Nitrogen, Phosphorus and Sulfur Co-Doped Pyrolyzed Bacterial Cellulose Nanofibers for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Heteroatom-Doped PBC
2.3. Characterization of Materials
2.4. Electrochemical Measurements
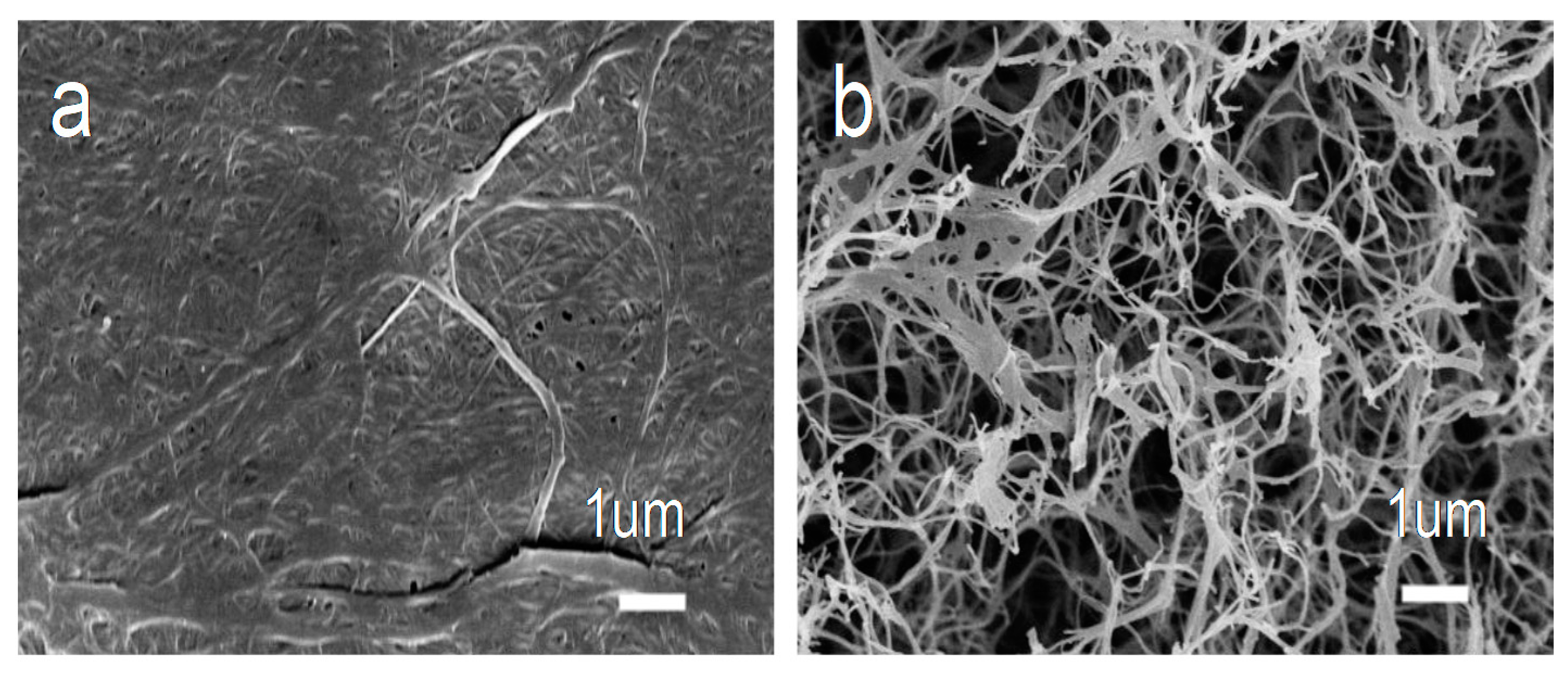
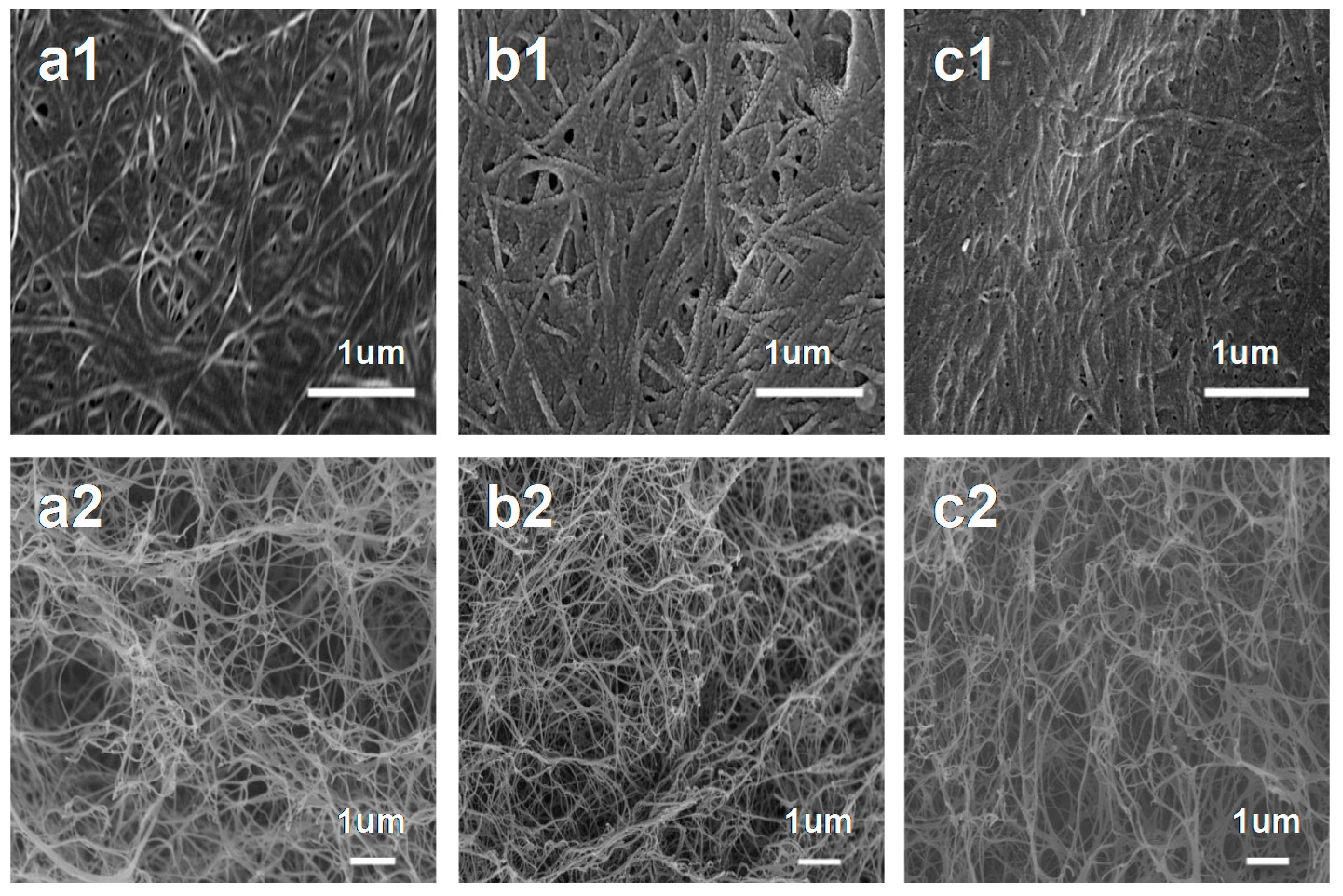
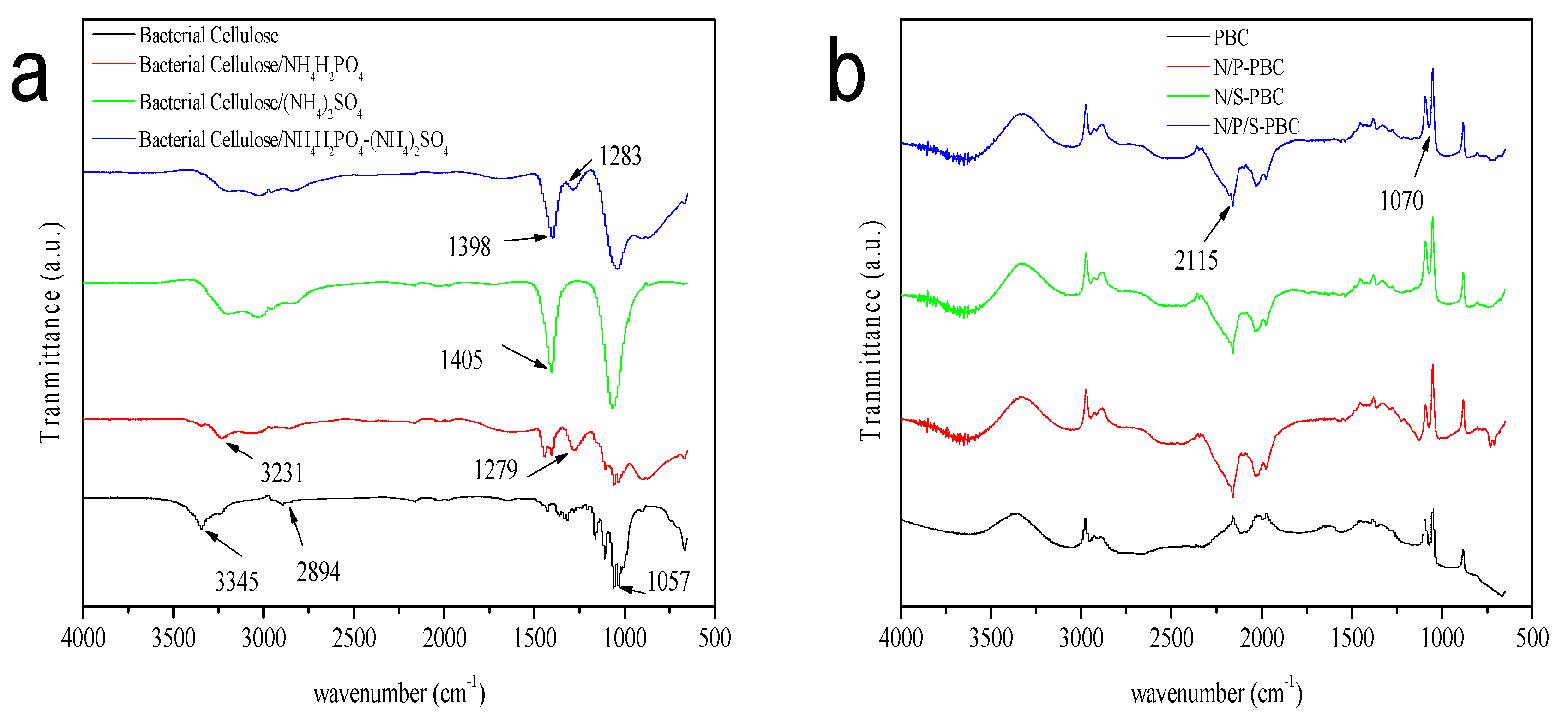
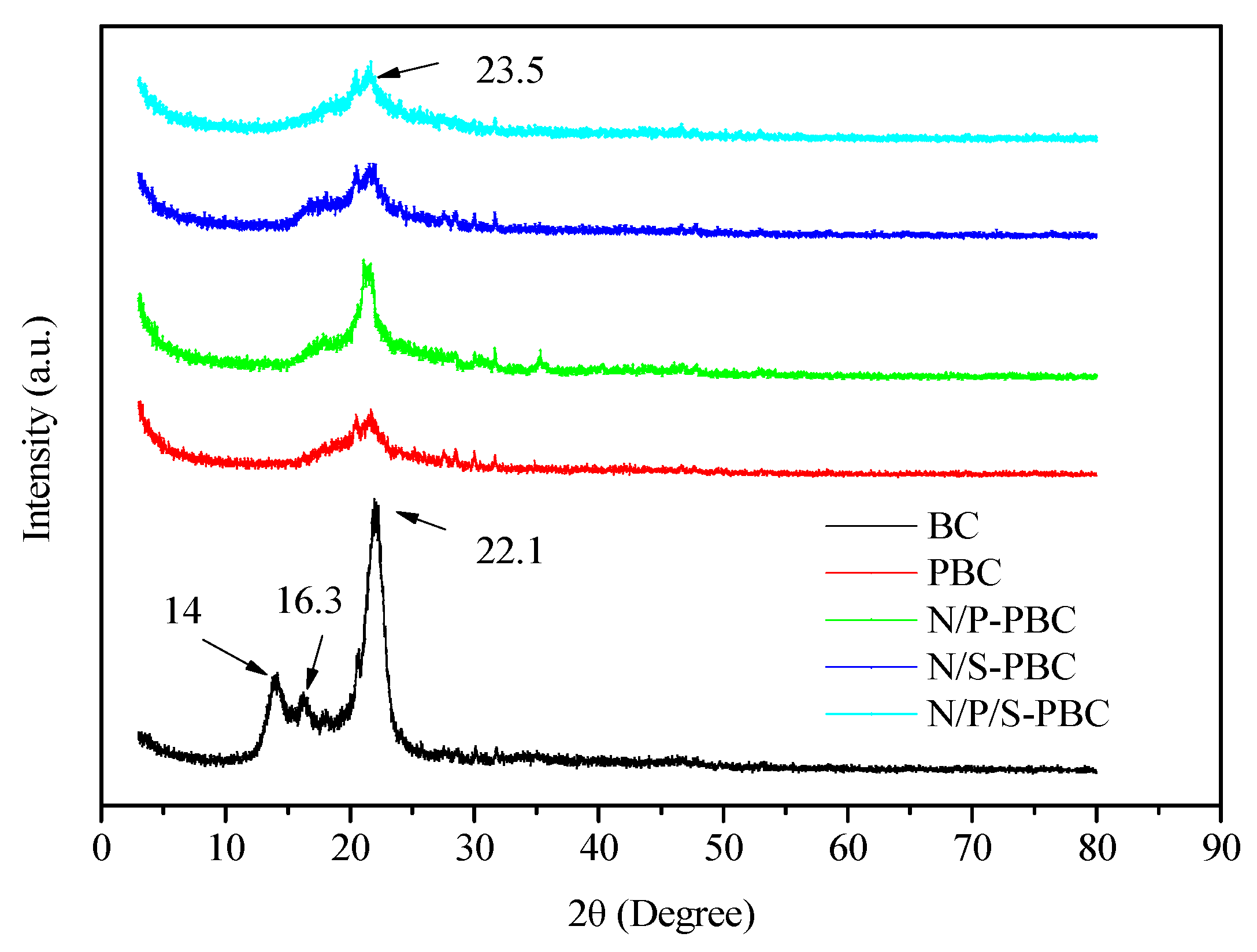
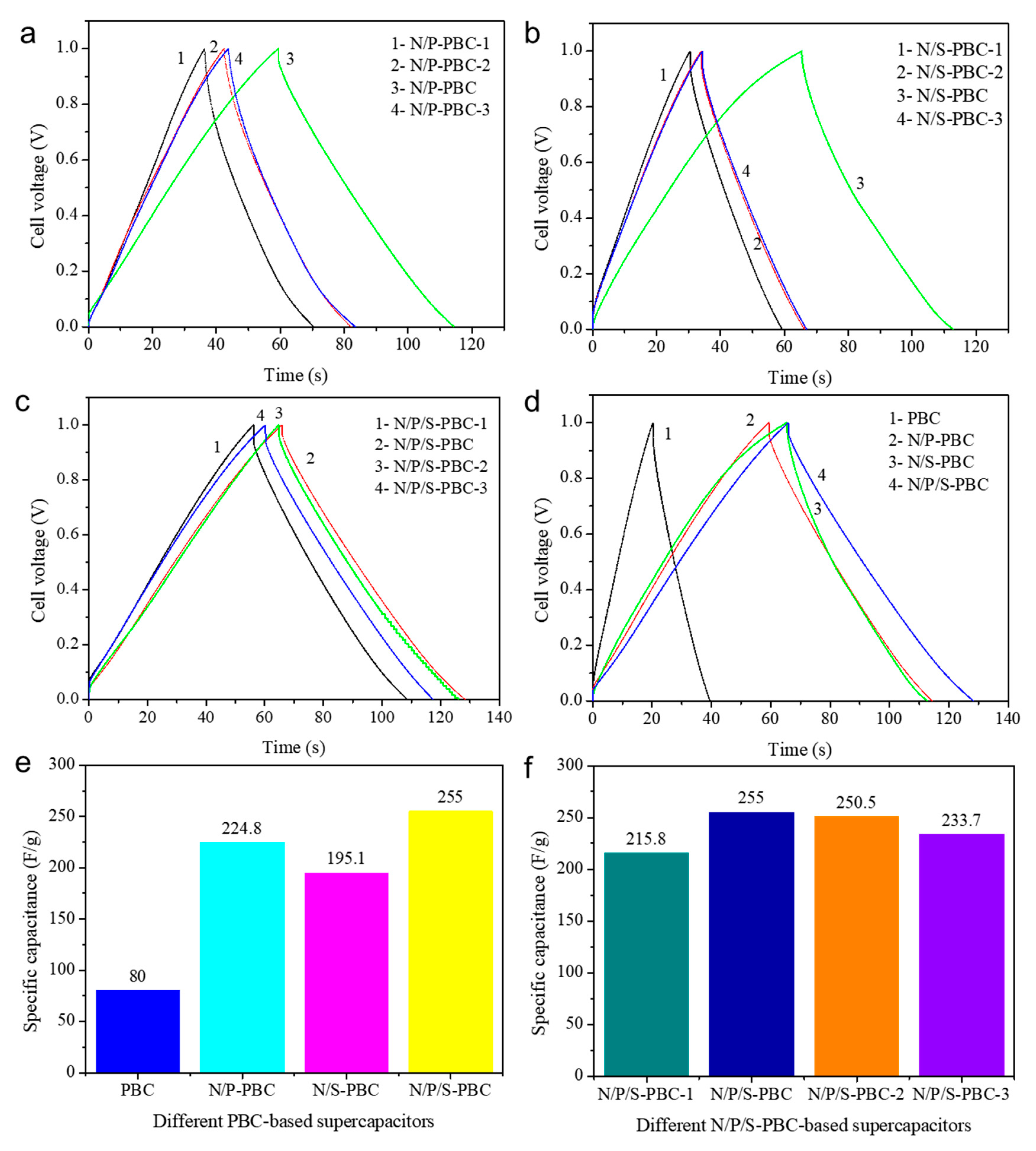
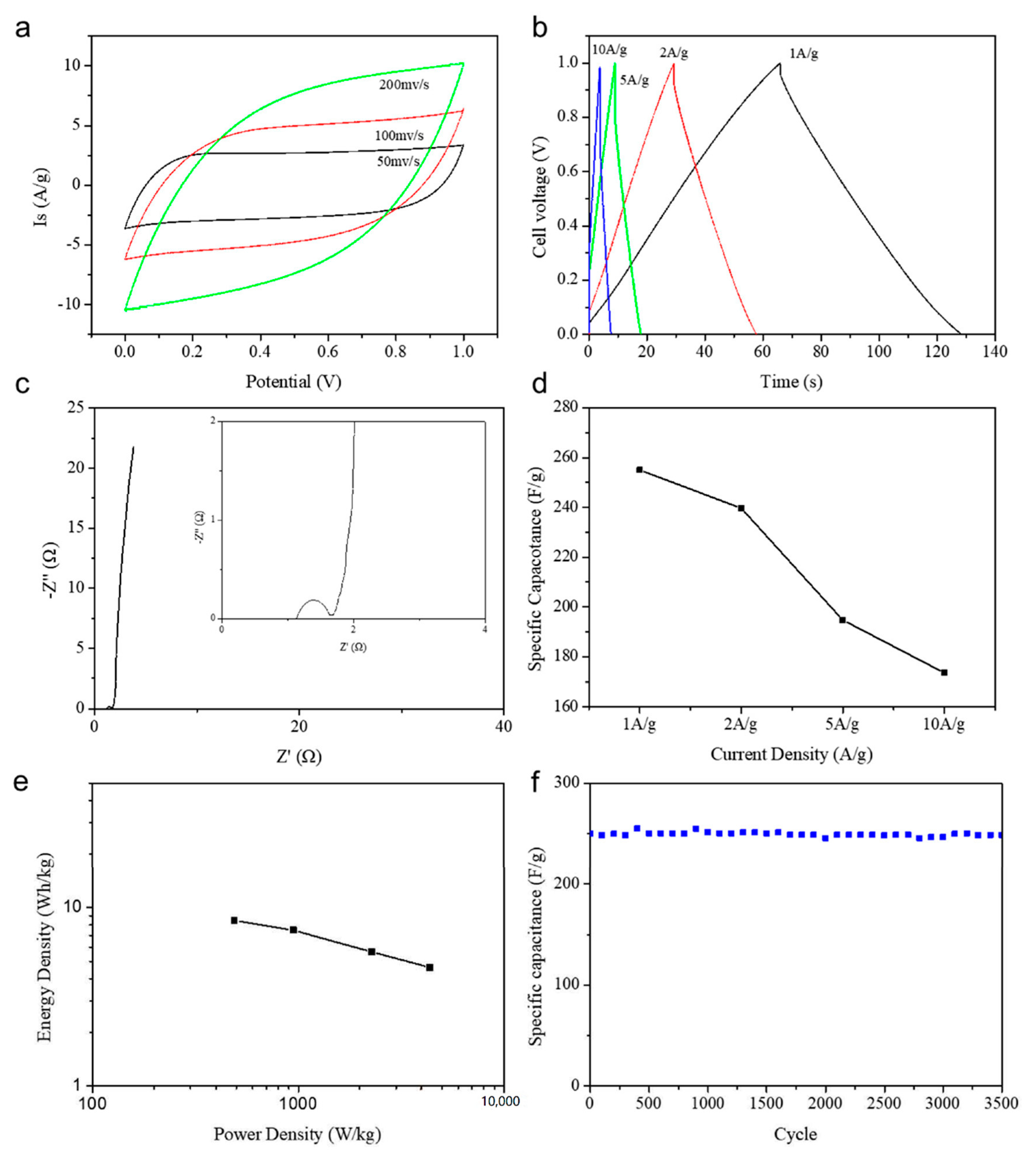
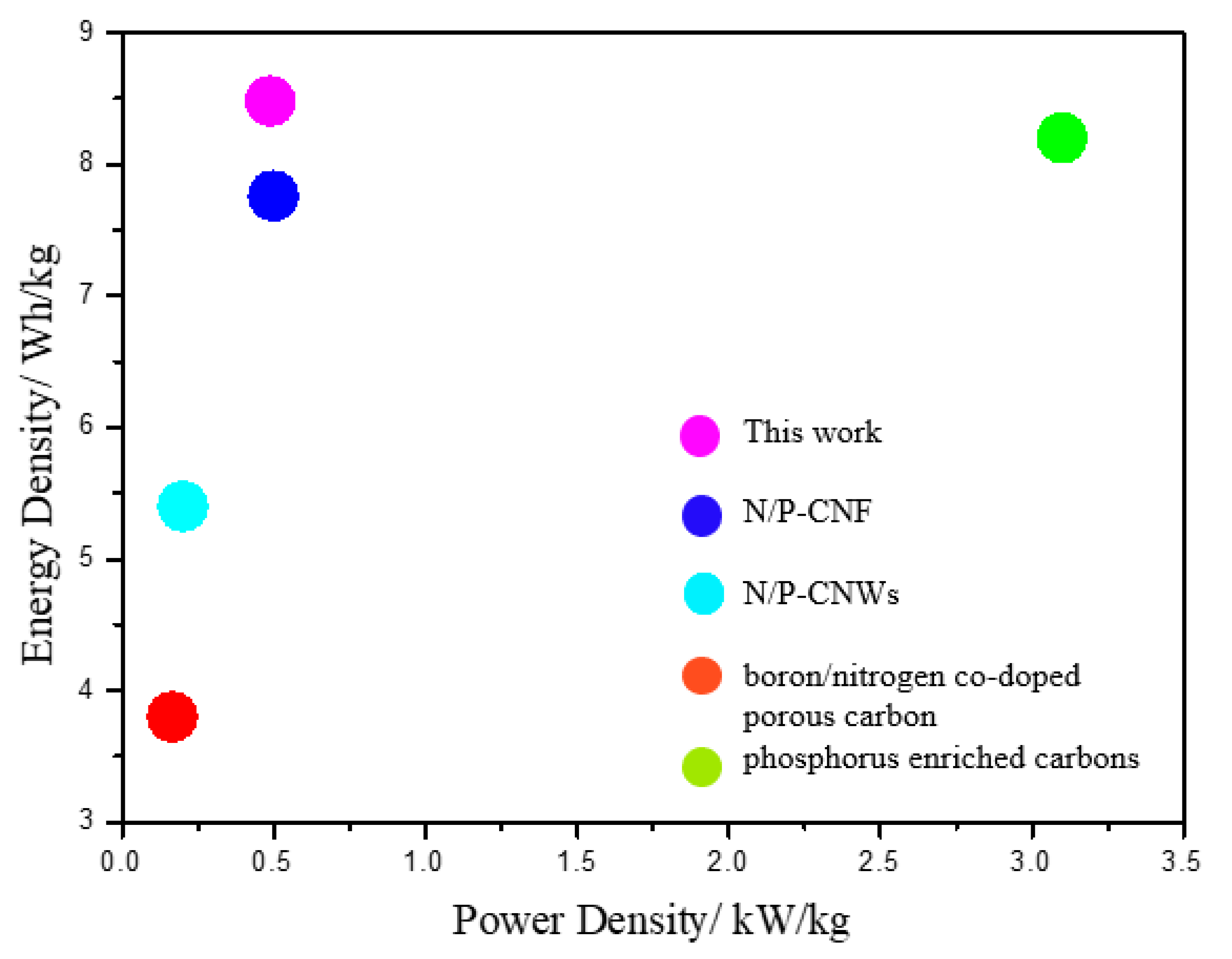
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.; Lee, D.; Lee, Y.; Chen, W.; Lee, S. Nanocellulose for Energy Storage Systems: Beyond the Limits of Synthetic Materials. Adv. Mater. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Lin, W.; Shao, X.; Hu, Z.; Li, R.; Yuan, D. High performance supercapacitor based on Ni3S2/carbon nanofibers and carbon nanofibers electrodes derived from bacterial cellulose. J. Power Sources 2014, 272, 137–143. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Zhai, X.; Xie, M.; Sun, Y.; Kang, H.; Tian, Q.; Qiu, H. Hierarchical porous carbon microrods derived from albizia flowers for high performance supercapacitors. Carbon 2019, 147, 242–251. [Google Scholar] [CrossRef]
