carbon dots and their nanohybrids for trichromatic white LEDs. J. Mater. Chem. C 2018, 6, 5957–5963. [Google Scholar] [CrossRef]
- Zhan, Y.; Lin, L.F.; Chen, M.; Wu, L.M. Facile synthesis of a terephthalic acid-based organic fluorophore with strong and colour-tunable emission in both solution and solid states for LED applications. ACS Appl. Mater. Interfaces 2018, 10, 33390–33398. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, K.; Han, Z.X.; Yin, Z.M.; Zhou, C.J.; Du, F.L.; Zhou, S.Y.; Chen, P.; Xie, Z. High colour rendering index trichromatic white and red LEDs prepared from silane-functionalized carbon dots. J. Mater. Chem. C 2017, 5, 9629–9637. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, F.; Wang, Y.L.; Yang, Y.Z.; Liu, X.G. Efficient resistance against solid-state quenching of carbon dots towards white light emitting diodes by physical embedding into silica. Carbon 2018, 126, 426–443. [Google Scholar] [CrossRef]
- Shao, J.R.; Zhu, S.J.; Liu, H.W.; Song, Y.B.; Tao, S.Y.; Yang, B. Full-colour emission polymer carbon dots with quench-resistant solid-state fluorescence. Adv. Sci. 2017, 4, 1700395. [Google Scholar] [CrossRef]
- Du, Q.; Zheng, J.X.; Wang, J.L.; Yang, Y.Z.; Liu, X.G. The synthesis of green fluorescent carbon dots for warm white LEDs. RSC Adv. 2018, 8, 19585–19595. [Google Scholar] [CrossRef] [Green Version]
- Qu, S.N.; Liu, X.Y.; Guo, X.Y.; Chu, M.H.; Zhang, L.G.; Shen, D.Z. Amplified spontaneous green emission and lasing emission from carbon nanoparticles. Adv. Funct. Mater. 2014, 24, 2689–2695. [Google Scholar] [CrossRef]
- Zhan, C.F.; Hu, Z.B.; Song, L.; Cui, Y.Y.; Liu, X.F. Valine-derived carbon dots with colour-tunable fluorescence for the detection of Hg2+ with high sensitivity and selectivity. New J. Chem. 2015, 39, 6201–6206. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Hu, Z.B.; Zhang, C.F.; Liu, X.F. Simultaneously enhancing up-conversion fluorescence and red-shifting down-conversion luminescence of carbon dots by a simple hydrothermal process. J. Mater. Chem. B 2014, 2, 6947–6952. [Google Scholar] [CrossRef]
- Yuan, B.; Guan, S.Y.; Sun, X.M.; Li, X.M.; Zeng, H.B.; Xie, Z.; Chen, P.; Zhou, S.Y. Highly efficient carbon dots with reversibly switchable green–red emissions for trichromatic white light-emitting diodes. ACS Appl. Mater. Interfaces 2018, 10, 16005–16014. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Zhang, M.R.; Liu, Y.; Cai, W.; Yang, W.J.; He, Z.Y.; Sun, X.L.; Luo, Y.; Liu, F. Synthesis of multi-functional green fluorescence carbon dots and their applications as a fluorescent probe for Hg2+ detection and zebrafish imaging. New J. Chem. 2018, 42, 10400–10405. [Google Scholar]
