On the Sensitivity of the Ni-rich Layered Cathode Materials for Li-ion Batteries to the Different Calcination Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of LiNi0.6Mn0.2Co0.2O2 (NMC) and LiNiO2 (LNO) Cathode Materials
2.2. Characterization Methods
2.3. Electrochemical Evaluation
3. Results and Discussion
3.1. Thermal Decomposition of the Prepared Precursors of Cathode Materials
- (i)
- lithium hydroxide monohydrate dehydrates to lithium hydroxide and water at 100 °C. The confirmation is a mass signal of water (m/e = 18).
- (ii)
- nickel–manganese–cobalt hydroxy carbonate decomposes from 150 to 500 °C, along with the decomposition of lithium hydroxide. These thermal decomposition reactions are associated with releasing water (m/e = 18) and carbon dioxide (m/e = 44).
- (iii)
- nickel–manganese–cobalt carbonate decomposes from 500 to 900 °C, along with decomposition of lithium carbonate (formed in the reaction between lithium hydroxide and carbon dioxide (contained in air) during the drying step). The confirmation is a mass signal of carbon dioxide (m/e = 44).
- (i)
- lithium hydroxide monohydrate dehydrates at 100 °C and water (m/e = 18) is released.
- (ii)
- lithium hydroxide decomposes to lithium oxide and water at 500 °C. The confirmation is a mass signal of water (m/e = 18).
- (iii)
- lithium hydroxy carbonate and lithium carbonate (formed in the reaction between lithium hydroxide and carbon dioxide (contained in air) during the drying step) decompose from 500 to 850 °C. These thermal decomposition reactions are related to the release of water (m/e = 18) and carbon dioxide (m/e = 44).
3.2. Physicochemical Properties
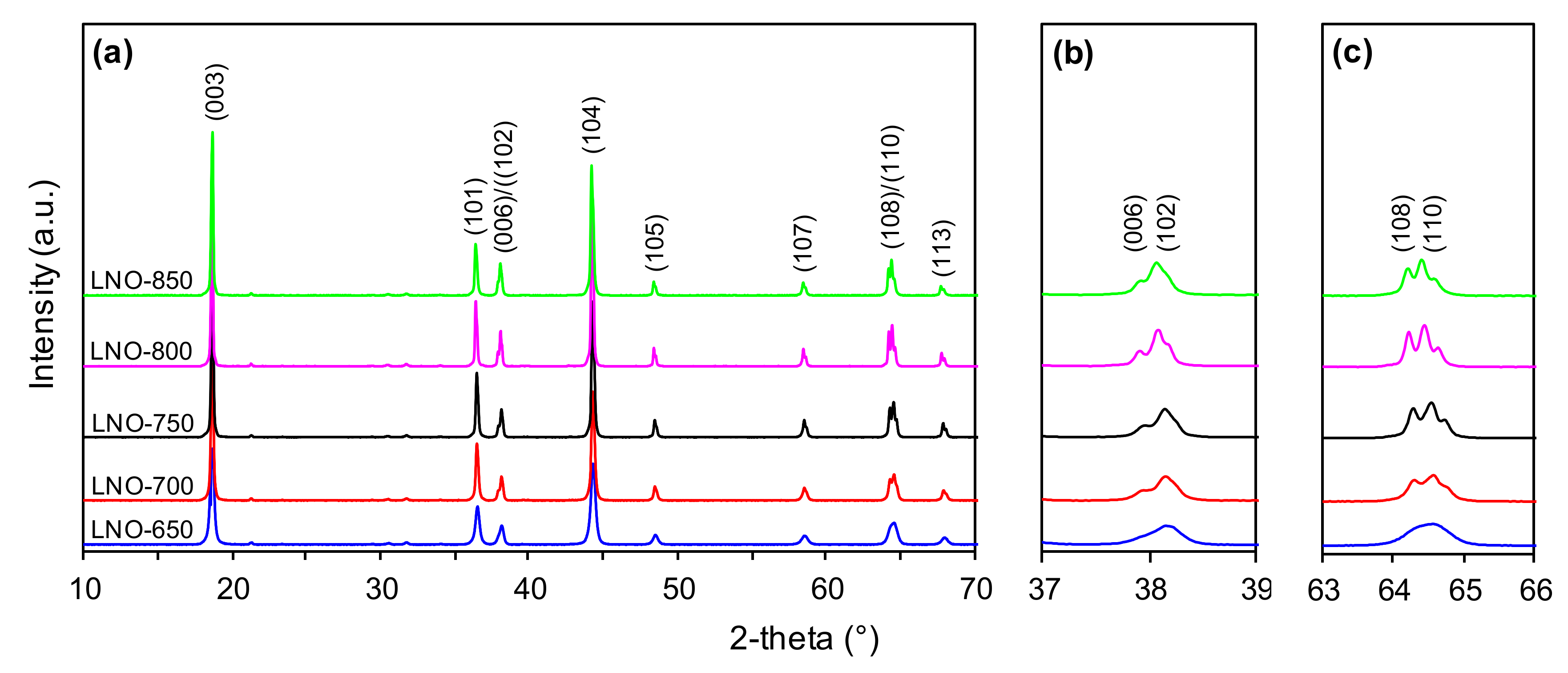
3.3. Structural Properties
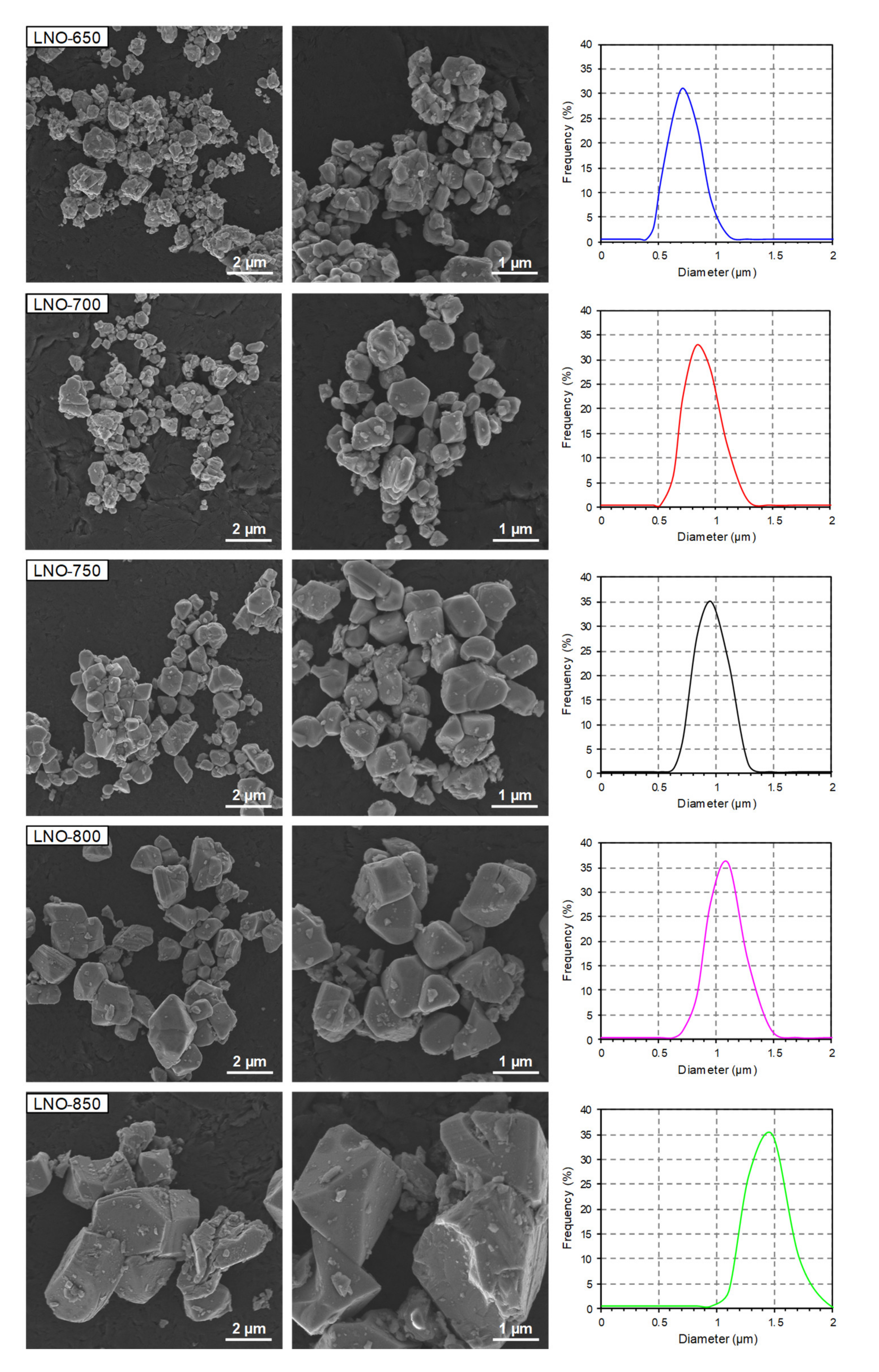
3.4. Morphological Properties
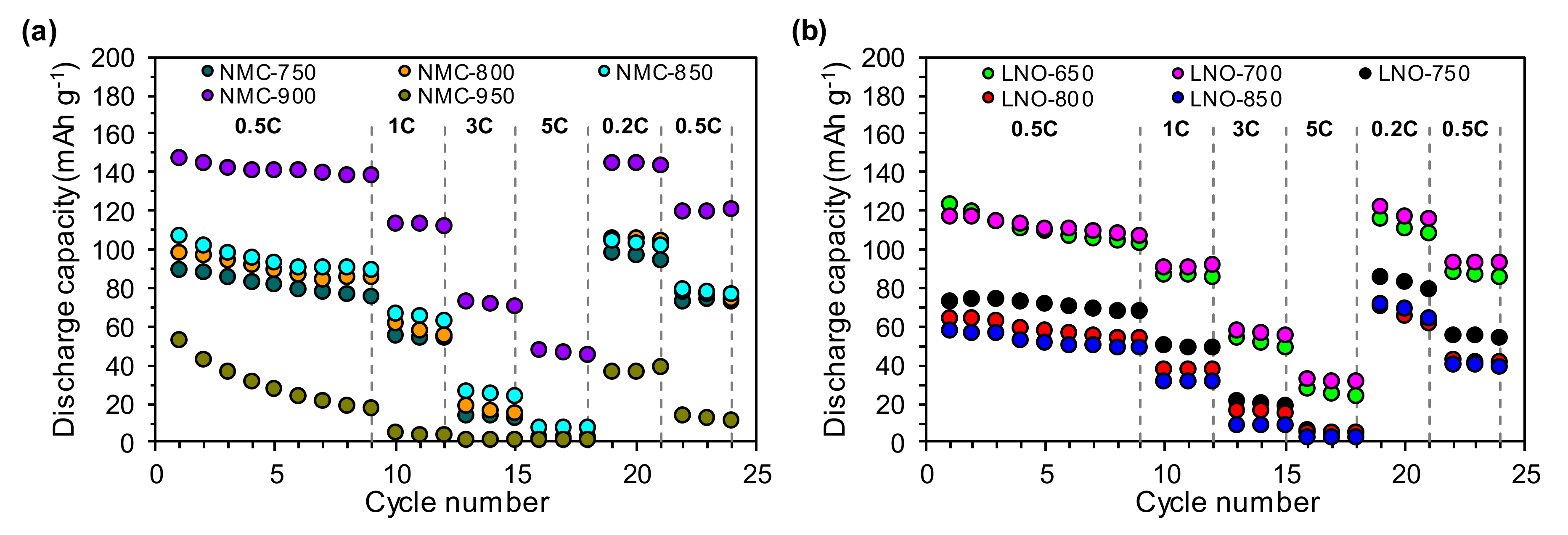
3.5. Electrochemical Performance
- (i)
- the charging voltage was too high, causing an irreversible phase transformation. The literature indicates that a course of charging process related to a successive phase transformation occurring in the sequence hexagonal (H1) → monoclinic (M) → hexagonal (H2) → hexagonal (H3) [19,26,62,63] is also important in the phenomenon of capacity fading. Depending on the composition (nickel content particularly) of the cathode material, the potential for the last irreversible H2 → H3 transformation decreases. For the NMC622 material, this potential is higher than 4.6 V vs. Li/Li+. However, for materials with higher nickel content (x ≥ 0.8), it is 4.2–4.3 V [19,61] and can be as low as 4.0 V vs. Li/Li+ for LNO materials [19,26]. This suggests that the charging of the studied materials during galvanostatic testing at high voltages of 4.4 V could also have an impact on the decrease of the capacity and stability of the LNO samples and lead to gradual cell degradation.
- (ii)
- the presence of impurities in the form of Li2CO3, which may significantly reduce the amount of working lithium ions during the charge/discharge process. Residual lithium compounds are inevitably present in the Ni-rich layered oxides, and their amount increases with the Ni content [19].