- Song, Z.; Duan, H.; Zhu, D.; Lv, Y.; Xiong, W.; Cao, T.; Li, L.; Liu, M.; Gan, L. Ternary-doped carbon electrodes for advanced aqueous solid-state supercapacitors based on a “water-in-salt” gel electrolyte. J. Mater. Chem. A 2019, 7, 15801–15811. [Google Scholar] [CrossRef]
- Peng, H.; Yao, B.; Wei, X.; Liu, T.; Kou, T.; Xiao, P.; Zhang, Y.; Li, Y. Pore and Heteroatom Engineered Carbon Foams for Supercapacitors. Adv. Energy Mater. 2019, 9, 1803665. [Google Scholar] [CrossRef]
- Chen, H.; Lu, X.; Wang, H.; Sui, D.; Meng, F.; Qi, W. Controllable fabrication of nitrogen-doped porous nanocarbons for high-performance supercapacitors via supramolecular modulation strategy. J. Energy Chem. 2020, 49, 348–357. [Google Scholar] [CrossRef]
- Strauss, V.; Marsh, K.; Kowal, M.D.; El Kady, M.; Kaner, R.B. A Simple Route to Porous Graphene from Carbon Nanodots for Supercapacitor Applications. Adv. Mater. 2018, 30, 1704449. [Google Scholar] [CrossRef]
- Kirubasankar, B.; Murugadoss, V.; Lin, J.; Ding, T.; Dong, M.; Liu, H.; Zhang, J.; Li, T.; Wang, N.; Guo, Z.; et al. In situ grown nickel selenide on graphene nanohybrid electrodes for high energy density asymmetric supercapacitors. Nanoscale 2018, 10, 20414–20425. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, H.; Wang, M.; Yang, M.; Guo, Q.; Wan, L.; Xia, H.; Yu, Y. High Energy and High Power Lithium-Ion Capacitors Based on Boron and Nitrogen Dual-Doped 3D Carbon Nanofibers as Both Cathode and Anode. Adv. Energy Mater. 