- Wang, W.; Li, Y.M.; Cheng, L.; Cao, Z.Q.; Liu, W.G. Water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J. Mater. Chem. B 2013, 2, 46–48. [Google Scholar] [CrossRef]
- Zheng, J.X.; Wang, J.L.; Wang, Y.L.; Yang, Y.Z.; Liu, X.G.; Xu, B.S. Facile and rapid synthesis of yellow-emission carbon dots for white light-emitting diodes. J. Electron. Mater. 2018, 47, 7497–7504. [Google Scholar] [CrossRef]
- Jin, S.H.; Kim, D.H.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the photoluminescence of graphene quantum dots through the charge transfer effect of functional groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef]
- Qu, S.N.; Zhou, D.; Li, D.; Ji, W.Y.; Jing, P.T.; Han, D.; Liu, L.; Zeng, H.B.; Shen, D.Z. Toward efficient orange emissive carbon nanodots through conjugated sp(2)-domain controlling and surface charges engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.Q.; Li, L.; Xu, F.; Xu, Z.H.; Wei, D.Q.; Feng, Y.J.; Wang, Y.M.; Jia, D.C.; Zhou, Y. Ultrahigh-yield synthesis of N-doped carbon nanodots that down-regulate ROS in zebrafish. J. Mater. Chem. B 2017, 5, 7848–7860. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Liu, J.H.; Hou, P.; Zhou, J.; Huang, C.Z. Preparation of nitrogen-doped carbon dots with high quantum yield from bombyx mori silk for Fe (iii) ions detection. RSC Adv. 2017, 7, 50584–50590. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.J.; Meng, Q.N.; Wang, L.; Zhang, J.H.; Song, Y.B.; Jin, H.; Zhang, K.; Sun, H.C.; Wang, H.Y.; Yang, B. Highly photoluminescent carbon dots for multicolour patterning, sensors, and bioimaging. Angew. Chem. 2013, 125, 4045–4049. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, X.Y.; Fan, Y.; Guo, X.Y.; Zhou, L.; Lv, Y.; Lin, J. One-step microwave synthesis of N-doped hydroxyl-functionalized carbon dots with ultra-high fluorescence quantum yields. Nanoscale 2016, 8, 15281–15287. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Liu, Z.X.; Deng, W.C.; Zhan, L.; Liu, M.L.; Huang, C.Z. Large-scale synthesis of photoluminescent carbon quantum dots: A self-exothermic reaction driving the formation of the nanocrystalline core at room temperature. Green Chem. 2016, 18, 5127–5132. [Google Scholar] [CrossRef]