- (iii)
- (iv)
- changes in the texture and microstructure of the materials, i.e., a deterioration of their specific surface area, as well as greater tendency for particles to aggregate/agglomerate, are highly adverse for the process of lithium ion diffusion, and are visible with increasing nickel content.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Sources 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Gröger, O.; Gasteiger, H.A.; Suchsland, J.-P. Review—Electromobility: Batteries or Fuel Cells? J. Electrochem. Soc. 2015, 162, A2605–A2622. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.G.; Yang, Z.; Lemmon, J.P.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Wang, C.; Xiao, J.; et al. Materials science and materials chemistry for large scale electrochemical energy storage: From transportation to electrical grid. Adv. Funct. Mater. 2013, 23, 929–946. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Myung, S.T.; Maglia, F.; Park, K.J.; Yoon, C.S.; Lamp, P.; Kim, S.J.; Sun, Y.K. Nickel-Rich Layered Cathode Materials for Automotive Lithium-Ion Batteries: Achievements and Perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Swiatowska, J.; Barboux, P. Lithium Battery Technologies: From the Electrodes to the Batteries; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128014172. [Google Scholar]
- Yoshino, A. Development of the Lithium-Ion Battery and Recent Technological Trends; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780444595133. [Google Scholar]
- Opitz, A.; Badami, P.; Shen, L.; Vignarooban, K.; Kannan, A.M. Can Li-Ion batteries be the panacea for automotive applications? Renew. Sustain. Energy Rev. 2017, 68, 685–692. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Mierlo, J. Van Cost projection of state of the art lithium-ion batteries for electric vehicles up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and Reality of Ni-Rich Cathode for Commercialization. Adv. Energy Mater. 2018, 8, 1–25. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Ruther, R.E.; Du, Z.; Self, E.C.; Meyer, H.M.; Daniel, C.; Belharouak, I.; Wood, D.L. Chemical stability and long-term cell performance of low-cobalt, Ni-Rich cathodes prepared by aqueous processing for high-energy Li-Ion batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.T.; Oh, S.M.; Sun, Y.K. Nickel-Rich and Lithium-Rich Layered Oxide Cathodes: Progress and Perspectives. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Zhang, S.S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Hou, P.; Yin, J.; Ding, M.; Huang, J.; Xu, X. Surface/Interfacial Structure and Chemistry of High-Energy Nickel-Rich Layered Oxide Cathodes: Advances and Perspectives. Small 2017, 13, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, D.; Chang, C. Improved electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials: Via incorporation of rubidium cations into the original Li sites. RSC Adv. 2017, 7, 51721–51728. [Google Scholar] [CrossRef]
- He, T.; Chen, L.; Su, Y.; Lu, Y.; Bao, L.; Chen, G.; Zhang, Q.; Chen, S.; Wu, F. The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni-Rich cathode materials. J. Power Sources 2019, 441, 227195. [Google Scholar] [CrossRef]
- Teichert, P.; Eshetu, G.G.; Jahnke, H.; Figgemeier, E. Degradation and aging routes of ni-rich cathode based li-ion batteries. Batteries 2020, 6, 8. [Google Scholar] [CrossRef]
- Kalyani, P.; Kalaiselvi, N. Various aspects of LiNiO2 chemistry: A review. Sci. Technol. Adv. Mater. 2005, 6, 689–703. [Google Scholar] [CrossRef]