2017, 7, 1701336. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Y.; Fan, H.; Liu, M.; Zhuo, O.; Wang, X.; Wu, Q.; Yang, L.; Ma, Y.; Hu, Z. Porous 3D Few-Layer Graphene-like Carbon for Ultrahigh-Power Supercapacitors with Well-Defined Structure-Performance Relationship. Adv. Mater. 2017, 29, 1604569. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W.; et al. High-Performance Energy Storage and Conversion Materials Derived from a Single Metal—Organic Framework/Graphene Aerogel Composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Prehal, C.; Koczwara, C.; Jäckel, N.; Schreiber, A.; Burian, M.; Amenitsch, H.; Hartmann, M.A.; Presser, V.; Paris, O. Quantification of ion confinement and desolvation in nanoporous carbon supercapacitors with modelling and in situ X-ray scattering. Nat. Energy 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, H.; Chen, L.; Hu, B.; Yu, S. Bacterial Cellulose: A Robust Platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Acc. Chem. Res. 2016, 49, 96–105. [Google Scholar] [CrossRef]
- Shu, Y.; Bai, Q.; Fu, G.; Xiong, Q.; Li, C.; Ding, H.; Shen, Y.; Uyama, H. Hierarchical porous carbons from polysaccharides carboxymethyl cellulose, bacterial cellulose, and citric acid for supercapacitor. Carbohydr. Polym. 