- Hou, J.; Wang, W.; Zhou, T.Y.; Wang, B.; Li, H.Y.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zheng, J.X.; Wang, J.L.; Yang, Y.Z.; Liu, X.G. Rapid microwave-assisted synthesis of highly luminescent nitrogen-doped carbon dots for white light-emitting diodes. Opt. Mater. 2017, 73, 319–329. [Google Scholar] [CrossRef]
- Song, Y.B.; Zhu, S.J.; Zhang, S.T.; Fu, Y.; Wang, L.; Zhao, X.H.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Niu, W.J.; Li, Y.; Zhu, R.H.; Shan, D.; Fan, Y.R.; Zhang, X.J. Ethylenediamine-assisted hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for sensitive biosensing and bioimaging. Sens. Actuator B Chem. 2015, 218, 229–236. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colourful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Kumar, G.S.; Roy, R.; Sen, D.; Ghorai, U.K.; Thapa, R.; Mazumder, N.; Saha, S.; Chattopadhyay, K.K. Amino-functionalized graphene quantum dots: Origin of tunable heterogeneous photoluminescence. Nanoscale 2014, 6, 3384–3391. [Google Scholar] [CrossRef]
- Tetsuka, H.; Asahi, R.; Nagoya, A.; Okamoto, K.; Tajima, I.; Ohta, R.; Okamoto, A. Optically tunable amino-functionalized graphene quantum dots. Adv. Mater. 2012, 24, 5333–5338. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhang, P.; Gao, M.X.; Liu, C.F.; Wang, W.; Leng, F.; Huang, C.Z. One-pot hydrothermal synthesis of highly luminescent nitrogen-doped amphoteric carbon dots for bioimaging from bombyx mori silk-natural proteins. J. Mater. Chem. B 2013, 1, 2868–2873. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, S.J.; Wang, H.Y.; Wang, Y.F.; Hao, Y.W.; Zhang, J.H.; Chen, Q.D.; Zhang, Y.L.; Han, W.; Yang, B.; et al. Unraveling bright molecule-like state and dark intrinsic state in green-fluorescence graphene quantum dots via ultrafast spectroscopy. Adv. Opt. Mater. 2013, 1, 264–271. [Google Scholar] [CrossRef]
- Dang, H.; Huang, L.K.; Zhang, Y.; Wang, C.F.; Chen, S. Large-scale ultrasonic fabrication of white fluorescent carbon dots. Ind. Eng. Chem. Res. 2016, 55, 5335–5341. [Google Scholar] [CrossRef]
- Zhong, H.Z.; Zhou, Y.; Ye, M.F.; He, Y.J.; Ye, J.P.; He, C.; Yang, C.H.; Li, Y.F. Controlled synthesis and optical properties of colloidal ternary chalcogenide CuInS2 nanocrystals. Chem. Mater. 2008, 20, 6434–6443. [Google Scholar] [CrossRef]