- Chen, H.; Dawson, J.A.; Harding, J.H. Effects of cationic substitution on structural defects in layered cathode materials LiNiO2. J. Mater. Chem. A 2014, 2, 7988–7996. [Google Scholar] [CrossRef]
- Yoon, C.S.; Jun, D.W.; Myung, S.T.; Sun, Y.K. Structural Stability of LiNiO2 Cycled above 4.2 V. ACS Energy Lett. 2017, 2, 1150–1155. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Cho, M.; Shim, J.; Rao, M.C. Structural and electrochemical properties of LiNiO2 cathodes prepared by solid state reaction method. Ionics 2020. [Google Scholar] [CrossRef]
- Arai, H.; Okada, S.; Sakurai, Y.; Yamaki, J.I. Reversibility of LiNiO2 cathode. Solid State Ionics 1997, 95, 275–282. [Google Scholar] [CrossRef]
- Dahn, J.R. Rechargeable LiNiO2/Carbon Cells Technical Papers Electrochemical Science and Technology Rechargeable LiNiO2/Carbon Cells. J. Electrochem. Soc. 1991, 138, 2207–2211. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and Structural Chemistry of LiNiO2 (R3m) for 4 Volt Secondary Lithium Cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Purwanto, A.; Yudha, C.S.; Ubaidillah, U.; Widiyandari, H.; Ogi, T.; Haerudin, H. NCA cathode material: Synthesis methods and performance enhancement efforts. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Gao, H.; Zeng, X.; Hu, Y.; Tileli, V.; Li, L.; Ren, Y.; Meng, X.; Maglia, F.; Lamp, P.; Kim, S.J.; et al. Modifying the Surface of a High-Voltage Lithium-Ion Cathode. ACS Appl. Energy Mater. 2018, 1, 2254–2260. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, Q.; Li, Y.; Chen, C.; Liu, X.; Yuan, M.; Wang, R.; Chen, S.; Xiao, S. Enhanced electrochemical performances of LiNi0.8Co0.1Mn0.1O2 by synergistic modification of sodium ion doping and silica coating. Solid State Ionics 2020, 346, 115214. [Google Scholar] [CrossRef]
- Xie, H.; Du, K.; Hu, G.; Peng, Z.; Cao, Y. The Role of Sodium in LiNi0.8Co0.15Al0.05O2 Cathode Material and Its Electrochemical Behaviors. J. Phys. Chem. C 2016, 120, 3235–3241. [Google Scholar] [CrossRef]
- Dixit, M.; Markovsky, B.; Aurbach, D.; Major, D.T. Unraveling the Effects of Al Doping on the Electrochemical Properties of LiNi0.5Co0.2Mn0.3O2 Using First Principles. J. Electrochem. Soc. 2017, 164, A6359–A6365. [Google Scholar] [CrossRef]
- Weigel, T.; Schipper, F.; Erickson, E.M.; Susai, F.A.; Markovsky, B.; Aurbach, D. Structural and Electrochemical Aspects of LiNi0.8Co0.1Mn0.1O2 Cathode Materials Doped by Various Cations. ACS Energy Lett. 2019, 4, 508–516. [Google Scholar] [CrossRef]
- Gan, C.; Hu, X.; Zhan, H.; Zhou, Y. Synthesis and characterization of Li1.2Ni0.6Co0.2Mn0.2O2+δ as a cathode material for secondary lithium batteries. Solid State Ionics 2005, 176, 687–692. [Google Scholar] [CrossRef]
- Liang, L.; Du, K.; Lu, W.; Peng, Z.; Cao, Y.; Hu, G. Synthesis and characterization of concentration-gradient LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. J. Alloys Compd. 2014, 613, 296–305. [Google Scholar] [CrossRef]
- Zybert, M.; Ronduda, H.; Szczęsna, A.; Trzeciak, T.; Ostrowski, A.; Żero, E.; Wieczorek, W.; Raróg-Pilecka, W.; Marcinek, M. Different strategies of introduction of lithium ions into nickel-manganese-cobalt carbonate resulting in LiNi0.6Mn0.2Co0.2O2 (NMC622) cathode material for Li-ion batteries. Solid State Ionics 2020, 348. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Szczęsna, A.; Trzeciak, T.; Ostrowski, A.; Wieciński, P.; Wieczorek, W.; Raróg-Pilecka, W.; Marcinek, M. Addition of yttrium oxide as an effective way to enhance the cycling stability of LiCoO2 cathode material for Li-ion batteries. Solid State Ionics 2020, 355, 115426. [Google Scholar] [CrossRef]
