Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications
Abstract
1. Introduction
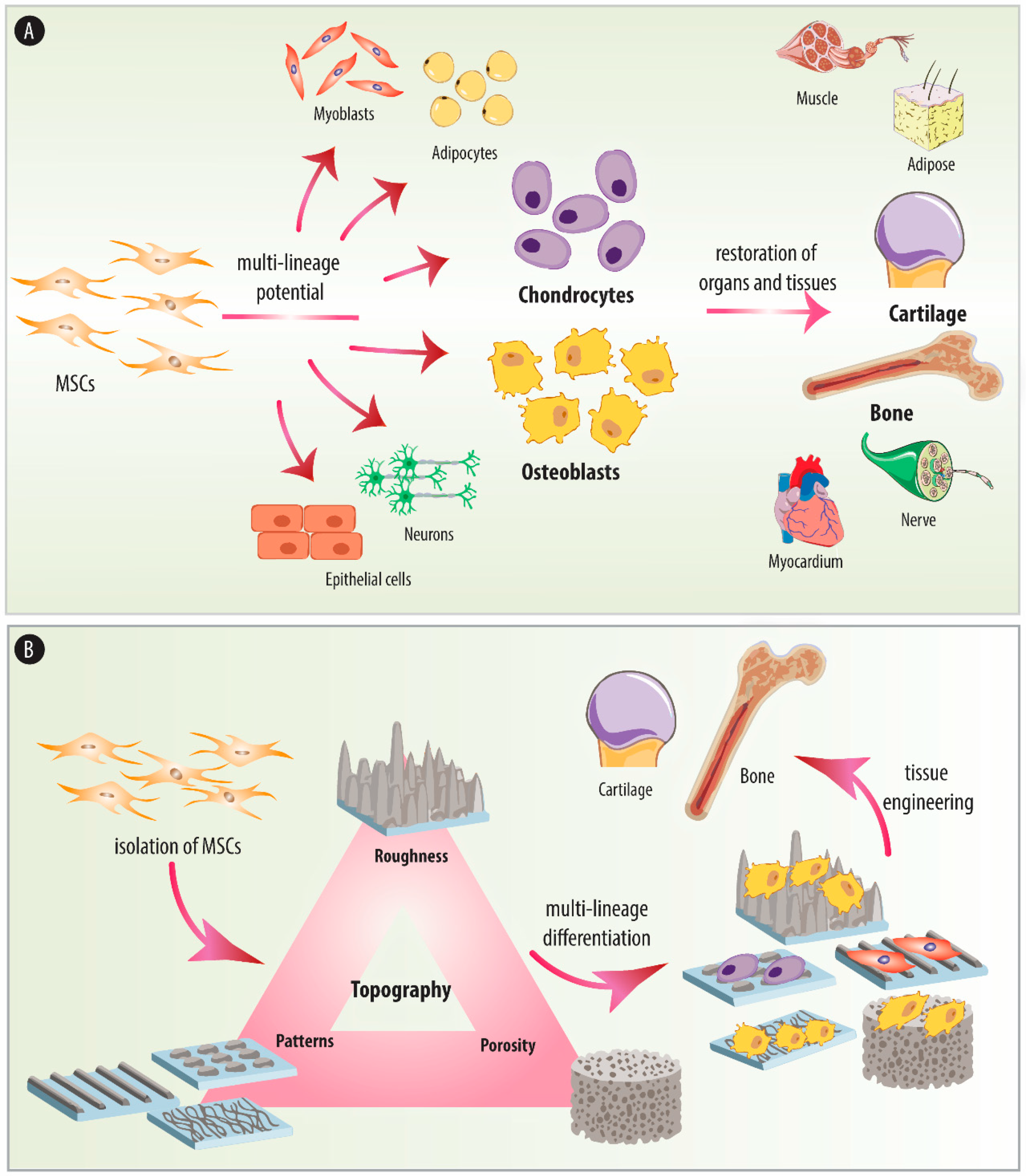
2. Origin and Relevance of MSCs
2.1. Isolation and Characterization
2.2. Differentiation Potential
2.3. Therapeutic Applications
3. Cell-Implant Surface Interactions
4. The Effect of Substrate Topography
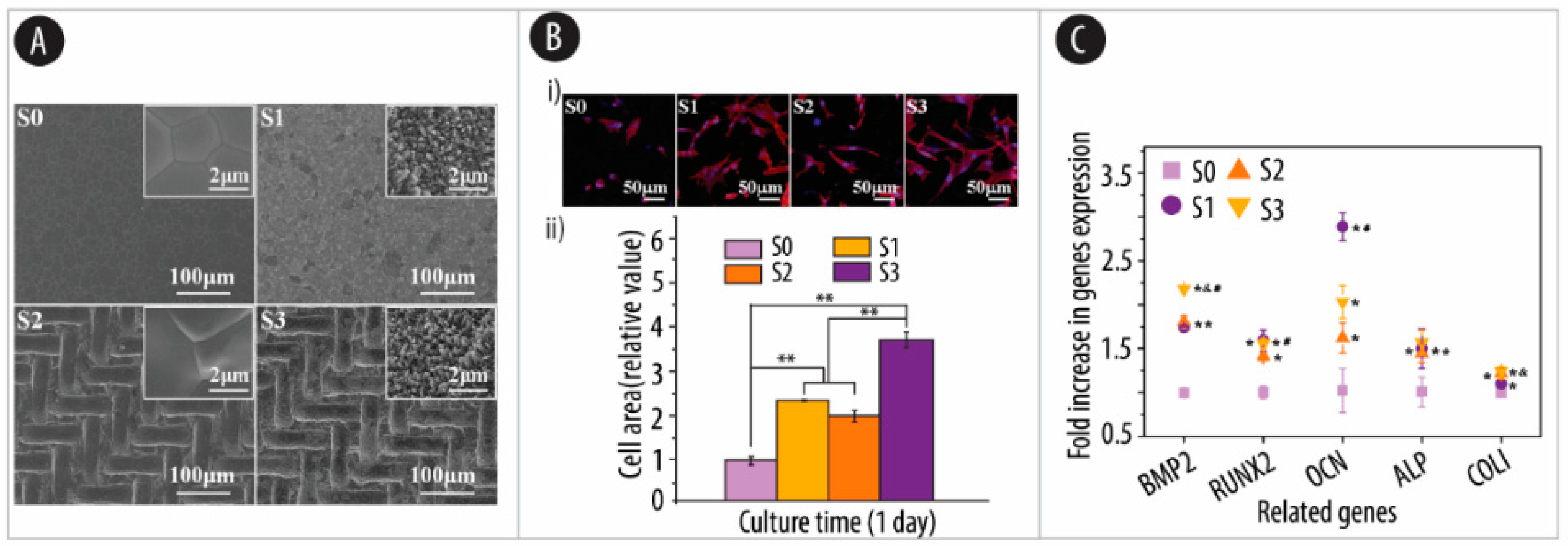
4.1. Substrate Roughness
4.1.1. Ceramic-Based Scaffolds
4.1.2. Titanium (Ti)-Based Scaffolds
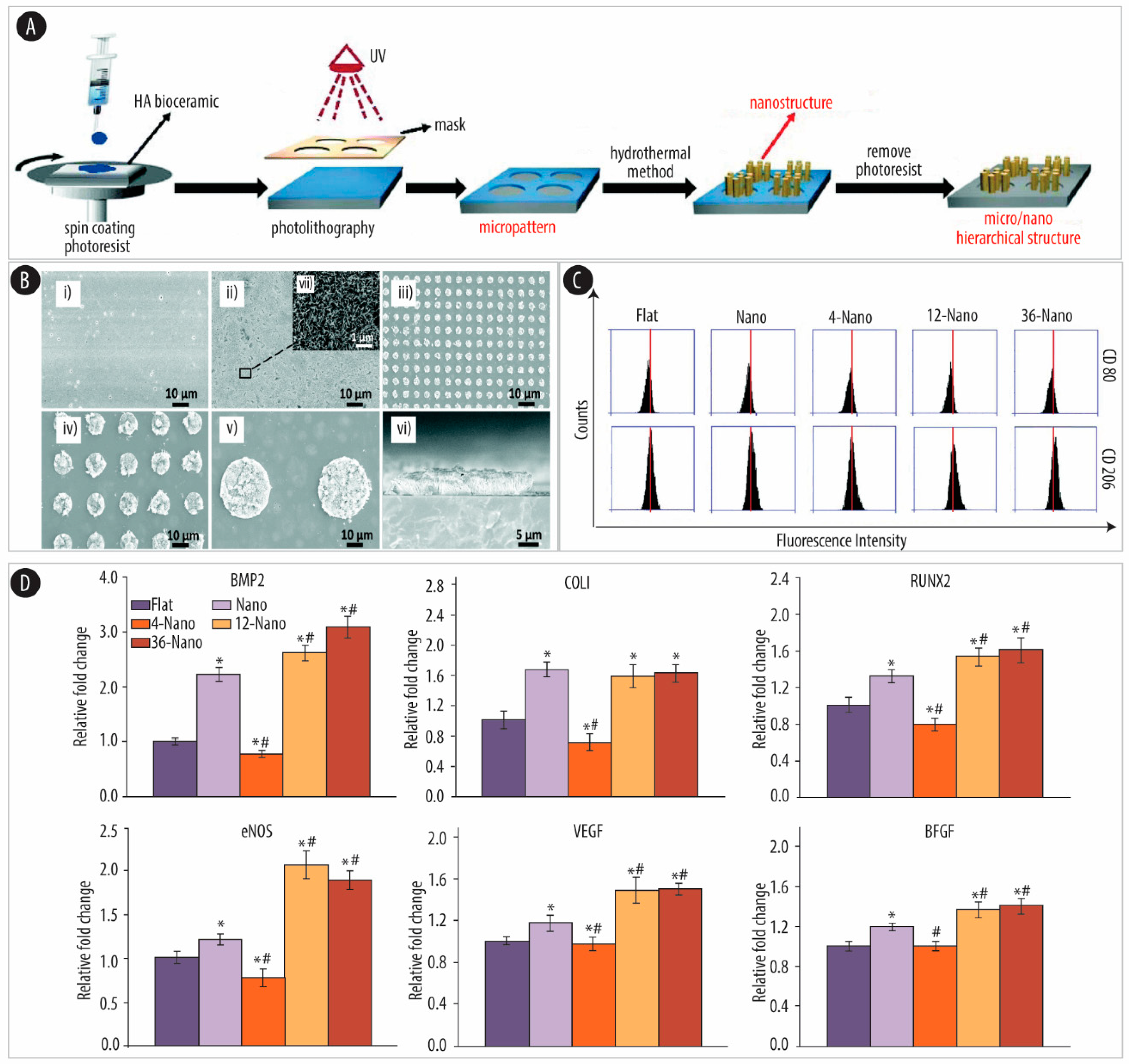
4.2. Substrate Patterns
4.2.1. Ceramic-Based Scaffolds
4.2.2. Ti-Based Scaffolds
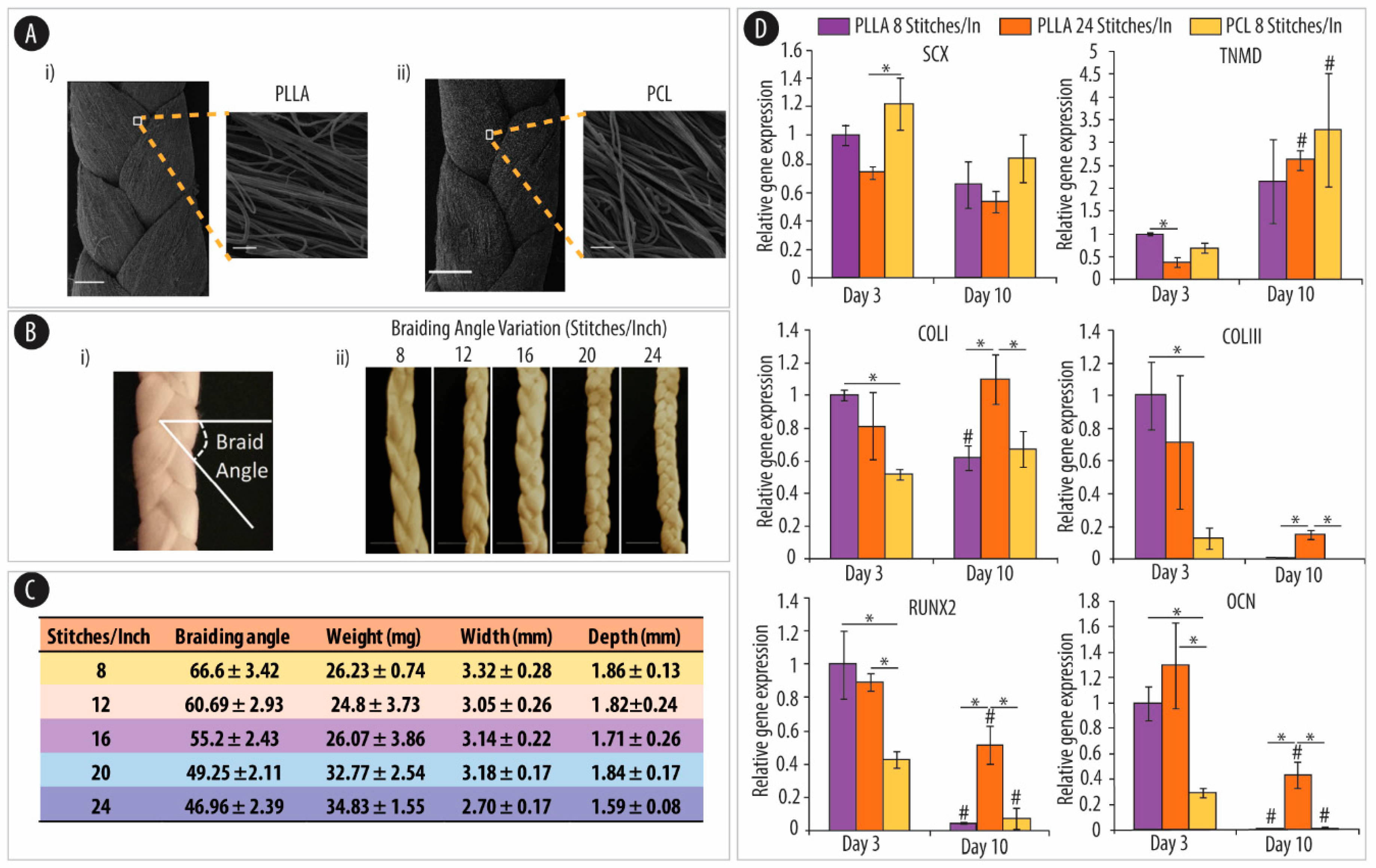
4.2.3. Polymeric Substrates
4.3. Porosity
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Curtis, A.S.G.; Varde, M. Control of cell behavior: Topological factors 2. J. Natl. Cancer Inst. 1964, 33, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-N.; Law, J.B.K.; He, A.Y.; Low, H.Y.; Hui, J.H.; Lim, C.T.; Yang, Z.; Lee, E.H. Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater. Sci. Eng. 2016, 2, 142–151. [Google Scholar] [CrossRef]
- Brafman, D.A. Constructing stem cell microenvironments using bioengineering approaches. Physiol. Genom. 2013, 45, 1123–1135. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, H.-K.; Kim, K.S. Intrinsic and extrinsic mechanical properties related to the differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2016, 473, 752–757. [Google Scholar] [CrossRef]
- Moghaddam, M.M.; Bonakdar, S.; Shariatpanahi, M.R.; Shokrgozar, M.A.; Faghihi, S. The effect of physical cues on the stem cell differentiation. Curr. Stem Cell Res. Ther. 2019, 14, 268–277. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, C. New depths in cell behaviour: Reactions of cells to nanotopography. Biochem. Soc. Symp. 1999, 65, 15–26. [Google Scholar]
- Norman, J.J.; Desai, T.A. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann. Biomed. Eng. 2006, 34, 89–101. [Google Scholar] [CrossRef]
