Abstract
Tm3+ has obvious emission characteristics in the near-infrared band. Thulium ions combined with different organic ligands lead to different fluorescent properties. In the near-infrared region, Tm3+ is a down-conversion fluorescent material that is unstable under high temperature and acidic conditions. Moreover, in those complex environments, the fluorescence from Tm3+ complex is usually degraded. In this work, two kinds of near-infrared fluorescent complexes, Tm(TTA)3phen and Tm(DBM)3phen, were prepared, and the intensity of their fluorescence is compared. The fluorescence intensity at 802 nm is greatly improved compared with Tm(TTA)3phen, and the intensity of the emission at 1235 nm and 1400–1500 nm is also enhanced. Moreover, the emission lifetime of SiO2-Tm(TTA)3phen is 50.38 μs. Tm(TTA)3phen complex and SiO2-Tm(TTA)3phen hybrid materials have better fluorescence than Tm(DBM)3phen and SiO2-Tm(DBM)3phen. Therefore, HTTA is a better choice of organic ligands for Tm3+. The NIR-fluorescent hybrid materials prepared have stronger fluorescence after combining with nano-SiO2compared with pure Tm3+ complexes, and have stronger structural stability compared with pure nano-SiO2.
1. Introduction
Rare earth ions complexes have special physical and chemical properties, which have attracted much attention thanks to their high purity, narrow emissivity, and high internal quantum efficiency. The fluorescent properties and mechanism of rare earth ion complexes have been studied for a long time. As early as 1942, Weissman investigated the fluorescence effect of β-diketone ligands on europium ions by ultraviolet radiation, and further studied the fluorescence efficiency of the complex [1,2,3,4]. At present, many organic ligands with large absorption cross sections in ultraviolet region have been found. The energy of the excited state can be transferred to the rare earth ions through the energy transfer inside these organic ligands, so the fluorescence intensity of rare earth ions can be greatly improved. Therefore, the rare earth ions fluorescent complexes formed by the combination of rare earth and organic ligand have excellent fluorescence [5,6,7]. However, the fluorescence properties of rare earth complexes can be damaged in complex environments. In the preparation of many fluorescent materials, rare earth ions cannot be prepared at a high temperature, and the solution concentration should be controlled. Owing to the large amount of coordination with rare earth ions, complexes are easy to coordinate with solution molecules, resulting in fluorescence quenching, and a decrease of the thermal stability. Therefore, high transparency, good rigidity, and three-dimensional network void structure of nano-SiO2 is chosen as the matrix, which can not only protect the stability of rare earth ions complexes, but also keep or increase the fluorescence of hybrid materials.
Therefore, the hybrid material prepared by the rare earth complex and nano-SiO2 is a promising strategy to protect the rare earth complex, improve their thermal stability, and to be used to prepare durable functional materials [6]. In order to be used in different fields, there has been a wealth of research on controlling the size of nano-SiO2. Rao et al. prepared nano-SiO2 with the size of 20–460 nm by the simple sol–gel method [8]. Rahman et al. found that, in the presence of a small amount of NH4Br, a single size of 20–34 nm could be synthesized [9]. Kim et al. reported that the introduction of dielectric could reduce the size of nano-SiO2 to 17.5 nm [10]. However, it is difficult to give consideration to both morphology and fluorescence because of the special amorphous structure of nano-SiO2, so there are few reports on the rare earth doped silica materials with special morphology [11].
By introducing different templates into the sol–gel method, the micro-morphology of SiO2 can be controlled. Among them, one template mainly includes surfactants, organic acids, and gel factors [12,13]. Another template mainly includes porous alumina film and insoluble helical polymer. For example, using dodecyl trimethylammonium bromide (CTAB) as a template, spherical and short rod-shaped nano-SiO2 materials were obtained by adjusting the amount of water. Moreover, different tartaric acid derivatives were used as templates to synthesize nano-SiO2 hollow microspheres, SiO2 nanoribbons, and SiO2 nanotubes [14,15,16]. Compared with previous reports, in order to reduce the influence of H2O on the fluorescence of Tm(TTA/DBM)3phen, we have improved the experimental method for preparing nano-SiO2, and changed the size of nano-SiO2 by adjusting the dosage of NH3·H2O.
Here, we report the synthesis of SiO2-Tm3+(SiO2-Tm(TTA)3phen and SiO2-Tm(DBM)3phen) hybrid materials via an improved method, which not only embeds Tm3+ complexes inside nano-SiO2, but also embeds a part of Tm3+ complexes on the surface of nano-SiO2. Thus, Tm3+ complexes (Tm(TTA)3phen, Tm(DBM)3phen) can be more richly filled in SiO2, and the fluorescence and stability of Tm3+ complexes can be enhanced.
Like ordinary electromagnetic waves, infrared light has wave–particle duality. The energy of infrared light is exactly equal to the energy difference between different energy states of molecules, so infrared absorption effect occurs. Because of the differences in fat content, sugar content, and freshness of food, as well as the differences in biological tissues, there are also differences in the absorption of infrared light waves in food and biological tissues. According to the differences in absorption, different food and biological tissues can be detected. Therefore, infrared fluorescent materials with good fluorescence properties provide favorable conditions for the development of biomedical and food detection.
2. Experimental and Characterization
2.1. Materials
Tetraethoxysilane (TEOS), 4,4,4-trifluoro-1-2-thenoyl-1,3-butanedione (HTTA), dibenzoylmethane (HDBM), ammonium hydroxide (NH3·H2O, 98%, AR), and thulium oxide (Tm2O3, 99.99%, AR) were purchased from Hu Shi Chemical Plant (Shanghai, China). 1,10-Phenanthrolinemonohydrate (Phen, 99%, AR) was purchased from China National Medicines Group (Beijing, China). TmCl3 ethanol solution (EtOH) was prepared as follows: Tm2O3 was dissolved in concentrated hydrochloric acid (HCl), and the surplus HCl was removed by evaporation. The residue was dissolved in anhydrous ethanol.
2.2. Synthesis of Tm3+ Complex
The Tm3+ complex (Tm(TTA)3phen, Tm(DBM)3phen)was prepared according to the following process. HTTA/HDBM and Phen in a stoichiometric molar ratio were dissolved in a suitable volume of anhydrous ethanol. The mass of HTTA/HDBM and Phen was 1.332 g/1.447 g and 0.396 g respectively, which were mixed, heated, and stirred for 1 h in a beaker. Then, an appropriate amount of 20 μL sodium hydroxide solution was added to the solution. A stoichiometric amount of TmCl3 ethanol solution was then added dropwise to the solution while stirring. The stirring temperature was 60 °C. The molar ratio of Tm3+/HTTA/HDBM/Phen was 1:3:1. Then, the pure complexes were collected by centrifugation and alcohol washing for three times. The final products were dried in oven at 50 °C and white powder was obtained.
2.3. Synthesis of SiO2-Tm3+ Hybrid Materials
An improved sol–gel process was employed in this work. Firstly, HTTA/HDBM and Phen were dissolved in a certain volume of anhydrous ethanol according to the stoichiometric molar ratio. The mass of HTTA/HDBM and Phen was 1.332 g/1.447 g and 0.396 g, respectively, and the solution was magnetically stirred for 0.5 h at 60 °C at 700 r/min. Next, 3 mL of NH3·H2O was added to 50 mL of ethanol. After 30 min, 3 mL TEOS was added to the above solution, then we adjusted the dosage of NH3·H2O, ethanol, and TEOS. Compared with previous research, no more water was added. The preparing method was improved and it saved water resources. Then, the proper amounts of silica gel solution, Tm(TTA/DBM)3phen solution, and Tm(TTA/DBM)3phen powder were mixed, heated, and stirred for 3 h, and then centrifuged (speed: 10,000 r/min, 10 min). The excessive Tm(TTA)3phen was removed by three times extensive washing with ethanol. The final products were dried in oven at 50 °C and white powder was obtained.
2.4. Characterization
A JEOL JEM-2100F transmission electron microscope was used for the identification of morphology and size of hybrid nanoparticles. The structure and crystal phases of Tm(TTA)3phen/Tm(DBM)3phen and SiO2-Tm(TTA)3phen/Tm(DBM)3phenwere determined by powder X-ray diffraction (XRD, Ultima IV, Rigaku Corporation, Japan). FT-IR of Tm(TTA)3phen/Tm(DBM)3phen, SiO2-Tm(TTA)3phen/Tm(DBM)3phen and SiO2 were performed using Nicolet5700. The elemental mapping was determined using an FEI (Field Electron and Ion Co.,) Talos F200i microscope (Thermo Fisher Scientific Inc., Waltham, MA, USA) operated at 200 KV. UV–vis absorption spectra were recorded on a Youke UV-755B spectrophotometer. The elemental composition was determined using scanning transmission electron microscopy with energy-dispersive X-ray spectroscopy (STEM-EDS), using an FEI Tecnai G2 F20 S-TWIN (FEI Inc., Hillsboro, OR, USA). Fluorescence spectra were measured by Edinburgh FLS-1000 steady-state transient fluorescence spectrometer (Edinburgh Inc., Livingston, UK) (342W Xe lamp).