- Song, M.; Kwon, I.; Kim, H.; Shim, S.; Mumm, D.R. Synthesis of LiNiO2 cathode by the combustion method. J. Appl. Electrochem. 2006, 36, 801–805. [Google Scholar] [CrossRef]
- Yue, P.; Wang, Z.; Peng, W.; Li, L.; Chen, W.; Guo, H.; Li, X. Spray-drying synthesized LiNi0.6Co0.2Mn0.2O2 and its electrochemical performance as cathode materials for lithium ion batteries. Powder Technol. 2011, 214, 279–282. [Google Scholar] [CrossRef]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Guohui, Y. A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries. J. Alloys Compd. 2020, 819, 153048. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Zou, J.; Liu, W.; Chen, Y. The structural mechanism of the improved electrochemical performances resulted from sintering atmosphere for LiNi0.5Co0.2Mn0.3O2 cathode material. J. Alloys Compd. 2013, 558, 172–178. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, P.; Estevez, L.; Wang, C.; Zhang, J.G. Effect of calcination temperature on the electrochemical properties of nickel-rich LiNi0.76Mn0.14Co0.10O2 cathodes for lithium-ion batteries. Nano Energy 2018, 49, 538–548. [Google Scholar] [CrossRef]
- Kong, J.Z.; Zhou, F.; Wang, C.B.; Yang, X.Y.; Zhai, H.F.; Li, H.; Li, J.X.; Tang, Z.; Zhang, S.Q. Effects of Li source and calcination temperature on the electrochemical properties of LiNi0.5Co0.2Mn0.3O2 lithium-ion cathode materials. J. Alloys Compd. 2013, 554, 221–226. [Google Scholar] [CrossRef]
- Xia, Y.F.; Nie, M.; Wang, Z.B.; Yu, F.D.; Zhang, Y.; Zheng, L.L.; Wu, J.; Ke, K. Structural, morphological and electrochemical investigation of LiNi0.6Co0.2Mn0.2O2 cathode material synthesized in different sintering conditions. Ceram. Int. 2015, 41, 11815–11823. [Google Scholar] [CrossRef]
- Ma, Q.; Peng, F.; Li, R.; Yin, S.; Dai, C. Effect of calcination temperature on microstructure and electrochemical performance of lithium-rich layered oxide cathode materials. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2016, 213, 123–130. [Google Scholar] [CrossRef]
- Gao, T.P.; Wong, K.W.; Fung, K.Y.; Zhang, W.; Ng, K.M. A rational three-step calcination strategy for synthesizing high-quality LiNi0.5Mn0.3Co0.2O2 cathode materials: The key role of suppressing Li2O formation. Electrochim. Acta 2018, 288, 153–164. [Google Scholar] [CrossRef]
- Deng, C.; Liu, L.; Zhou, W.; Sun, K.; Sun, D. Effect of synthesis condition on the structure and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 prepared by hydroxide co-precipitation method. Electrochim. Acta 2008, 53, 2441–2447. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Muta, T.; Li, D.; Noguchi, H.; Yoshio, M.; Ma, R.; Takada, K.; Sasaki, T. The effects of extra Li content, synthesis method, sintering temperature on synthesis and electrochemistry of layered LiNi1/3Mn1/3Co1/3O2. J. Power Sources 2006, 162, 629–635. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Ohzuku, T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119–121, 171–174. [Google Scholar] [CrossRef]
- Ngala, J.K.; Chernova, N.A.; Ma, M.; Mamak, M.; Zavalij, P.Y.; Whittingham, M.S. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound. J. Mater. Chem. 2004, 14, 214–220. [Google Scholar] [CrossRef]