- Nie, Z.; Kumacheva, E. Patterning surfaces with functional polymers. Nat. Mater. 2008, 7, 277–290. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Herzyk, P.; Agheli, H.; Sutherland, D.S.; Wilkinson, C.D. Group analysis of regulation of fibroblast genome on low-adhesion nanostructures. Biomaterials 2007, 28, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Shukla, K.P.; Moctezuma, M.; Tang, L. Cellular and molecular responses of smooth muscle cells to surface nanotopography. J. Nanosci. Nanotechnol. 2007, 7, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; McCloy, D.; Robertson, M.; Agheli, H.; Sutherland, D.S.; Affrossman, S.; Oreffo, R.O. Osteoprogenitor response to semi-ordered and random nanotopographies. Biomaterials 2006, 27, 2980–2987. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef]
- Yim, E.K.; Darling, E.M.; Kulangara, K.; Guilak, F.; Leong, K.W. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010, 31, 1299–1306. [Google Scholar] [CrossRef]
- Hoch, A.I.; Mittal, V.; Mitra, D.; Vollmer, N.; Zikry, C.A.; Leach, J.K. Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells. Biomaterials 2016, 74, 178–187. [Google Scholar] [CrossRef]
- Leach, J.K.; Whitehead, J. Materials-directed differentiation of mesenchymal stem cells for tissue engineering and regeneration. ACS Biomater. Sci. Eng. 2017, 4, 1115–1127. [Google Scholar] [CrossRef]
- Dobbenga, S.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Nanopattern-induced osteogenic differentiation of stem cells-A systematic review. Acta Biomater. 2016, 46, 3–14. [Google Scholar] [CrossRef]
- Mashinchian, O.; Turner, L.-A.; Dalby, M.J.; Laurent, S.; Shokrgozar, M.A.; Bonakdar, S.; Imani, M.; Mahmoudi, M. Regulation of stem cell fate by nanomaterial substrates. Nanomedicine 2015, 10, 829–847. [Google Scholar] [CrossRef]
