Biomimetic ZnO for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. ZnO Powder Synthesis
2.2. ZnO Characterization
2.3. Solar Cells Assembly and Performance Measurement
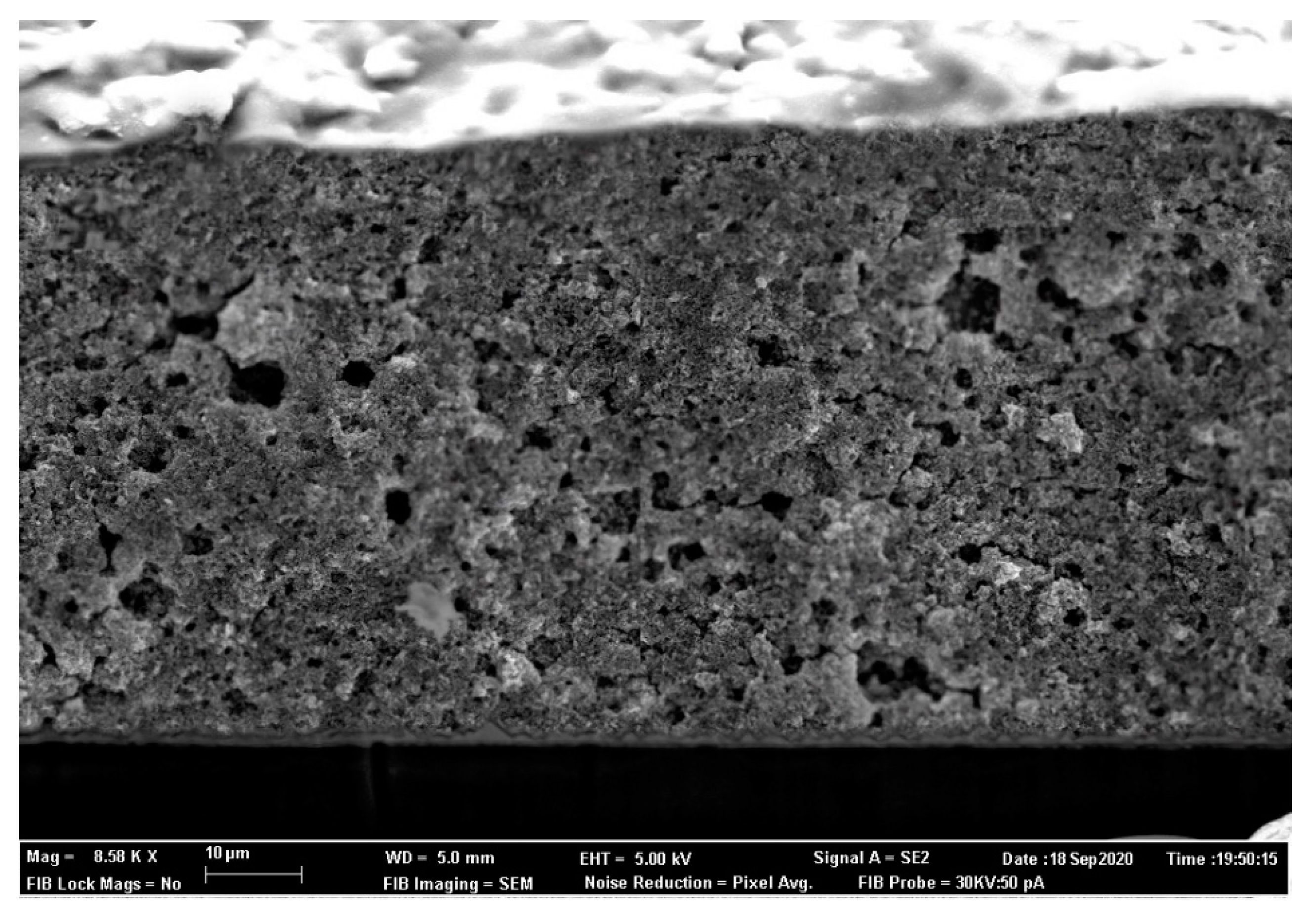
3. Results
3.1. ZnO Characterization
3.2. Solar Cells Characterization
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Sabu, U.; Tripathi, N.; Logesh, G.; Rashad, M.; Joy, M.; Balasubramanian, M. Development of biomorphic C-ZnO with in situ formation of ZnS using eggshell membrane as bio-template. Ceram. Int. 2020, 46, 22869–22875. [Google Scholar] [CrossRef]
- Camaratta, R.; Orozco-Messana, J.; Pérez Bergmann, C. Synthesis of ZnO through biomimetization of eggshell membranes using different precursors and its characterization. Ceram. Int. 2015, 41, 14826–14833. [Google Scholar] [CrossRef]
- Ingole, V.; Vuherer, T.; Maver, U.; Vinchurkar, A.; Ghule, A.; Kokol, V. Mechanical Properties and Cytotoxicity of Differently Structured Nanocellulose-hydroxyapatite Based Composites for Bone Regeneration