2020, 227, 115346. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Yu, Y. A facile strategy toward sodium-ion batteries with ultra-long cycle life and high initial Coulombic Efficiency: Free-standing porous carbon nanofiber film derived from bacterial cellulose. Energy Storage Mater. 2019, 22, 105–112. [Google Scholar] [CrossRef]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Müllen, K. Efficient Synthesis of Heteroatom (N or S)-Doped Graphene Based on Ultrathin Graphene Oxide-Porous Silica Sheets for Oxygen Reduction Reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Puziy, A.M.; Poddubnaya, O.I.; Suárez-García, F.; Tascón, J.M.D.; Lu, G.Q. Highly Stable Performance of Supercapacitors from Phosphorus-Enriched Carbons. J. Am. Chem. Soc. 2009, 131, 5026–5027. [Google Scholar] [CrossRef]
- Miao, L.; Duan, H.; Liu, M.; Lu, W.; Zhu, D.; Chen, T.; Li, L.; Gan, L. Poly(ionic liquid)-derived, N, S-codoped ultramicroporous carbon nanoparticles for supercapacitors. Chem. Eng. J. 2017, 317, 651–659. [Google Scholar] [CrossRef]
- Jin, H.; Feng, X.; Li, J.; Li, M.; Xia, Y.; Yuan, Y.; Yang, C.; Dai, B.; Lin, Z.; Wang, J.; et al. Heteroatom-Doped Porous Carbon Materials with Unprecedented High Volumetric Capacitive Performance. Angew. Chem. Int. Ed. 2019, 58, 2397–2401. [Google Scholar] [CrossRef]