3. Results and Discussions
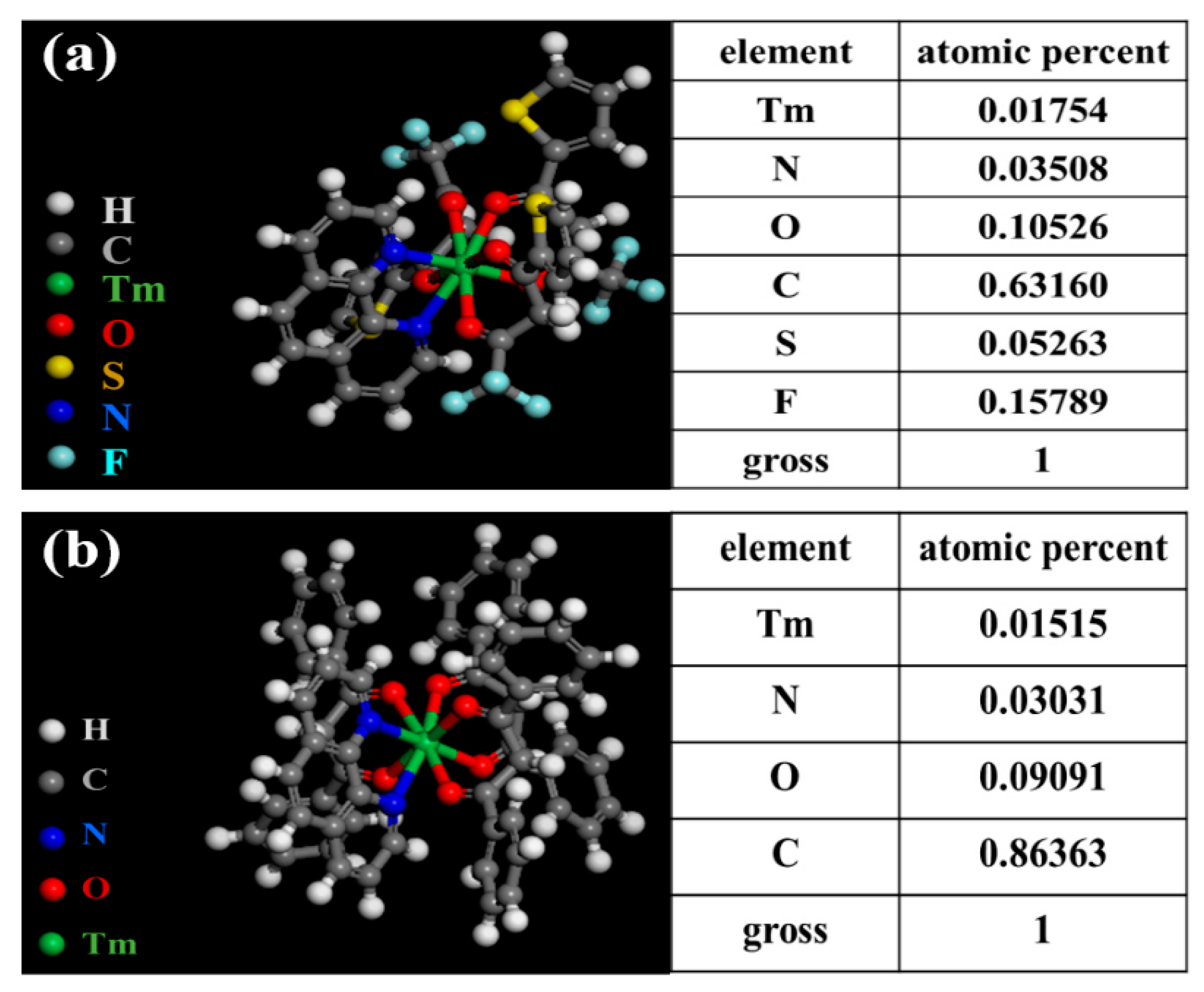
The structure and composition of Tm(TTA)3phen were investigated by EDS (Energy dispersive spectrometer) analysis. Figure 1a shows the predicted structure of Tm(TTA)3phen and the EDS clearly shows that S, F, N, O, C, and thulium elements are present in the Tm(TTA)3phen. According to the element composition obtained by EDS, F and S elements belong to HTTA, while N elements belong to Phen. By calculating the elements content of F, S, N, and thulium, we obtain the ratio between different groups to be Tm3+/HTTA/Phen = 1:3:1 [10]. The structure and composition of Tm(DBM)3phen are similar to Tm(TTA)3phen. N elements belong to Phen and O elements belong to HDBM. By calculating the elements content of C, N, O, and thulium, we obtain the ratio Tm3+/DBM/Phen = 1:3:1 [17].

Figure 1.
Predicted structure and EDS of (a) Tm(TTA)3phen; (b) Tm(DBM)3phen.
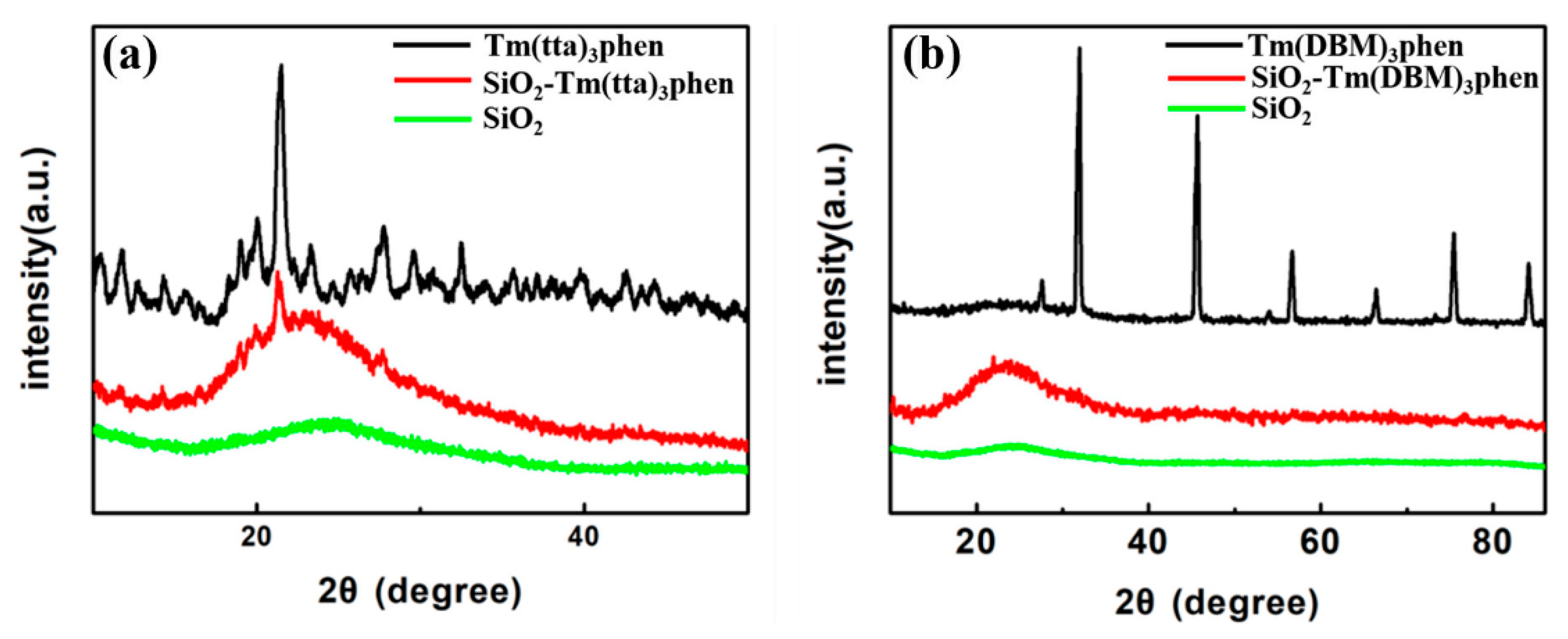
Figure 2 shows the XRD of SiO2, Tm(TTA/DBM)3phen, and SiO2-Tm(TTA/DBM)3phen. Both SiO2-Tm(TTA)3phen and SiO2-Tm(DBM)3phen have a wide diffraction peak at 2θ = 20~25 in Figure 2a,b, which can be assigned to the amorphous silica, and the diffraction peak coincides with the characteristic peak of PDF#47-0715, without significant impurities. It is found that the diffracted intensity of SiO2 in SiO2-Tm(TTA/DBM)3phen hybrid materials is significantly higher than that of pure nano-silica. This is because Tm(TTA/DBM)3phen is embedded in the nano-SiO2, so the bond angle and bond length of SiO2 are changed, which improves the structural symmetry of nano-SiO2. Therefore, the hybrid materials have better structural stability. However, the diffraction peak of nano-SiO2 in SiO2-Tm(TTA)3phen is obviously stronger than that in SiO2-Tm(DBM)3phen, thus the structural stability of SiO2-Tm(TTA)3phen nano hybrid materials is stronger than that of SiO2-Tm(DBM)3phen. Furthermore, in Figure 2a, at the diffraction angle of 2θ = 21.731 and 2θ = 28.337, both of Tm(TTA)3phen and SiO2-Tm(TTA)3phen show a sharp and strong crystallization peak, which indicates that both of Tm(TTA)3phen and SiO2-Tm(TTA)3phen nanoparticles have high crystallinity. Figure 2b indicates that both Tm(DBM)3phen and SiO2-Tm(DBM)3phen nanoparticles have low crystallinity. Therefore, in terms of structure, the structural stability and composite degree of SiO2-Tm(TTA)3phen are obviously stronger than that of SiO2-Tm(DBM)3phen.