- Engler, A.J.; Sweeney, H.L.; Discher, D.E.; Schwarzbauer, J.E. Extracellular matrix elasticity directs stem cell differentiation. J. Musculoskelet. Neuronal Interact. 2007, 7, 335. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Kang, E.-S.; Kim, D.-S.; Suhito, I.R.; Lee, W.; Song, I.; Kim, T.-H. Two-dimensional material-based bionano platforms to control mesenchymal stem cell differentiation. Biomater. Res. 2018, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Hwang, S.J.; Bae, Y.C.; Jung, J.S. miR-21 Regulates adipogenic differentiation Through the modulation of TGF-β signaling in mesenchymal stem cells derived from human adipose tissue. STEM CELLS 2009, 27, 3093–3102. [Google Scholar] [CrossRef]
- Nieden, N.I.Z.; Kempka, G.; Rancourt, D.E.; Ahr, H.-J. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: Effect of cofactors on differentiating lineages. BMC Dev. Biol. 2005, 5, 1. [Google Scholar] [CrossRef]
- Derfoul, A.; Perkins, G.L.; Hall, D.J.; Tuan, R.S. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. STEM CELLS 2006, 24, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Sun, M.; Xu, H.; Gao, Y.; Liu, J.; Li, M. Characterization and therapeutic applications of mesenchymal stem cells for regenerative medicine. Tissue Cell 2020, 64, 101330. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. Development 1966, 16, 381–390. [Google Scholar]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! STEM CELLS Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal stem cells: From roots to boost. STEM CELLS 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ke, M.; Xu, L.; Liu, L.; Chen, X.; Xia, W.; Li, X.; Chen, Z.; Ma, J.; Liao, D.; et al. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation. Transplantation 2013, 95, 161–168. [Google Scholar] [CrossRef]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The Influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Golpanian, S.; El-Khorazaty, J.; Mendizabal, A.; Difede, D.L.; Suncion, V.Y.; Karantalis, V.; Fishman, J.E.; Ghersin, E.; Balkan, W.; Hare, J.M. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J. Am. Coll. Cardiol. 2015, 65, 125–132. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, K.S.; Jeon, J.H.; Lee, D.R.; Shim, S.H.; Kim, J.K.; Cha, D.-H.; Yoon, T.K.; Kim, G.J. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011, 346, 53–64. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Sibov, T.T.; Tobo, P.R.; Marti, L.C.; Pavon, L.F.; Oliveira, D.M.; Tobo, P.R.; Campos, A.H.; Paes, A.T.; Amaro, E.; Gamarra, L.F.; et al. Mesenchymal stem cells from umbilical cord blood: Parameters for isolation, characterization and adipogenic differentiation. Cytotechnology 2012, 64, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-X.; Sun, Y.; Shi, J.; Li, C.-L.; Fang, S.-B.; Wang, D.; Deng, X.-Q.; Wen, W.; Fu, Q.-L. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res. Ther. 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Higher chondrogenic potential of fibrous synovium-and adipose synovium–derived cells compared with subcutaneous fat–derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006, 54, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Milan, P.B.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 106, 61–72. [Google Scholar] [CrossRef]
- Burrow, K.L.; Hoyland, J.A.; Richardson, S.M. Human adipose-derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor-matched bone marrow mesenchymal stem cells during expansion in vitro. Stem Cells Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Oreffo, R.O.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef]
- Khojasteh, A.; Fahimipour, F.; Jafarian, M.; Sharifi, D.; Jahangir, S.; Khayyatan, F.; Eslaminejad, M.B. Bone engineering in dog mandible: Coculturing mesenchymal stem cells with endothelial progenitor cells in a composite scaffold containing vascular endothelial growth factor. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1767–1777. [Google Scholar] [CrossRef]
- Katagiri, W.; Watanabe, J.; Toyama, N.; Osugi, M.; Sakaguchi, K.; Hibi, H. Clinical study of bone regeneration by conditioned medium from mesenchymal stem cells after maxillary sinus floor elevation. Implant. Dent. 2017, 26, 607–612. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Liebergall, M.; Schroeder, J.; Mosheiff, R.; Gazit, Z.; Yoram, Z.; Rasooly, L.; Daskal, A.; Khoury, A.; Weil, Y.; Beyth, S. Stem cell–based therapy for prevention of delayed fracture union: A randomized and prospective preliminary study. Mol. Ther. 2013, 21, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.L.; Kimura, H.A.; Pinheiro, C.C.G.; Shimomura, K.; Nakamura, N.; Ferreira, J.R.; Gomoll, A.H.; Hernandez, A.J.; Bueno, D.F. Human synovial mesenchymal stem cells good manufacturing practices for articular cartilage regeneration. Tissue Eng. Part C Methods 2018, 24, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Bougioukli, S.; Sugiyama, O.; Pannell, W.; Ortega, B.; Tan, M.H.; Tang, A.H.; Yoho, R.; Oakes, D.A.; Lieberman, J.R. Gene therapy for bone repair using human cells: Superior osteogenic potential of bone morphogenetic protein 2–transduced mesenchymal stem cells derived from adipose tissue compared to bone marrow. Hum. Gene Ther. 2018, 29, 507–519. [Google Scholar] [CrossRef]
- Wakitani, S.; Goto, T.; Pineda, S.J.; Young, R.G.; Mansour, J.M.; Caplan, A.I.; Goldberg, V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am. Vol. 1994, 76, 579–592. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef]
- De Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; Broek, M.P.H.V.D.; Nizak, R.; Van Rijen, M.H.P.; De Weger, R.A.; Dhert, W.J.A.; Saris, D.B. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. STEM CELLS 2016, 35, 256–264. [Google Scholar] [CrossRef]
- Orozco, L.; Soler, R.; Morera, C.; Alberca, M.; Sánchez, A.; García-Sancho, J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: A pilot study. Transplant. 2011, 92, 822–828. [Google Scholar] [CrossRef]
- Pettine, K.A.; Suzuki, R.K.; Sand, T.T.; Murphy, M.B. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int. Orthop. 2017, 41, 2097–2103. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am. J. Sports Med. 2016, 45, 82–90. [Google Scholar] [CrossRef]