Application. Nanomaterials 2020, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Su, H.; Zang, D.; Cao, W.; Wang, N. Biogenic Synthesis of Tubular SnO2 with Hierarchical Intertextures by an Aqueous Technique Involving Glycoprotein. Langmuir 2007, 23, 8108–8113. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Qi, L.; Ma, J. Eggshell Membrane Templating of Hierarchically Ordered Macroporous Networks Composed of TiO2 Tubes. Adv. Mater. 2002, 14, 1543–1546. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Q.; Ding, Y. Morphologies-controlled synthesis of CaWO4 crystals by a novel supramolecular template method. J. Cryst. Growth 2005, 279, 410–414. [Google Scholar] [CrossRef]
- Su, H.; Dong, Q.; Han, J.; Zang, D.; Guo, Q. Biogenic Synthesis and Photocatalysis of Pd−PdO Nanoclusters Reinforced Hierarchical TiO2 Films with Interwoven and Tubular Conformations. Biomacromolecules 2008, 9, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Moritaka, T.; Yamashita, Y.; Tojo, T.; Inada, R.; Sakurai, Y. Characterization of Sn4P3–Carbon Composite Films for Lithium-Ion Battery Anode Fabricated by Aerosol Deposition. Nanomaterials 2019, 9, 1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhu, X.; Li, S.; Chen, M.; Lu, H.; Yang, Y. Ag@SiO2 Core-shell Nanoparticles Embedded in a TiO2 Mesoporous Layer Substantially Improve the Performance of Perovskite Solar Cells. Nanomaterials 2018, 8, 701. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Domínguez, D.; Pico, M.; Álvarez-Serrano, I.; López, M. New Fe2O3-Clay@C Nanocomposite Anodes for Li-Ion Batteries Obtained by Facile Hydrothermal Processes. Nanomaterials 2018, 8, 808. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, F.I.; Buraidah, M.H.; Arof, A.K.; Mellander, B.-E.; Noor, I.M. Impact of tetrabutylammonium, iodide and triiodide ions conductivity in polyacrylonitrile based electrolyte on DSSC performance. Solar Energy 2020, 196, 379–388. [Google Scholar] [CrossRef]