Figure 2.
X-ray diffraction (XRD) patterns of (a) Tm(TTA)3phen, SiO2-Tm(TTA)3phen, and Nano-SiO2; (b) Tm(DBM)3phen, SiO2-Tm(DBM)3phen, and nano-SiO2.
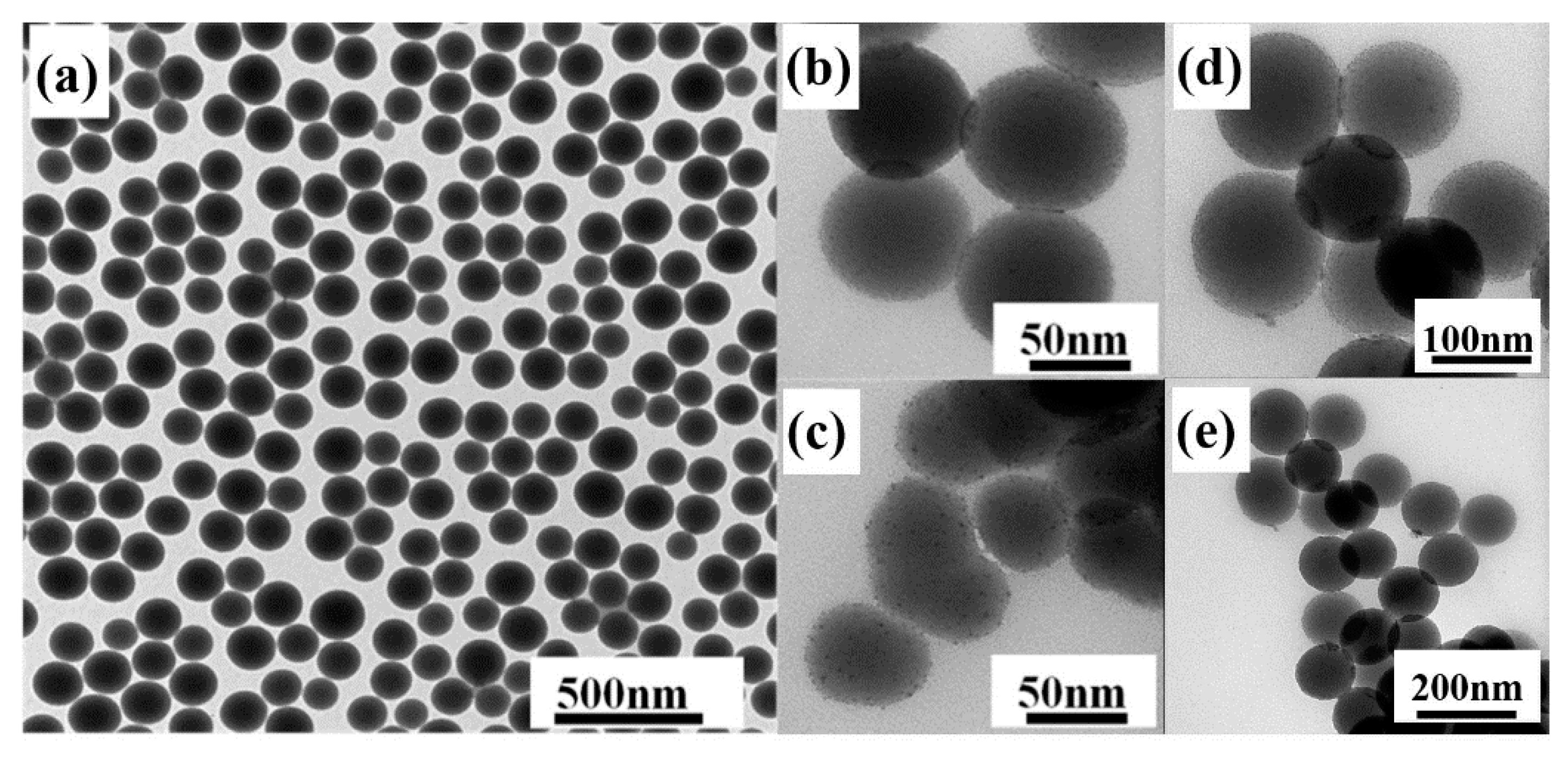
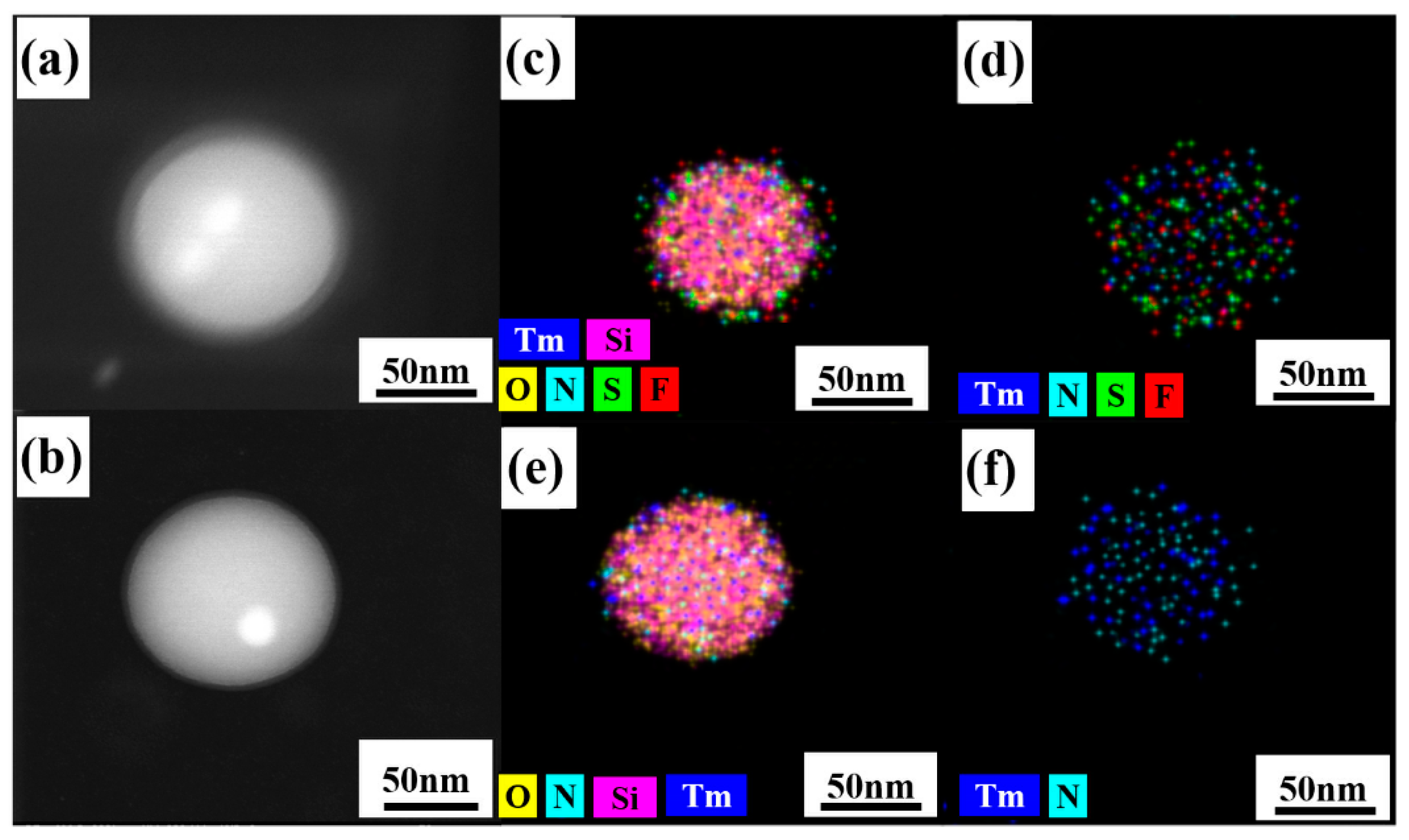
The structure and morphology of the samples were examined by TEM. From Figure 3a, we can see that the nano hybrid materials prepared by the improved method have good uniformity and dispersion in shape and size. The surface of nano-SiO2 is smooth and the nanospheres have a diameter of 80 nm. It is clear from Figure 3b–e that Tm3+ complexes were successfully attached to the surface of nano-SiO2 and some of Tm3+ complexes were embedded into SiO2. Figure 4c–f show elemental mapping of Si (pink), O (yellow), F (red), S (green), Tm (blue), and N (wathet). The existence of S, Tm, F, and N confirms the presence of the Tm(TTA/DBM)3phen complexes in the nano-SiO2.

Figure 3.
Transmission electron microscopy (TEM) image of (a) SiO2 nanoparticles; (b,d) SiO2-Tm(TTA)3phen; (c,e) SiO2-Tm(DBM)3phen.

Figure 4.
Scanning transmission electron microscopy (STEM) dark-field (DF) image of (a) SiO2-Tm(TTA)3phen and (b) SiO2-Tm(DBM)3phen; (c,d) elemental mapping of Si, O F, S, Tm, and N elements of SiO2-Tm(TTA)3phen; (e,f) elemental mapping of Tm, Si, O, and N elements of SiO2-Tm(DBM)3phen.