- Bao, L.; Liu, C.; Zhang, Z.L.; Pang, D.W. Photoluminescence-tunable carbon nanodots: Surface-state energy-gap tuning. Adv. Mater. 2015, 27, 1663–1667. [Google Scholar] [CrossRef]
- Wang, D.U.; Khan, W.; Tang, Z.B.; Wang, Y.H. Applicability evaluation of a bright green emitting carbon dots in solid states for white light emitting diodes. Chem. Asian J. 2017, 13, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, Z.M.; Du, F.L. Green light–emitting diodes with high efficiency organosilane-functionalized carbon dots. Integr. Ferroelectr. 2017, 181, 70–77. [Google Scholar] [CrossRef]
- Zhai, Y.C.; Shen, F.Z.; Zhang, X.T.; Jing, P.T.; Li, D.; Yang, X.D.; Zhou, D.; Xu, X.W.; Qu, S.N. Synthesis of green emissive carbon dots@montmorillonite composites and their application for fabrication of light-emitting diodes and latent fingerprints markers. J. Colloid Interface Sci. 2019, 554, 344–352. [Google Scholar] [CrossRef]
- Boonsin, R.; Barros, A.; Donat, F.; Boyer, D.; Chadeyron, G.; Schneider, R.; Boutinaud, P.; Mahiou, R. Optical Properties and Reliability Studies of Gradient Alloyed Green Emitting (CdSe)x(ZnS)1-x and Red Emitting (CuInS2)x(ZnS)1-x Quantum Dots for White Light Emitting Diodes. ACS Photonics 2018, 5, 462–470. [Google Scholar] [CrossRef]
- Dai, P.; Cao, J.; Zhang, X.; Liu, Y. Bright and High Color Rendering White Light Emitting Diode Using Color Tunable Oxychloride and Oxyfluoride Phosphors. J. Phys. Chem. C 2016, 120, 18713–18720. [Google Scholar] [CrossRef]
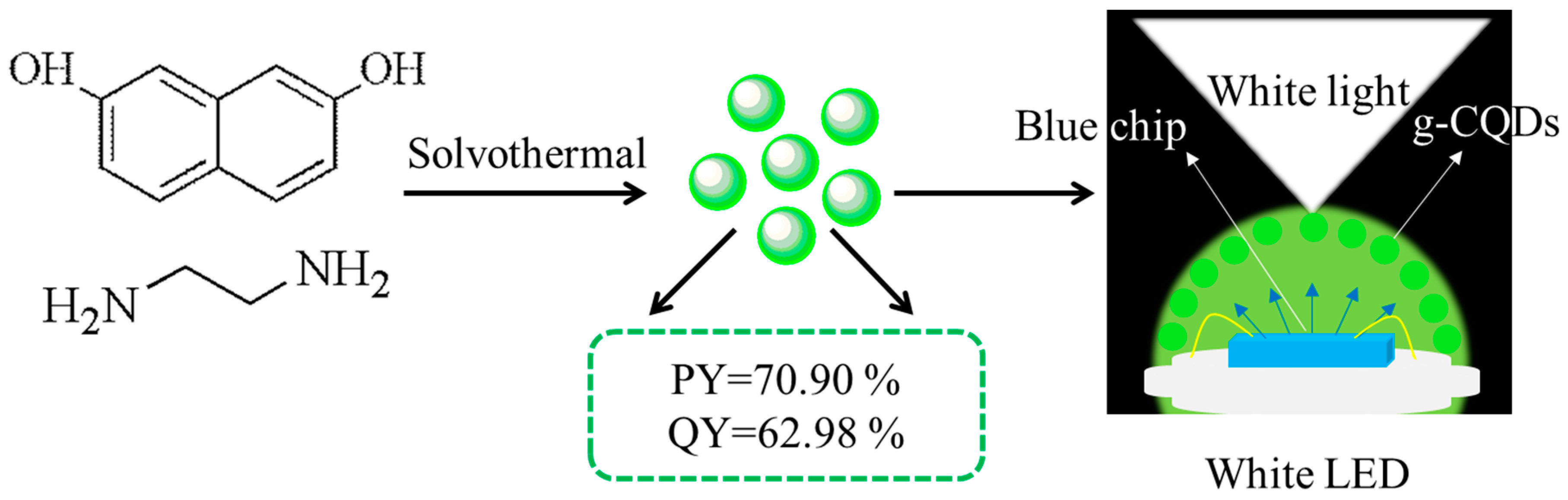

| V(EDA)/mL | Reaction Temperature/°C | Reaction Duration/h | PY/% | QY/% |
|---|---|---|---|---|
| 0 | 180 | 12 | 0.60 | 5.29 |
| 2 | 88.58 | 41.07 | ||
| 4 | 70.90 | 62.98 | ||
| 8 | 51.60 | 36.22 | ||
| 4 | 160 | 12 | 21.15 | 15.07 |
| 180 | 70.90 | 62.98 | ||
| 200 | 50.50 | 43.05 | ||
| 4 | 180 | 10 | 34.90 | 25.42 |
| 12 | 70.90 | 62.98 | ||
| 14 | 37.10 | 24.02 |
| Year | Experimental Materials | Synthesis Methods | PY/% | QY/% | References |
|---|---|---|---|---|---|
| 2018 | 3,4,9,10-tetranitroperylene | solvothermal | - | 80 | [12] |
| 2018 | citric acid, N-(2-aminoethyl)-3- aminopropyltrimethoxysilane (AEATMS) | solvothermal | 49 | [3] | |
| 2018 | urea, aniline, ethylenediamine | hydrothermal | - | 2.46 | [13] |
| 2017 | citric acid, N-(β-aminoethyl)-γ-aminopropyl trimethoxysilane (AEAPMS) | solvothermal | - | 16.4 | [5] |