- Ian, Y.; Bu, Y. Improving the performance of dye-sensitized solar cells by incoporating a novel ZnO:Al charge injection layer. Optik 2020, 205, 164242. [Google Scholar] [CrossRef]
- Prochowicz, D.; Mahdi, M.; Małgorzata, T.; Wolska-Pietkiewicz, M.J.; Trivedi, S.; Kumar, M.; Zakeeruddin, S.M.; Lewiński, J.; Graetzel, M.; Yadav, P. Suppressing recombination in perovskite solar cells via surface engineering of TiO2 ETL. Solar Energy 2020, 197, 50–57. [Google Scholar] [CrossRef]
- Seow, Z.L.S.; Wong, A.S.W.; Thavasi, V.; Jose, R.; Ramakrishna, S.; Ho, G.W. Controlled synthesis and application of ZnO nanoparticles, nanorods and nanospheres in dye-sensitized solar cells. Nanotechnology 2008, 20, 045604. [Google Scholar] [CrossRef] [PubMed]
- Nakade, S.; Saito, Y.; Kubo, W.; Kitamura, T.; Wada, Y.; Yanagida, S. Influence of TiO2 Nanoparticle Size on Electron Diffusion and Recombination in Dye-Sensitized TiO2 Solar Cells. J. Phys. Chem. B 2003, 107, 8607–8611. [Google Scholar] [CrossRef]