According to TEM and XRD characterization, it can be concluded that SiO2 in SiO2-Tm(TTA)3phen hybrid materials provides better protection for Tm(TTA)3phen;combined with the TGA (Thermo Gravimetric Analysis) and fluorescence spectra, it can be concluded that the SiO2-Tm(TTA)3phen has better structural stability and better fluorescent performance than SiO2-Tm(DBM)3phen.
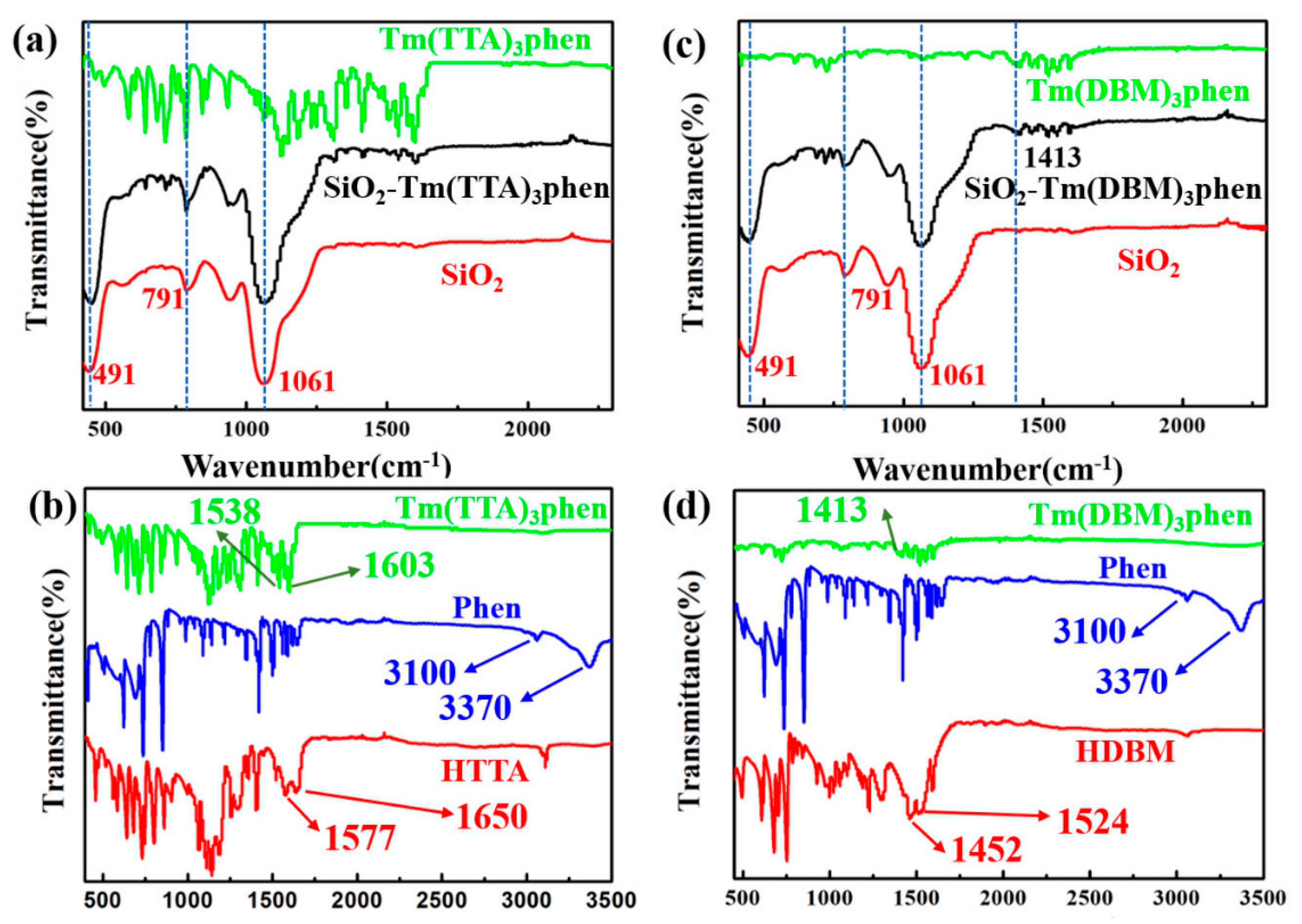
Figure 5 shows the FT-IR spectra of the SiO2-Tm(TTA/DBM)3phen hybrid materials, Tm(TTA/DBM)3phen complexes, and nano-SiO2. Figure 5a displays the FT-IR spectra of the Tm(TTA)3phen and SiO2-Tm3+ hybrid nanoparticles and SiO2. Obviously, the absorption peak of 1061 cm−1 is due to the frequency band of Si-O-Si symmetric telescopic vibration, and 491 cm−1 corresponds to the band of bending vibration of Tm-O-Si. The absorption peak at 791 cm−1 is the bending vibration peak of Si-OH. FT-IR spectra indicate that Tm(TTA)3phen and SiO2 combine to a hybrid material with a stable structure. Figure 5b shows the FT-IR spectra of the HTTA, Phen, and Tm(TTA)3phen. For Tm(TTA)3phen, the characteristic bands at 1577 cm−1 and 1656 cm−1 of HTTA disappear. At the same time, a strong absorption peak (C=O) appears at 1603 cm−1, indicating that the carbonyl group in HTTA is coordinated with Tm3+, and HTTA is confirmed as a non-negative bidentate ligand to coordinate with Tm3+. The coordination bond between Tm3+ and Phen is also formed in Tm(TTA)3phen. The absorption peak (C=N) of Phen appears around 1538 cm−1. The absorption peaks (N-H) at 3370 cm−1 and 3100 cm−1 in Phen disappear and form new coordination bonds with thulium ions. This indicates that the coordination between the two carbon atoms of Phen and thulium ions is bidentate coordination. FT-IR spectra indicate that Tm(TTA)3phen and SiO2 combine to form a hybrid material with a stable structure [18]. Similarly, when Tm(DBM)3phen is successfully combined with SiO2 in Figure 5c,d, the absorption peaks (N-H) at 3370 cm−1 and 3100 cm−1 in Phen disappear and form new coordination bonds with thulium ions. The stretching vibration of C=N originally located at 1413 cm−1 is red shifted, indicating that C=N is weakened to a certain extent. C=N and nano silicon hydroxyl on the surface of silicon are bonded by hydrogen to provide a site for the Tm3+ complex, while its strength is weakened. The absorption peak at 791 cm−1 is the bending vibration peak of Si-OH, while the peak at 491 cm−1corresponds to the band of bending vibration of Tm-O-Si [18].

Figure 5.
FT-IR spectra of (a) SiO2-Tm(TTA)3phen, Tm(TTA)3phen, and nano-SiO2; (b) Tm(TTA)3phen, Phen, and 4,4,4-trifluoro-1-2-thenoyl-1,3-butanedione (HTTA); (c) SiO2-Tm(DBM)3phen, Tm(DBM)3phen, and nano-SiO2; (d) Tm(DBM)3phen, Phen, and dibenzoylmethane (HDBM).