- Guo, P.; Xiao, F.; Liu, Q.; Liu, H.; Guo, Y.; Gong, J.R.; Wang, S.; Liu, Y. One-Pot Microbial Method to Synthesize Dual-Doped Graphene and Its Use as High-Performance Electrocatalyst. Sci. Rep. 2013, 3, 3499. [Google Scholar] [CrossRef] [Green Version]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Park, S.H.; Woo, S.I. B, N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J. Mater. Chem. A 2013, 1, 3694. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Liang, H.; Guan, Q.; Zhu, Z.; Song, L.; Yao, H.; Lei, X.; Yu, S. Highly conductive and stretchable conductors fabricated from bacterial cellulose. NPG Asia Mater. 2012, 4, e19. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Huang, Z.; Liang, H.; Gao, H.; Yu, S. Three-Dimensional Heteroatom-Doped Carbon Nanofiber Networks Derived from Bacterial Cellulose for Supercapacitors. Adv. Funct. Mater. 2014, 24, 5104–5111. [Google Scholar] [CrossRef]
- Wu, Z.; Li, C.; Liang, H.; Chen, J.; Yu, S. Ultralight, Flexible, and Fire-Resistant Carbon Nanofiber Aerogels from Bacterial Cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef]
- Wu, Z.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.; Müllen, K. Three-Dimensional Nitrogen and Boron Co-doped Graphene for High-Performance All-Solid-State Supercapacitors. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Chen, Z.; Lu, G.Q.; Cheng, H. Synthesis and Electrochemical Property of Boron-Doped Mesoporous Carbon in Supercapacitor. Chem. Mater. 2008, 20, 7195–7200. [Google Scholar] [CrossRef]
- Yuan, D.; Huang, X.; Yan, J.; Yu, W.; Meng, H.; Rong, J. Porous Carbon Nanofibers Derived from Bacterial Cellulose for Sustainable Energy Storage. Sci. Adv. Mater. 2013, 5, 1694–1700. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Zhang, B.; Chen, C.; Wang, A.; Zhang, T.; Su, D.S. Dual-heteroatom-modified ordered mesoporous carbon: Hydrothermal functionalization, structure, and its electrochemical performance. J. Mater. Chem. 2012, 22, 4963. [Google Scholar] [CrossRef] [Green Version]
- Paraknowitsch, J.P.; Zhang, Y.; Wienert, B.; Thomas, A. Nitrogen- and phosphorus-co-doped carbons with tunable enhanced surface areas promoted by the doping additives. Chem. Commun. 2013, 49, 1208. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.A.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Su, F.; Poh, C.K.; Chen, J.S.; Xu, G.; Wang, D.; Li, Q.; Lin, J.; Lou, X.W. Nitrogen-containing microporous carbon nanospheres with improved capacitive properties. Energy Environ. Sci. 2011, 4, 717–724. [Google Scholar] [CrossRef]