- Hashem, A.M.A.; Abdel-Ghany, A.E.; Eid, A.E.; Trottier, J.; Zaghib, K.; Mauger, A.; Julien, C.M. Study of the surface modification of LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion battery. J. Power Sources 2011, 196, 8632–8637. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Q.; Huang, Z.; He, Z.; Wang, Z.; Guo, H.; Li, X. Synthesis and electrochemical study of Zr-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion battery. Ceram. Int. 2016, 42, 263–269. [Google Scholar] [CrossRef]
- Choi, Y.M.; Pyun, S.I.; Moon, S.I. Effects of cation mixing on the electrochemical lithium intercalation reaction into porous LiNiCoO2 electrodes. Solid State Ionics 1996, 89, 43–52. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, F.; Liu, B.; Zhou, H.; Zhang, Q.; Kong, J.; Wang, Q. Progress in Preparation and Modification of LiNi0.6Mn0.2Co0.2O2 Cathode Material for High Energy Density Li-Ion Batteries. Int. J. Electrochem. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries. Electrochem. Energ. Rev. 2020, 3. [Google Scholar] [CrossRef]
- Dixit, M.; Markovsky, B.; Schipper, F.; Aurbach, D.; Major, D.T. Origin of Structural Degradation during Cycling and Low Thermal Stability of Ni-Rich Layered Transition Metal-Based Electrode Materials. J. Phys. Chem. C 2017, 121, 22628–22636. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity fading of Ni-rich Li[NixCoyMn1−x−y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Bak, S.M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.J.; Kim, K.B.; Chung, K.Y.; Yang, X.Q.; Nam, K.W. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Zhang, S.S. Understanding of performance degradation of LiNi0.80Co0.10Mn0.10O2 cathode material operating at high potentials. J. Energy Chem. 2020, 41, 135–141. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Du, C.Q.; Zhang, F.; Ma, C.X.; Wu, J.W.; Tang, Z.Y.; Zhang, X.H.; Qu, D. Synthesis and electrochemical properties of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material for lithium-ion battery. Ionics 2016, 22, 209–218. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |




| Cathode Material | Heat-Treatment (Calcination) Conditions | Electrochemical Performance | Ref. | |||
|---|---|---|---|---|---|---|
| Initial Discharge Capacity (mAh g−1) | Capacity Retention (%) | Voltage Range (V vs. Li/Li+) | ||||
| LiNi0.5Co0.2Mn0.3O2 | two-step | 500 °C (4 h)/800–900 °C (12 h) | 150–163 (0.1C) | >99 (30 cycles, 0.2C) | 2.5–4.3 | [46] |
| LiNi0.6Co0.2Mn0.2O2 | two-step | 650 °C (6 h)/750–850 °C (10–12 h) | 130–165 (1C) | >99 (100 cycles, 1C) | 3.0–4.3 | [47] |
| Li1.2Mn0.56Ni0.16Co0.08O2 | one-step | 750–950 °C (20 h) | 255–269 (0.1C) | 84–102 (100 cycles, 0.1C) | 2.0–4.8 | [48] |
| LiNi0.76Mn0.14Co0.10O2 | two-step | 500 °C (10 h)/725–900 °C (20 h) | 200–215 (0.1C) | 42–80 (200 cycles, 0.3C) | 2.7–4.5 | [45] |
| LiNi0.5Mn0.3Co0.2O2 | one-step | 900 °C (15 h) | 161 (0.1C) | 59 (30 cycles, 0.1C) | 2.8–4.3 | [49] |
| LiNi0.5Mn0.3Co0.2O2 | two-step | 450 °C (6 h)/900 °C (15 h) | 171 (0.1C) | 69 (30 cycles, 0.1C) | 2.8–4.3 | [49] |
| LiNi0.5Mn0.3Co0.2O2 | two-step | 750 °C (6 h)/900 °C (15 h) | 165 (0.1C) | 63 (30 cycles, 0.1C) | 2.8–4.3 | [49] |
| LiNi0.5Mn0.3Co0.2O2 | three-step | 450 °C (3 h)/560 °C (3 h)/900 °C (15 h) | 179 (0.1C) | 85 (30 cycles, 0.1C) | 2.8–4.3 | [49] |
| Samples | Calcination Conditions | Li/TM Molar Ratio 1 | |
|---|---|---|---|
| 1st Step | 2nd Step | ||
| NMC-750 | 500 °C, 5 h | 750 °C, 15 h | 1.024 |
| NMC-800 | 500 °C, 5 h | 800 °C, 15 h | 1.019 |
| NMC-850 | 500 °C, 5 h | 850 °C, 15 h | 1.012 |
| NMC-900 | 500 °C, 5 h | 900 °C, 15 h | 1.007 |
| NMC-950 | 500 °C, 5 h | 950 °C, 15 h | 1.002 |