| 2015 | L-valine, H3PO4 (85%) | solvothermal | - | 44.8 | [10] |
| 2014 | citric acid, urea | solvothermal | - | 36 | [9] |
| 2014 | ammonium citrate, ammonium hydroxide, H2O2 | hydrothermal | 34 | 16.5 | [11] |
| 2013 | phytic acid, ethylenediamine | microwave oven | - | 21.65 | [14] |
| 2018 | 2,7-dihydroxynaphthalene, ethylenediamine, H2O2 | solvothermal | 70.90 | 62.98 | this article |
| Sample | N | C | H | O |
|---|---|---|---|---|
| g-CQDs/% | 20.31 | 55.15 | 5.44 | 19.10 |
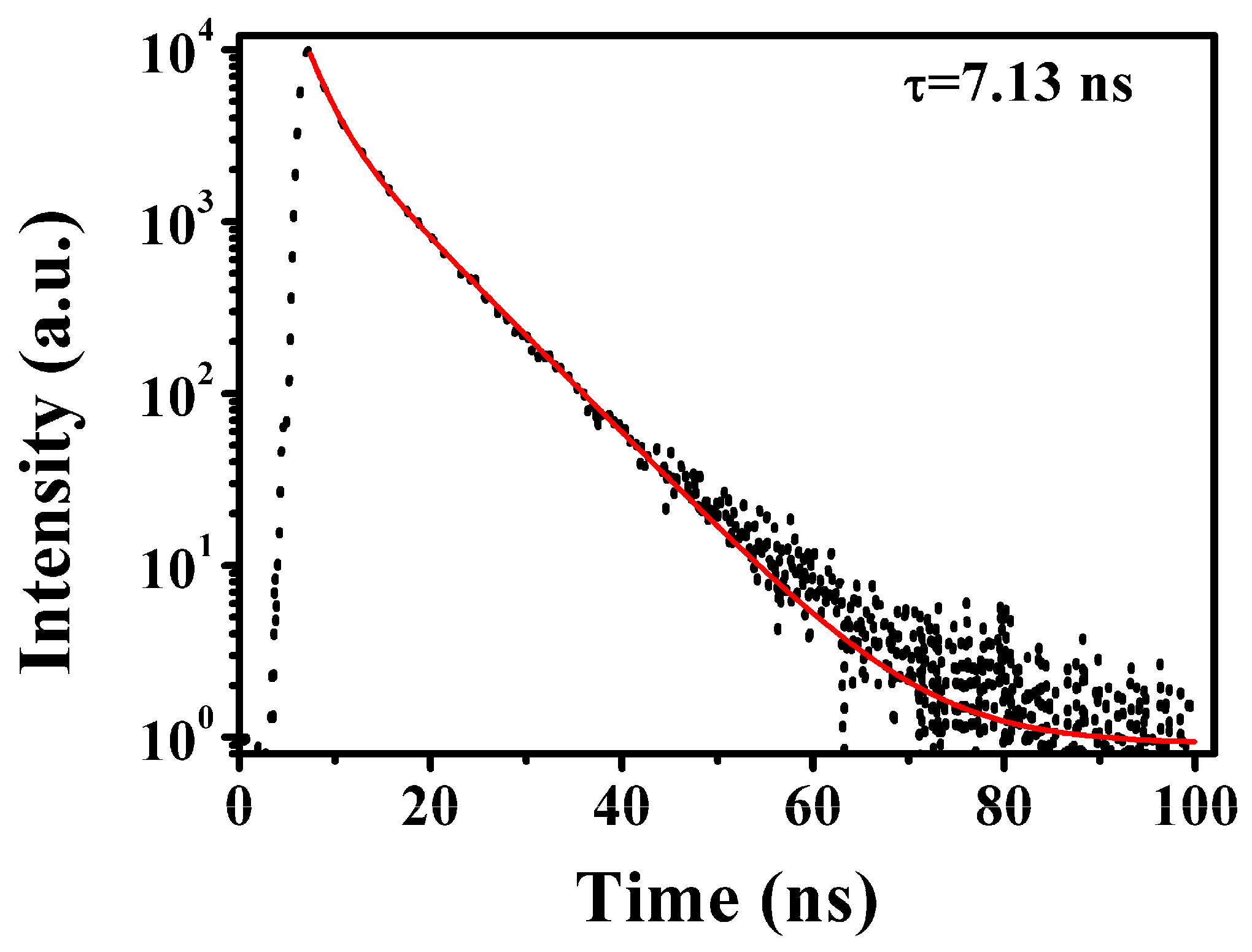
| Sample | α1 | τ1 (ns) | α2 | τ2 (ns) | χ2 | <τ> (ns) |
|---|---|---|---|---|---|---|
| g-CQDs | 0.28 | 2.18 | 0.72 | 7.68 | 1.28 | 7.13 |
| Products | PY/% | QY/% | λex/nm | λem/nm | WLEDs | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Phosphor | CIE Coordinates | CRI | CCT | ||||||
| CDs | - | 16.5 | 400 | 500 | CDs + CaAlSiN3:Eu2+ | (0.382, 0.391) | 86.9 | 3863 | [35] |
| SiCDs | - | - | 450 | 524 | SiCDs | (0.3353, 0.5647) | - | - | [36] |
| G-CDs | - | 80 | 460 | 508 | G-CDs/MTES + R-CDs/APTES | (0.4046, 0.4028) | 92.9 | 3610 | [12] |
| g-CDs | - | 14 | 405 | 522 | g-CDs@MMT composites | (0.46, 0.49) | - | 3232 | [37] |
| (CdSe)x(ZnS)1−x | - | 54 | 365 | 517 | (CdSe)x(ZnS)1−x + (CuInS2)x(ZnS)1−x | (0.437, 0.432) | 65.5 | 3220 | [38] |
| Sr7.95Si4O12Cl8: 0.05Eu2+ | - | 81 | 395 | 500–557 | Sr7.95Si4O12Cl8:0.05 Eu2+ + Ca2+/Sr2+/Mn2+ | ~ | 90.3 | ~ | [39] |
| g-CQDs | 70.90 | 62.98 | 460 | 513 | g-CQDs | (0.45, 0.37) | 87 | 2520 | this article |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Xie, Y.; Wei, Y.; Yang, Y.; Liu, X.; Chen, Y.; Xu, B. An Efficient Synthesis and Photoelectric Properties of Green Carbon Quantum Dots with High Fluorescent Quantum Yield. Nanomaterials 2020, 10, 82. https://doi.org/10.3390/nano10010082
Zheng J, Xie Y, Wei Y, Yang Y, Liu X, Chen Y, Xu B. An Efficient Synthesis and Photoelectric Properties of Green Carbon Quantum Dots with High Fluorescent Quantum Yield. Nanomaterials. 2020; 10(1):82. https://doi.org/10.3390/nano10010082
Chicago/Turabian StyleZheng, Jingxia, Yanting Xie, Yingying Wei, Yongzhen Yang, Xuguang Liu, Yongkang Chen, and Bingshe Xu. 2020. "An Efficient Synthesis and Photoelectric Properties of Green Carbon Quantum Dots with High Fluorescent Quantum Yield" Nanomaterials 10, no. 1: 82. https://doi.org/10.3390/nano10010082