- Hu, Z.; Li, S.; Cheng, P.; Yu, W.; Li, R.; Shao, X.; Lin, W.; Yuan, D. N,P-co-doped carbon nanowires prepared from bacterial cellulose for supercapacitor. J. Mater. Sci. 2016, 51, 2627–2633. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Liang, H.; Kong, M.; Guan, Q.; Chen, P.; Wu, Z.; Yu, S. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef]
- Guo, H.; Gao, Q. Boron and nitrogen co-doped porous carbon and its enhanced properties as supercapacitor. J. Power Sources 2009, 186, 551–556. [Google Scholar] [CrossRef]


| Sample Codes | Concentration of NH4H2PO4 Solution (mol/L) | Concentration of (NH4)2SO4 Solution (mol/L) |
|---|---|---|
| N/P-PBC-1 | 0.02 | 0 |
| N/P-PBC-2 | 0.05 | 0 |
| N/P-PBC | 0.1 | 0 |
| N/P-PBC-3 | 0.2 | 0 |
| N/S-PBC-1 | 0 | 0.005 |
| N/S-PBC-2 | 0 | 0.01 |
| N/S-PBC | 0 | 0.05 |
| N/S-PBC-3 | 0 | 0.1 |
| N/P/S-PBC-1 | 0.05 | 0.025 |
| N/P/S-PBC | 0.1 | 0.025 |
| N/P/S-PBC-2 | 0.05 | 0.05 |
| N/P/S-PBC-3 | 0.1 | 0.05 |
| Sample | Area Percentage (%) | ||||||
|---|---|---|---|---|---|---|---|
| N-5 | N-6 | P-C | P-N | P-O | S2p3/2 | S2p1/2 | |
| N/P/S-PBC | 89.18 | 10.82 | 23.37 | 58.20 | 18.43 | 94.17 | 5.827 |
| Sample | N1s | P2p | S2p | O1s |
|---|---|---|---|---|
| PBC | 1.4 | 0 | 0 | 1.85 |
| N/P-PBC | 3.67 | 2.41 | 0 | 3.57 |
| N/S-PBC | 3.27 | 0 | 1.34 | 2.6 |
| N/P/S-PBC-1 | 3.64 | 0.96 | 0.71 | 2.82 |
| N/P/S-PBC | 3.90 | 1.22 | 0.62 | 3.97 |