| LNO-650 | 500 °C, 5 h | 650 °C, 15 h | 1.048 |
| LNO-700 | 500 °C, 5 h | 700 °C, 15 h | 1.041 |
| LNO-750 | 500 °C, 5 h | 750 °C, 15 h | 1.033 |
| LNO-800 | 500 °C, 5 h | 800 °C, 15 h | 1.026 |
| LNO-850 | 500 °C, 5 h | 850 °C, 15 h | 1.018 |
| Samples | Temperature Range (°C) | Weight Loss (%) |
|---|---|---|
| NMC-750 | 30–750 | 24.1 |
| NMC-800 | 30–800 | 24.1 |
| NMC-850 | 30–850 | 24.2 |
| NMC-900 | 30–900 | 24.4 |
| NMC-950 | 30–950 | 24.5 |
| LNO-650 | 30–650 | 3.8 |
| LNO-700 | 30–700 | 4.4 |
| LNO-750 | 30–750 | 4.9 |
| LNO-800 | 30–800 | 5.1 |
| LNO-850 | 30–850 | 5.5 |
| Samples | SSA 1 (m2 g−1) | Dav 2 (µm) | Tap Density (cm3 g−1) |
|---|---|---|---|
| NMC-750 | 8.9 | 0.4 | 1.31 |
| NMC-800 | 8.1 | 0.5 | 1.35 |
| NMC-850 | 5.5 | 0.6 | 1.39 |
| NMC-900 | 1.5 | 0.7 | 1.51 |
| NMC-950 | 1.2 | 0.9 | 1.78 |
| LNO-650 | 1.6 | 0.7 | 2.16 |
| LNO-700 | 1.2 | 0.8 | 2.17 |
| LNO-750 | 0.9 | 1.0 | 2.20 |
| LNO-800 | 0.7 | 1.1 | 2.22 |
| LNO-850 | 0.6 | 1.5 | 2.29 |
| Samples | a (Å) | c (Å) | V (Å3) | c/a | R 1 | dav 2 (nm) |
|---|---|---|---|---|---|---|
| NMC-750 | 2.881 | 14.237 | 102.34 | 4.942 | 1.09 | 17 |
| NMC-800 | 2.879 | 14.235 | 102.18 | 4.944 | 1.13 | 25 |
| NMC-850 | 2.878 | 14.235 | 102.09 | 4.946 | 1.19 | 37 |
| NMC-900 | 2.879 | 14.243 | 102.20 | 4.947 | 1.21 | 63 |
| NMC-950 | 2.880 | 14.248 | 102.33 | 4.947 | 1.03 | 94 |
| LNO-650 | 2.880 | 14.205 | 102.07 | 4.932 | 1.16 | 28 |
| LNO-700 | 2.882 | 14.208 | 102.19 | 4.923 | 1.18 | 56 |
| LNO-750 | 2.883 | 14.207 | 102.28 | 4.928 | 1.12 | 75 |
| LNO-800 | 2.886 | 14.212 | 102.54 | 4.924 | 1.08 | 96 |
| LNO-850 | 2.889 | 14.218 | 102.73 | 4.921 | 1.06 | 124 |
| Samples | Charge Capacity (mAh g−1) | Discharge Capacity (mAh g−1) | Coulombic Efficiency (%) |
|---|---|---|---|
| NMC-750 | 201 | 147 | 73 |
| NMC-800 | 192 | 145 | 76 |
| NMC-850 | 185 | 132 | 71 |
| NMC-900 | 245 | 186 | 76 |
| NMC-950 | 166 | 120 | 72 |
| LNO-650 | 225 | 182 | 81 |
| LNO-700 | 215 | 183 | 85 |
| LNO-750 | 202 | 156 | 77 |
| LNO-800 | 164 | 116 | 71 |
| LNO-850 | 146 | 110 | 75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronduda, H.; Zybert, M.; Szczęsna-Chrzan, A.; Trzeciak, T.; Ostrowski, A.; Szymański, D.; Wieczorek, W.; Raróg-Pilecka, W.; Marcinek, M. On the Sensitivity of the Ni-rich Layered Cathode Materials for Li-ion Batteries to the Different Calcination Conditions. Nanomaterials 2020, 10, 2018. https://doi.org/10.3390/nano10102018
Ronduda H, Zybert M, Szczęsna-Chrzan A, Trzeciak T, Ostrowski A, Szymański D, Wieczorek W, Raróg-Pilecka W, Marcinek M. On the Sensitivity of the Ni-rich Layered Cathode Materials for Li-ion Batteries to the Different Calcination Conditions. Nanomaterials. 2020; 10(10):2018. https://doi.org/10.3390/nano10102018
Chicago/Turabian StyleRonduda, Hubert, Magdalena Zybert, Anna Szczęsna-Chrzan, Tomasz Trzeciak, Andrzej Ostrowski, Damian Szymański, Władysław Wieczorek, Wioletta Raróg-Pilecka, and Marek Marcinek. 2020. "On the Sensitivity of the Ni-rich Layered Cathode Materials for Li-ion Batteries to the Different Calcination Conditions" Nanomaterials 10, no. 10: 2018. https://doi.org/10.3390/nano10102018
APA StyleRonduda, H., Zybert, M., Szczęsna-Chrzan, A., Trzeciak, T., Ostrowski, A., Szymański, D., Wieczorek, W., Raróg-Pilecka, W., & Marcinek, M. (2020). On the Sensitivity of the Ni-rich Layered Cathode Materials for Li-ion Batteries to the Different Calcination Conditions. Nanomaterials, 10(10), 2018. https://doi.org/10.3390/nano10102018