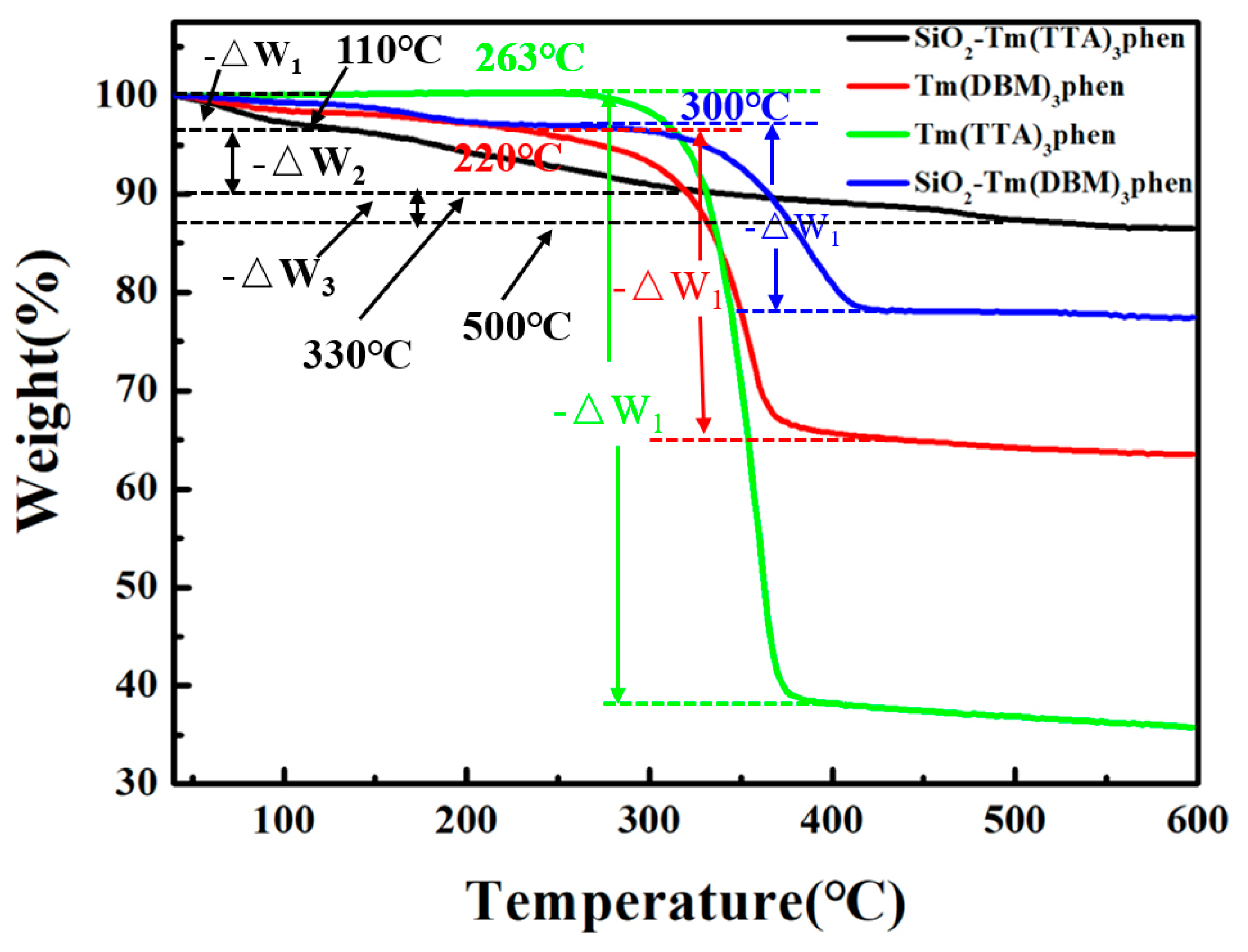
Figure 6 shows the TGA curves of SiO2-Tm(DBM)3phen, SiO2-Tm(TTA)3phen, Tm(TTA)3phen, and Tm(DBM)3phen, respectively. By thermo gravimetric analysis of SiO2-Tm(DBM)3phen, SiO2-Tm(TTA)3phen, Tm(TTA)3phen, and Tm(DBM)3phen, the thermal stability of the Tm(TTA/DBM)3phen and the SiO2-Tm(TTA/DBM)3phen was obtained, respectively. As can be seen from Figure 6, the thermal decomposition of Tm(TTA)3phen complexes shows 60% weight loss in the range of 263 °C to 380 °C, which is analyzed as the decomposition of two organic ligands in the Tm(TTA)3phen. After 400 °C, all the complexes decompose to thulium oxide. In addition, the thermal decomposition of SiO2-Tm(TTA)3phen is divided into three stages. In the first stage, there is 3% weight loss in the range of 40 °C to 100 °C, which is due to the removal of the adsorbed water on the surface of SiO2-Tm(TTA)3phen nanosphere. In the second stage, there is 7% weight loss in the range of 110 °C to 330 °C, which is due to the dehydration of water of colloidal nano-SiO2 in SiO2-Tm(TTA)3phen molecule. In the third stage, there is 3% weight loss in the range of 330 °C to 500 °C, which is due to the dehydration of crystal water in SiO2-Tm(TTA)3phen molecules. Thus, it can be seen that the thermal stability of Tm(TTA)3phen is greatly improved after combination with nano-SiO2. Moreover, by analyzing the thermal weight of SiO2-Tm(DBM)3phen and Tm(DBM)3phen, we can see that the initial decomposition temperature of the two nanomaterials is 220 °C and 300 °C, respectively. Therefore, the thermal stability of SiO2-Tm(DBM)3phen and Tm(DBM)3phen is less stable than SiO2-Tm(TTA)3phen; this is consistent with the results of TEM images analysis.

Figure 6.
TGA curves of SiO2-Tm(DBM)3phen, SiO2-Tm(TTA)3phen, Tm(TTA)3phen, and Tm(DBM)3phen in nitrogen atmosphere(10 °C/min).
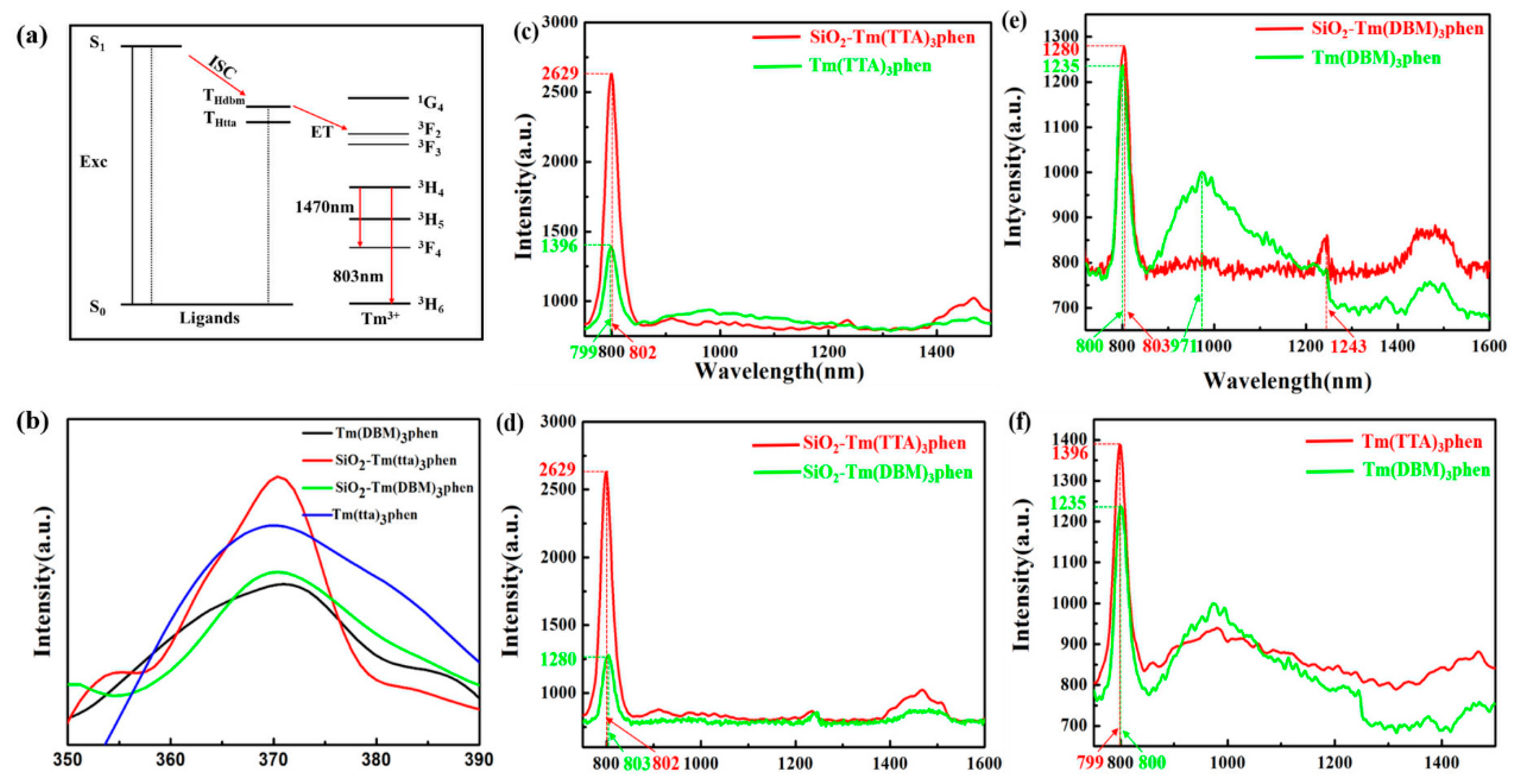
Figure 7 shows the Jablonski diagram of prepared samples. The intra-molecular energy transfer mechanism shown in Figure 7a is the energy transfer process in Tm3+ complexes, which most scientists agree with Crosby’s theory [19] on the energy transfer mechanism of organic ligands transferring to Tm3+. In this process, Tm3+ is no longer first excited by the external excitation energy, but the organicligands of the Tm3+ complex first absorb energy, which is excited from the ground state S0 to the first excited state S1. Then, the molecules are internally transformed to the low-excited single state S1. In the next step of the process, the energy is transferred from the lowest excited singlet S1 to the excited triplet T1. Next, the energy is transferred from the lowest excited triplet T1 to the Tm3+ by virtue of the chemical bond of the Tm3+ complex itself. Thulium ion receives energy and is stimulated to produce an energy level transition, emitting characteristic fluorescence as they return to the ground state. If the energy level of the lowest excited triplet (T1) is lower than that of the excited state of rare earth ion, effective energy transfer cannot occur [20]. Recursively, the effect of this organic ligand′s energy transfer to the fluorescence material central ion is called the “antenna effect” [21,22]. Because of the stronger absorption capacity of HTTA in ultraviolet region and higher excitation state energy transfer efficiency than HDBM, HTTA helps to shorten the transition state of thulium ion and enhance the absorption coefficient of thulium ion in ultraviolet region. As the absorption coefficient of lanthanide ions is extremely low, and f-f level transitions of rare earth ions are forbidden, the ions must be combined with appropriate ligands. In this case, the excitation energy generated by the ligands absorbing photons in the ultraviolet region is transferred to the 4f resonance level [23]. Basically, intra-molecular energy transfer in thulium ion complex molecule is strongly affected by the energy difference between T1 of donor and 4f electronic levels of thulium ions acceptor [24,25,26]. In the energy transfer process from organic ligands to thulium ion, the optimal energy difference is conducive to effective energy transfer. TheT1 of HTTA and HDBM are 20,400 cm−1 and 21,700 cm−1, respectively. Moreover, the energy difference between the 3F2 level of HTTA and thulium ion is 7900 cm−1 [27], which is obviously lower than the energy difference between the 3F2 level of HDBM and thulium ion. Therefore, Tm(TTA)3phen will have stronger fluorescence than that of Tm(DBM)3phen.