- Song, D.; Kim, H.; Suh, J.; Jun, B.; Rho, W. Multi-Shaped Ag Nanoparticles in the Plasmonic Layer of Dye-Sensitized Solar Cells for Increased Power Conversion Efficiency. Nanomaterials 2017, 7, 136. [Google Scholar] [CrossRef] [PubMed]
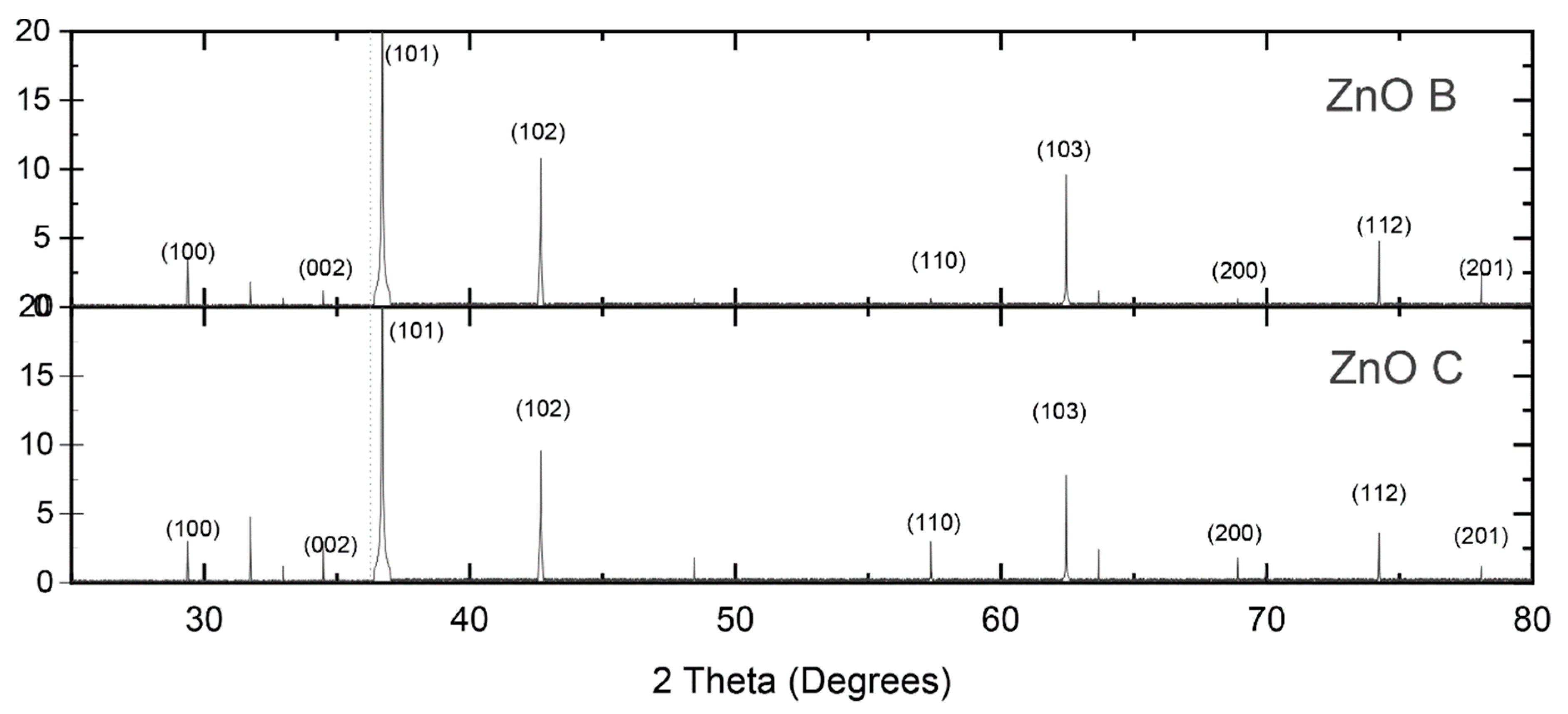
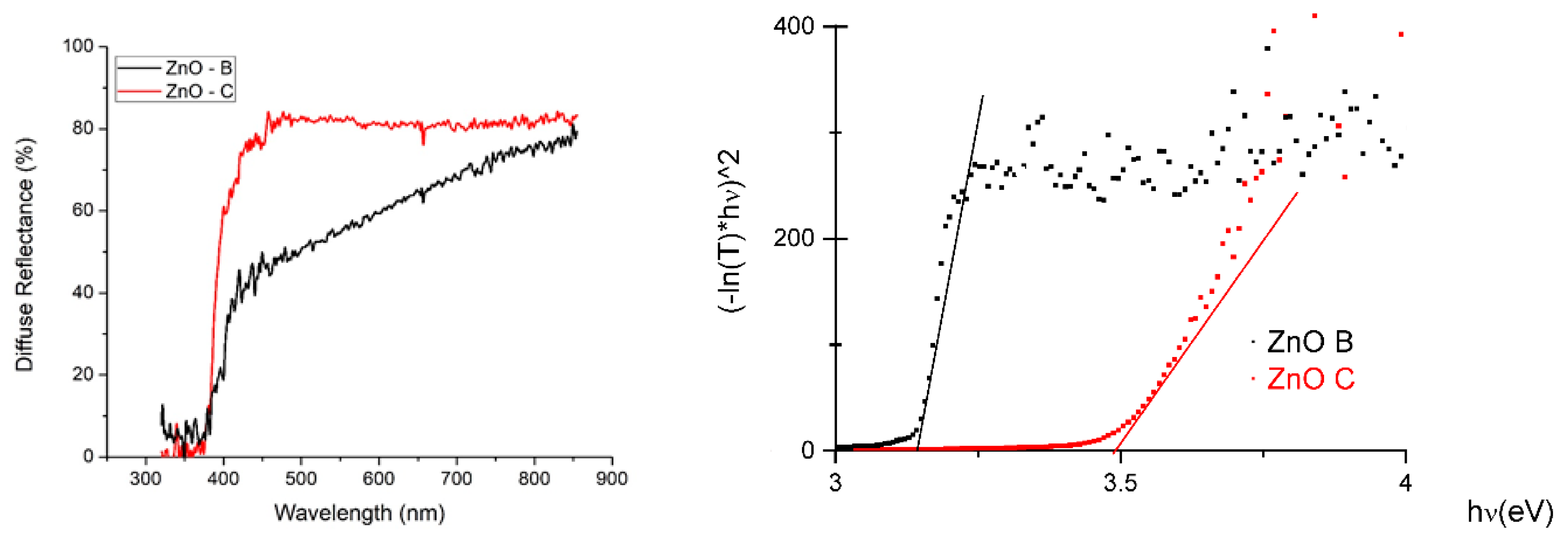
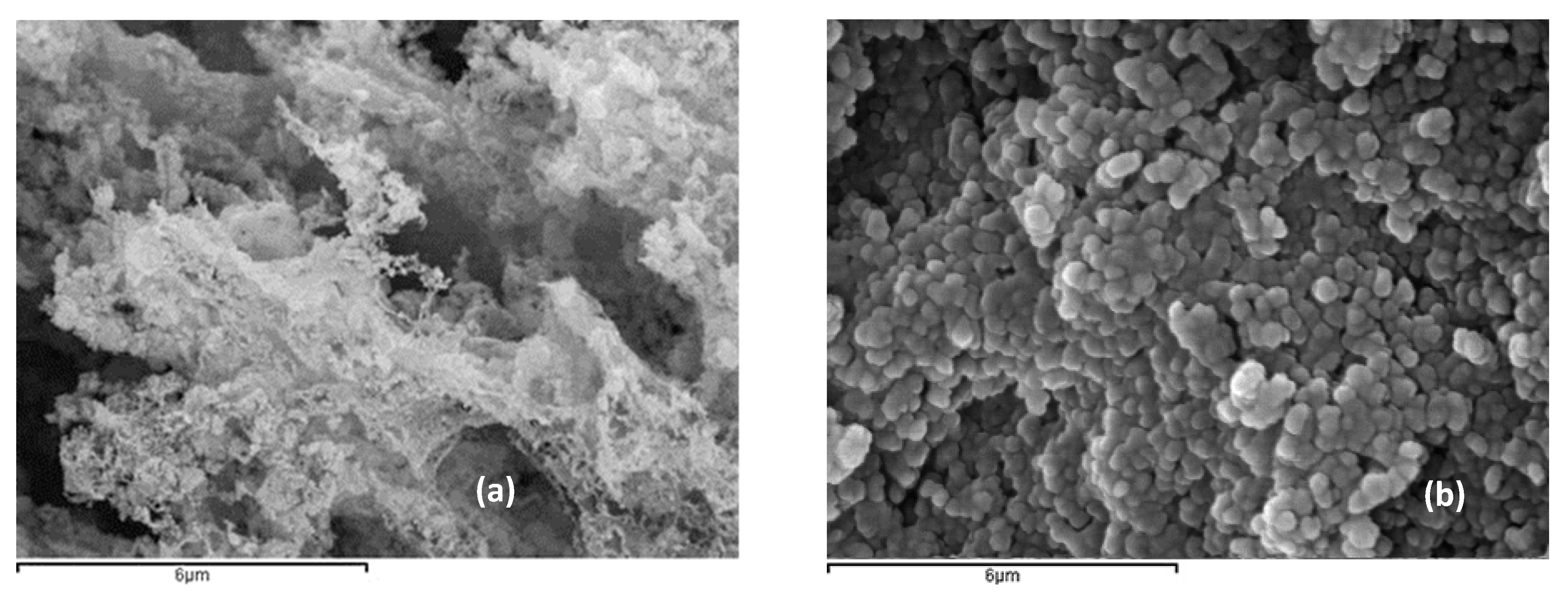
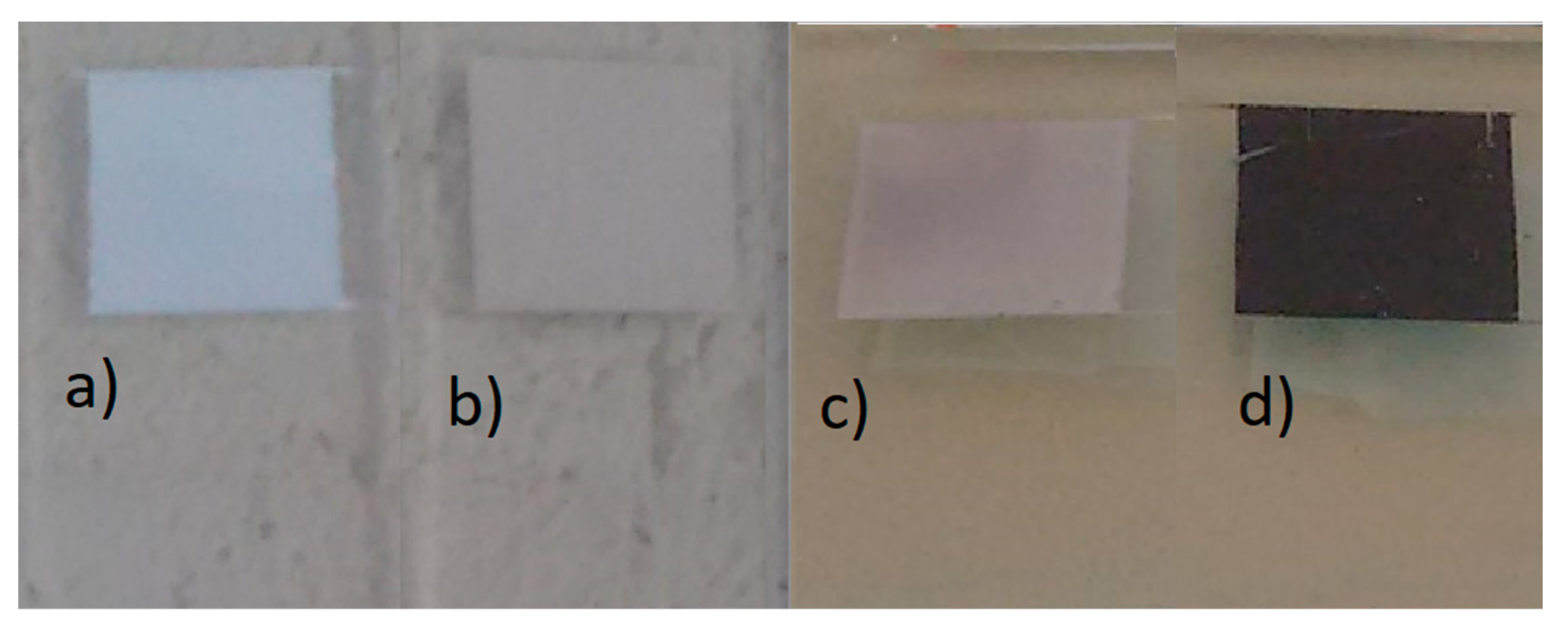

| ZnO | Biomimetic | Commercial |
|---|---|---|
| Naming convention | ZnO-B | ZnO-C |
| ZnO type | ZnO-B | ZnO-C |
|---|---|---|
| Crystallite size (nm) | 10.2 | 75.5 |
| Surface area (m2/g) | 20.6 | 8.9 |
| Bandgap (eV) | 3.18 | 3.49 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Messana, J. Biomimetic ZnO for Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 1907. https://doi.org/10.3390/nano10101907
Orozco-Messana J. Biomimetic ZnO for Dye-Sensitized Solar Cells. Nanomaterials. 2020; 10(10):1907. https://doi.org/10.3390/nano10101907
Chicago/Turabian StyleOrozco-Messana, Javier. 2020. "Biomimetic ZnO for Dye-Sensitized Solar Cells" Nanomaterials 10, no. 10: 1907. https://doi.org/10.3390/nano10101907