| N/P/S-PBC-2 | 3.83 | 0.82 | 1.14 | 3.61 |
| N/P/S-PBC-3 | 4.18 | 1.34 | 1.20 | 3.84 |
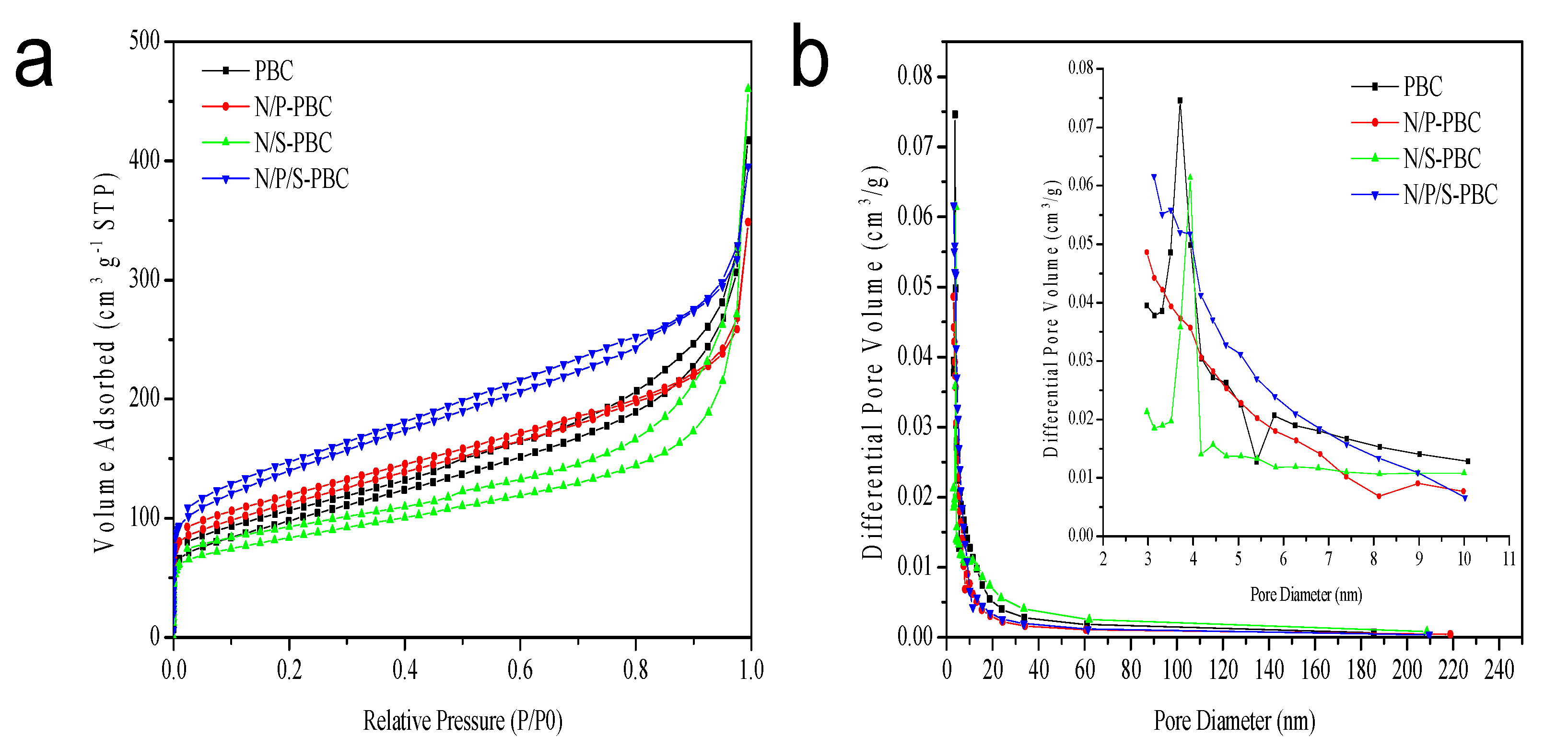
| Sample | SBET (m2/g) | VT (cm3/g) | dM (nm) |
|---|---|---|---|
| PBC | 350 | 0.65 | 3.71 |
| N/P-PBC | 397 | 0.54 | 2.97 |
| N/S-PBC | 296 | 0.71 | 3.93 |
| N/P/S-PBC | 498 | 0.61 | 3.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, Y.; Xia, W.; Gong, J.; Jia, S.; Zhang, J. Nitrogen, Phosphorus and Sulfur Co-Doped Pyrolyzed Bacterial Cellulose Nanofibers for Supercapacitors. Nanomaterials 2020, 10, 1912. https://doi.org/10.3390/nano10101912
Li Z, Wang Y, Xia W, Gong J, Jia S, Zhang J. Nitrogen, Phosphorus and Sulfur Co-Doped Pyrolyzed Bacterial Cellulose Nanofibers for Supercapacitors. Nanomaterials. 2020; 10(10):1912. https://doi.org/10.3390/nano10101912
Chicago/Turabian StyleLi, Zheng, Yaogang Wang, Wen Xia, Jixian Gong, Shiru Jia, and Jianfei Zhang. 2020. "Nitrogen, Phosphorus and Sulfur Co-Doped Pyrolyzed Bacterial Cellulose Nanofibers for Supercapacitors" Nanomaterials 10, no. 10: 1912. https://doi.org/10.3390/nano10101912
APA StyleLi, Z., Wang, Y., Xia, W., Gong, J., Jia, S., & Zhang, J. (2020). Nitrogen, Phosphorus and Sulfur Co-Doped Pyrolyzed Bacterial Cellulose Nanofibers for Supercapacitors. Nanomaterials, 10(10), 1912. https://doi.org/10.3390/nano10101912