Figure 7.
(a) Schematic energy diagram for the indirect excitation mechanism of theTm3+-ligands system; (b) excitation spectra of Tm(DBM)3phen, SiO2-Tm(TTA)3phen, SiO2-Tm(DBM)3phen, and Tm(TTA)3phen (λem = 802 nm). Fluorescent spectra of (c) Tm(TTA)3phen and SiO2-Tm(TTA)3phen, (d) SiO2-Tm(DBM)3phenand SiO2-Tm(TTA)3phen, (e) Tm(DBM)3phen and SiO2-Tm(DBM)3phen, and (f) Tm(TTA)3phen and Tm(DBM)3phen (λex = 370 nm) (solid samples, λex = 370 nm, slit width: 5 × 2 nm, liquid nitrogen refrigeration temperature: −80 °C).
The excitation spectra of Tm3+ complexes and SiO2-Tm3+ hybrid materials are shown in Figure 7b. The excitation spectra of two thulium ions complexes containing different organic ligands have wide absorption bands, which are mainly attributed to the π–π* electron transfer of the organic ligands. However, the absorption band of HTTA is significantly stronger than that of HDBM. In addition, the fluorescence sensitization of HTTA is much more effective than that of HDBM. The excitation spectrum of SiO2-Tm(TTA)3phen is narrower and stronger than that of Tm(TTA)3phen, Tm(DBM)3phen, and SiO2-Tm(DBM)3phen, but the location of the excitation peak and the absorption band position of the organic ligands remain unchanged. This suggests that the introduction of SiO2 does not alter the absorption bands of the organic ligands. Moreover, the coordination bond formed by SiO2 and Tm3+ can carry out more efficient energy transfer in thulium ions complexes molecules. Four samples with the strongest fluorescence spectra were detected at the excitation wavelength of 370 nm.
Figure 7c–f show the emission spectra of Tm(TTA/DBM)3phen and SiO2-Tm(TTA/DBM)3phen hybrid materials. In Figure 7c, the emission peak of SiO2-Tm(TTA)3phen is basically unchanged compared with Tm(TTA)3phen, while the fluorescence intensity is obviously enhanced. Tm(TTA)3phen and SiO2-Tm(TTA)3phen exhibit sharp peaks at 799 nm and 802 nm that are ascribed to 3H4→3H6 transitions of Tm3+ [28]. A wide emission peak appears at 1400 nm–1550 nm, which is caused by the 3H4→3F4 transitions of Tm3+. The emission peak of SiO2-Tm(TTA)3phen is higher and wider than that of Tm(TTA)3phen [29,30,31]. The highest intensity of Tm(TTA)3phen in the curve is 1387 (a.u.), while the highest intensity of SiO2-Tm(TTA)3phen in the curve is 2630 (a.u.), thus there is an enhancement about two times compared with pure complex; the data are given in Table 1. When HDBM is introduced as the ligand of trivalent thulium ion, the highest intensity of Tm(DBM)3phen in the curve is 1235 (a.u.), while the highest intensity of SiO2-Tm(DBM)3phen in the curve is 1280 (a.u.). Thus, there is also an enhancement compared with pure complex; the data are also shown in Table 1. In summary, the NIR-fluorescent hybrid materials have stronger fluorescence after combination with nano-SiO2 compared with pure Tm3+ complexes, and SiO2-Tm(TTA)3phen is more fluorescent than SiO2-Tm(DBM)3phen [32].

Table 1.
Enhancement of fluorescence intensity of Tm3+ complexes and SiO2-Tm3+ hybrid materials.
In addition, from Figure 7e, we can see the emission peak position of SiO2-Tm(DBM)3phen is basically unchanged compared with Tm(DBM)3phen, but it is different from Figure 7c; that is, the emission intensity of SiO2-Tm(DBM)3phen near 800 nm is slightly higher than that of Tm(DBM)3phen. The emission peak of SiO2-Tm(DBM)3phen at 1243 nm is originally the emission peak of Tm(DBM)3phen at 971nm. The fluorescence intensity of SiO2-Tm(DBM)3phen near 1400–1500 nm is higher than Tm(DBM)3phen. Figure 7d,f show that the fluorescence intensity of both Tm(DBM)3phen and SiO2-Tm(DBM)3phen is much lower than that of Tm(TTA)3phen and SiO2-Tm(TTA)3phen.
The UV–vis absorption spectra of Tm(TTA)3phen and Tm(DBM)3phen are shown in Figure S1. It can be found that the absorption peak of Tm(TTA)3phen and Tm(DBM)3phen is the strongest near 370 nm. According to the ultraviolet absorption spectrum analysis, the strong absorption capacity of HTTA/HDBM, and the high energy transfer efficiency of excited state in the ultraviolet region, HTTA/HDBM can help shorten the transition state of thulium ion in the ultraviolet region and improve the absorption coefficient of thulium ion in the ultraviolet region. The absorptive bandwidth of Tm(TTA)3phen is wider than that of Tm(DBM)3phen, combined with UV–vis absorption spectra shown in Figure S1 of Tm(DBM)3phen and Tm(TTA)3phen, which indicates that HTTA absorbs energy and delivers it more efficiently to Tm3+, resulting in better sensitized radiation of Tm3+. Therefore, HTTA has a better matching degree with Tm3+ than HDBM, which also indicates that Tm(TTA)3phen has a higher fluorescence yield than Tm(DBM)3phen.
The increase of fluorescence intensity of SiO2-Tm3+ hybrid materials is due to the carrying effect of SiO2-Tm3+. The amorphous nano-SiO2 provide a microenvironment for Tm3+ complex to limit energy consumption by reducing non-radiative transitions, leading to an increase in the fluorescence intensity of SiO2-Tm3+. According to the analysis of fluorescence spectra(Figure 7) and ultraviolet absorption spectrum(Figure S1), we find that Tm(TTA)3phen and SiO2-Tm(TTA)3phen hybrid nanoparticles have higher fluorescence than those of Tm(DBM)3phen and SiO2-Tm(DBM)3phen. These results show that HTTA is a better choice of organic ligands for Tm3+, and SiO2-Tm(TTA)3phen still has better fluorescence after the combination with nano-SiO2.
To further investigate the fluorescence properties of thulium ion complexes, the room-temperature fluorescence decay curves were measured in Figure S2, excited at 370 nm, and monitored at 802 nm. The decay curve of SiO2-Tm(TTA)3phen fits a single exponential function: D(t) = c0exp(−t/τ), with a lifetime of 50.38 μs.
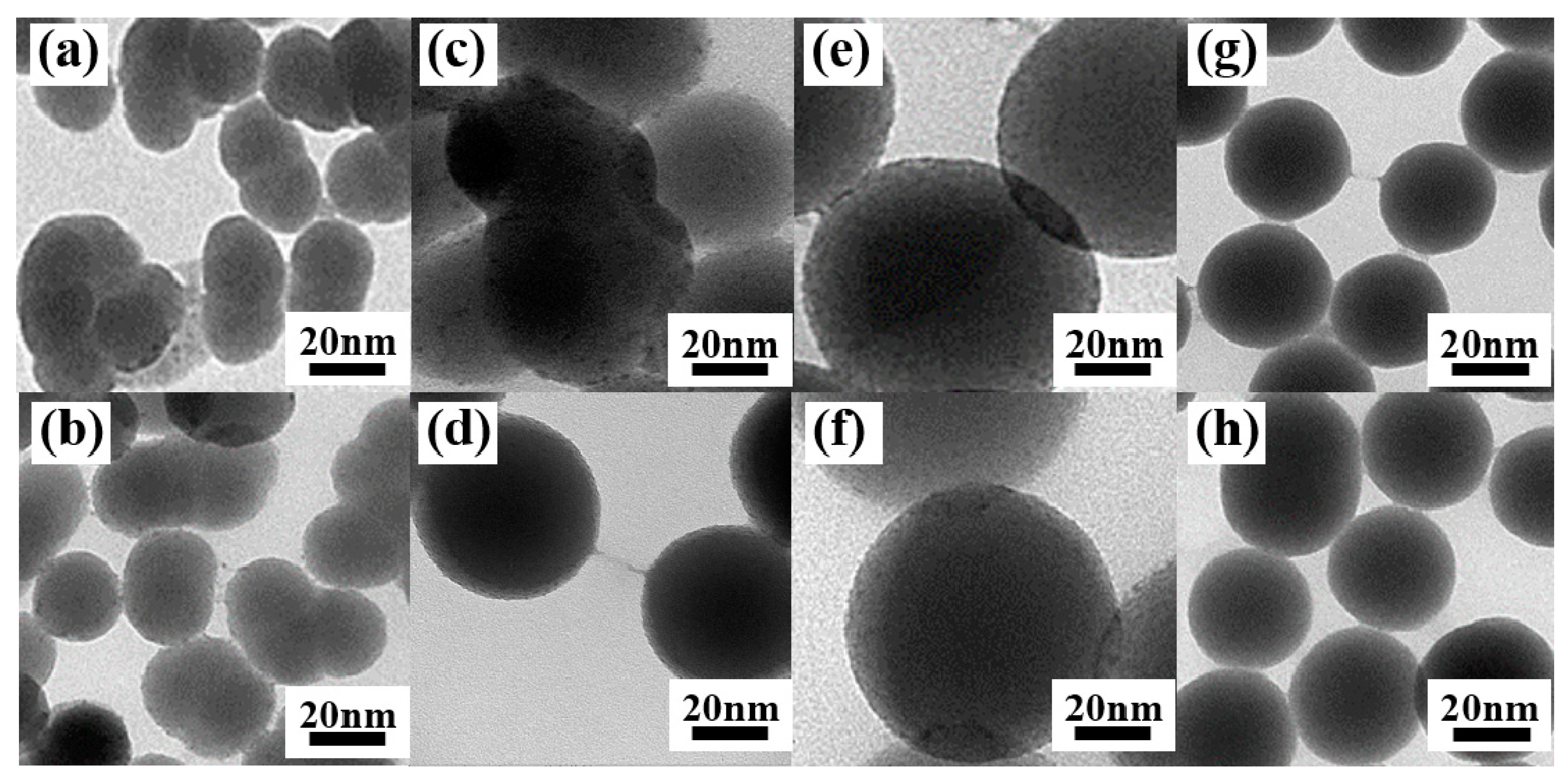
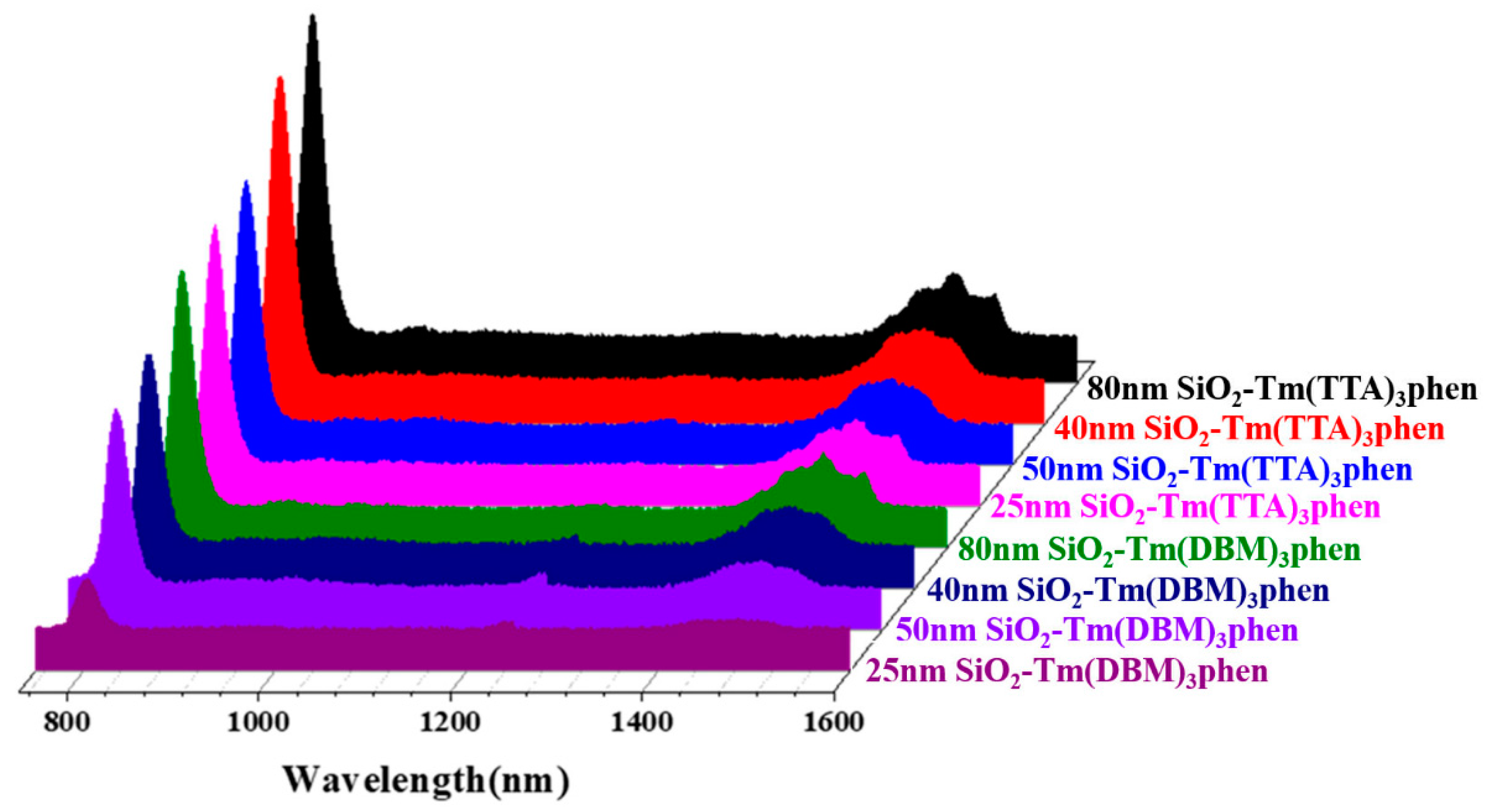
Figure 8 shows the TEM images of SiO2-Tm3+ hybrid fluorescent materials with different particle size prepared under different NH3·H2O conditions. The measurements show that, when the dosage of NH3·H2O is increased from 1 mL to 3 mL, the diameters of SiO2-Tm3+ nanospheres are gradually increased and the dispersibility of the nanospheres are improved. Moreover, as can be seen in Figure 8, when the diameter of the SiO2-Tm3+ nanoparticles is 80 nm, the morphology of the hybrid fluorescent materials is uniform and maintains good dispersity. More Tm3+ complexes are attached to the nano-SiO2 nanoparticles in this case.

Figure 8.
SiO2-Tm(DBM)3phen with a diameter of (a) 25 nm, (c) 50 nm, (e) 80 nm, and (g) 40 nm; SiO2-Tm(TTA)3phen with a diameter of (b) 25 nm, (d) 50 nm, (f) 80 nm, and (h) 40 nm.
The fluorescence spectra of SiO2-Tm3+ hybrid fluorescent materials (SiO2-Tm(TTA)3phen and SiO2-Tm(DBM)3phen) of different particle sizes are shown in Figure 9. Of the four different sizes of SiO2-Tm(TTA)3phen, when the diameter of nanoparticles is 80 nm, the fluorescence intensity of SiO2-Tm(TTA)3phen is the highest. Similarly, of the four different sizes of SiO2-Tm(DBM)3phen, when the diameter of nanoparticles is 80 nm, the fluorescence intensity of SiO2-Tm(DBM)3phen is the highest. However, the fluorescence intensity of SiO2-Tm3+ hybrid fluorescent material with a diameter of 40 nm is higher than that of SiO2-Tm3+ hybrid fluorescent material with a diameter of 50 nm. This is because, when the content of NH3·H2O is high, the hydrolysis process of TEOS is accelerated. As the nano-SiO2 with a diameter of 40 nm has better dispersion, more Tm(TTA)3phen complexes get attached to each nano-SiO2 and embedded in the nanospheres in samples of the same volume. This gives higher fluorescence intensity.

Figure 9.
Fluorescence spectra of SiO2-Tm(DBM)3phen and SiO2-Tm(TTA)3phen with different diameters.
4. Conclusions
In summary, SiO2-Tm(TTA/DBM)3phen NIR-fluorescent hybrid materials were prepared by an improved sol–gel method. By comparing the thulium ion complexes of two different organic ligands, we found that Tm(TTA)3phen has better fluorescent performance than that of Tm(DBM)3phen. In addition, the hybrid materials ofSiO2-Tm(TTA)3phen have better structural stability and thermal stability than that of SiO2-Tm(DBM)3phen. Moreover, fluorescence intensity of SiO2-Tm(TTA)3phen at 802 nm, 1231 nm, and 1400–1500 nm is significantly higher than that of Tm(TTA)3phen, Tm(DBM)3phen, and SiO2-Tm(DBM)3phen. Tm(TTA)3phen and SiO2-Tm(TTA)3phendisplay better fluorescent performance than that of Tm(DBM)3phen and SiO2-Tm(DBM)3phen. Therefore, HTTA is a better choice of organic ligand for Tm3+, and SiO2-Tm(TTA)3phen still has better fluorescent performance after the combination with nano-SiO2. In addition, the fluorescence intensity of SiO2-Tm(TTA/DBM)3phen of different sizes was studied, and it was found that it was the highest when the diameter of SiO2-Tm(TTA/DBM)3phen was 80 nm. We believe this kind of near-infrared fluorescent hybrid material with a more stable structure and stronger fluorescence will have prospects for food detection, biological detection, and biological imaging.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-4991/10/10/1964/s1, Figure S1: UV–vis absorption spectra of (a) Tm(DBM)3phen and (b) Tm(TTA)3phen, Figure S2: Fluorescence decay curve of SiO2-Tm(tta)3phen when excited at 370 nm and monitored at 803 nm.
Author Contributions
Conceptualization, Y.W. (Yanxin Wang) and Q.S.; methodology, Q.S.; software, J.H., Y.W. (Yao Wang); validation, Y.W. (Yanxin Wang), J.H. and L.B.; formal analysis, P.L.; investigation, Z.Z.; resources, J.T.; data curation, Y.W. (Yanxin Wang); writing—original draft preparation, Q.S.; writing—review and editing, Y.W. (Yanxin Wang); visualization, X.W.; supervision, Y.W. (Yanxin Wang); project administration, Y.W. (Yanxin Wang); funding acquisition, Y.W. (Yanxin Wang), J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant no 52070104, 51878361, 51503112), Natural Scientific Foundation of Shandong Province (Grant no ZR2019MEM048),State Key Project of International Cooperation Research (2016YFE0110800, 2017YFE0108300), the National Program for Introducing Talents of Discipline to Universities (“111” plan), 1st class discipline program of Materials Science of Shandong Province, and The Double-Hundred Foreign Expert Program of Shandong Province (2019–2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chu, X.-Y.; Wang, W.-M.; Nie, Y.-Y.; Cui, J.-Z.; Gao, H.-L. Regulating the luminescent and magnetic properties of rare-earth complexes with β-diketonatecoligands. New J. Chem. 2018, 42, 11417–11429. [Google Scholar] [CrossRef]
- Feng, J.; Song, S.; Fan, W.; Sun, L.-N.; Guo, X.-M.; Peng, C.-Y.; Yu, J.; Yu, Y.-N.; Zhang, H. Near-infrared luminescent mesoporous MCM-41 materials covalently bonded with ternary thulium complexes. Microporous Mesoporous Mater. 2009, 117, 278–284. [Google Scholar] [CrossRef]
- Liu, X.; Wang, N.; Suo, Q. Synthesis and luminescence properties of rare earth ternary complexes consisting of Eu(III), β-diketones and 1,10-phenanthroline. J. Rare Earths 2008, 26, 778–782. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Gong, M. Luminescence and energy transfer of La5Si2BO13: A (A = Ce3+/Tb3+/Eu3+/Sm3+) phosphors under UV excitation. Mater. Lett. 2015, 143, 71–74. [Google Scholar] [CrossRef]
- Carlos, L.D.; Ferreira, R.A.S.; Bermudez, V.D.Z.; Ribeiro, S.J. ChemInform Abstract: Lanthanide-Containing Light-Emitting Organic-Inorganic Hybrids: A Bet on the Future. Adv. Mater. 2009, 40, 509–534. [Google Scholar] [CrossRef]
- Ramakrishna, V.V.; Patil, S.K.; Reddy, L.K.; Reddy, A.S. Solvent extraction of Tm(III) by sulfoxides and mixtures of DPSO (di-N-pentyl sulfoxide) and HTTA (thenoyl-trifluoroacetone) from perchlorate and thiocyanate media. J. Radioanal. Nucl. Chem. 1978, 47, 57–76. [Google Scholar] [CrossRef]
- Shukla, R. Solvent extraction of metals with potassium-dihydro-bispyrazolyl-borate. Talanta 2002, 57, 633–639. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B. Eu3+, Tb3+/β-diketonate functionalized mesoporous SBA-15/GaN composites: Multi-component chemical bonding assembly, characterization, and luminescence. J. Colloid Interface Sci. 2013, 395, 145–153. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A novel method for synthesis of silica nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Rahman, I.; Vejayakumaran, P.; Sipaut, C.; Ismail, J.; Abu Bakar, N.; Adnan, R.; Chee, C. Effect of anion electrolytes on the formation of silica nanoparticles via the sol–gel process. Ceram. Int. 2006, 32, 691–699. [Google Scholar] [CrossRef]
- Kim, S.-S.; Kim, H.-S.; Kim, W.-S.; Kim, S.G. Effect of electrolyte additives on sol-precipitated nano silica particles. Ceram. Int. 2004, 30, 171–175. [Google Scholar] [CrossRef]
- Horikawa, T.; Do, D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Tagaya, M.; Ikoma, T.; Yoshioka, T.; Motozuka, S.; Xu, Z.; Minami, F.; Tanaka, J. Synthesis and luminescence properties of Eu(III)-doped nanoporous silica spheres. J. Colloid Interface Sci. 2011, 363, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zou, H.; Wang, G.; Sun, Y.; Huo, Q.; Xu, X.; Sheng, Y. Synthesis and luminescence properties ofmonodisperse SiO2 @SiO2:Eu3+ microspheres. Opt. Mater. 2014, 37, 583–588. [Google Scholar] [CrossRef]
- Xie, M.; Zeng, L.; Zhou, X.; Xie, F. Synthesis and photoluminescence properties of novel red emitting phosphor Sr2LiSiO4F: Eu3+. Solid State Sci. 2015, 39, 6–9. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H.; Song, S.; Li, Z.-F.; Sun, L.-N.; Xing, Y.; Guo, X.-M. Syntheses, crystal structures, visible and near-IR luminescent properties of ternary lanthanide (Dy3+, Tm3+) complexes containing 4,4,4-trifluoro-1-phenyl-1,3-butanedione and 1,10-phenanthroline. J. Lumin. 2008, 128, 1957–1964. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Tang, J.; Huang, L.; Wang, Y.; Huang, Z.; Liu, J.; Xu, Q.; Shen, W.; Belfiroe, L.A. Enhanced emission of nanoSiO2-carried Eu3+ complexes and highly luminescent hybrid nanofibers. Opt. Mater. 2013, 35, 1395–1403. [Google Scholar] [CrossRef]
- Burdick, G.W.; Downer, M.C. The role of linear crystal-field terms in hypersensitive Eu3+ optical-transition intensities. Eur. J. Solid State Inorg. Chem. 1991, 28, 217–220. [Google Scholar]
- Shi, J.; Wang, Y.; Huang, L.; Lu, P.; Sun, Q.; Wang, Y.; Tang, J.; Belfiore, L.A.; Kipper, M.J. Polyvinylpyrrolidone Nanofibers Encapsulating an Anhydrous Preparation of Fluorescent SiO₂⁻Tb3+ Nanoparticles. Nanomaterials 2019, 9, 510. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Lehn, J.-M. Luminescent lanthanide complexes as photochemical supramolecular devices. Coord. Chem. Rev. 1993, 123, 201–228. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K. From Antenna to Assay: Lessons Learned in Lanthanide Luminescence. Accounts Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, M.R.; Norouzi, P.; Akbari-Adergani, B. Thulium(III) Ions Monitoring by a Novel Thulium(III) Microelectrode Based on a S-N Schiff Base. Electroanaysis 2007, 19, 1145–1151. [Google Scholar] [CrossRef]
- Zang, F.X.; Hong, Z.R.; Li, W.L.; Li, M.T.; Sun, X.Y. 1.4 μm band electroluminescence from organic light-emitting diodes based on thulium complexes. Appl. Phys. Lett. 2004, 84, 2679. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Lin, J.; Wang, S.; Yang, K. Preparation and luminescence properties of ormosil material doped with Eu(TTA)3phen complex. J. Non-Crystalline Solids 2000, 278, 218–222. [Google Scholar] [CrossRef]
- Sager, W.F.; Filipescu, N.; Serafin, F.A. Substituent effects on intramolecular energy transfer. I. absorption and phosphorescence spectra of rare earth p-diketone chelates. J. Phys. Chem. 1965, 69, 1092–1100. [Google Scholar] [CrossRef]
- Du, P.; Huang, X.; Yu, J.S. Facile synthesis of bifunctional Eu3+ -activated NaBiF 4 red-emitting nanoparticles for simultaneous white light-emitting diodes and field emission displays. Chem. Eng. J. 2018, 337, 91–100. [Google Scholar] [CrossRef]
- Dejneka, M.; Streltsov, A.; Pal, S.; Frutos, A.G.; Powell, C.L.; Yost, K.; Yuen, P.; Müller, U.; Lahiri, J. Rare earth-doped glass microbarcodes. In Proceedings of the National Academy of Sciences. Proc. Natl. Acad. Sci. USA 2003, 100, 389–393. [Google Scholar] [CrossRef]
- Hakeem, D.A.; Pi, J.W.; Kim, S.W.; Park, K. New Y2LuCaAl2SiO12:Ln (Ln = Ce3+, Eu3+, and Tb3+) phosphors for white LED applications. Inorg. Chem. Front. 2018, 5, 1336–1345. [Google Scholar] [CrossRef]
- Kobwittaya, K.; Oishi, Y.; Torikai, T.; Yada, M.; Watari, T.; Luitel, H.N. Nearly pure NIR to NIR upconversion luminescence in Tm3+, Yb3+ co-doped ZnO-TiO2 composite phosphor powder. Vacuum 2018, 148, 286–295. [Google Scholar] [CrossRef]
- Dou, A.; Shen, L.; Wang, N.; Cai, Y.; Cai, M.; Guo, Y.; Huang, F.; Tian, Y.; Xu, S.; Zhang, J. Investigation of Tm3+/Yb3+ co-doped germanate–tellurite glasses for efficient 2.1 μm mid-infrared laser materials. Appl. Phys. B 2018, 124, 86. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Huang, L.; Lian, S.; Wang, Y.; Tang, J.; Belfiore, L.A.; Kipper, M.J. Tb3+/Eu3+ Complex-Doped Rigid Nanoparticles in Transparent Nanofibrous Membranes Exhibit High Quantum Yield Fluorescence. Nanomaterials 2020, 10, 694. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).