- Chen, M.; Guo, W.; Gao, S.; Hao, C.; Shen, S.; Zhang, Z.; Wang, Z.; Li, X.; Jing, X.; Zhang, X.; et al. Biomechanical stimulus based strategies for meniscus tissue engineering and regeneration. Tissue Eng. Part B Rev. 2018, 24, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Gülecyüz, M.F.; Macha, K.; Pietschmann, M.F.; Ficklscherer, A.; Sievers, B.; Roßbach, B.P.; Jansson, V.; Müller, P.E. Allogenic myocytes and mesenchymal stem cells partially improve fatty rotator cuff degeneration in a rat model. Stem Cell Rev. Rep. 2018, 14, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med. J. 2011, 52, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 2015, 78, 436–447. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, H.; Dai, G.; Wang, X.; Hua, R.; Liu, X.; Wang, P.; Chen, G.; Yue, W.; An, Y. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013, 1532, 76–84. [Google Scholar] [CrossRef]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I trial of intrathecal mesenchymal stem cell-derived neural progenitors in progressive multiple sclerosis. EBioMedicine 2018, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shichinohe, H.; Kawabori, M.; Iijima, H.; Teramoto, T.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Terasaka, S.; Arato, T.; Houkin, K. Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): A study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Venkatesh, K.; Sen, D. mesenchymal stem cells as a source of dopaminergic neurons: A potential cell based therapy for Parkinson’s disease. Curr. Stem Cell Res. Ther. 2017, 12, 326–347. [Google Scholar] [CrossRef]
- Tse, H.-F.; Kwong, Y.-L.; Chan, J.K.F.; Lo, G.; Ho, C.-L.; Lau, C.-P. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 2003, 361, 47–49. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Petersen, B.E. Bone marrow as a potential source of hepatic oval cells. Science 1999, 284, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Von Der Mark, K.; Park, J. Engineering biocompatible implant surfaces. Prog. Mater. Sci. 2013, 58, 327–381. [Google Scholar] [CrossRef]
- Lehnert, D.; Wehrle-Haller, B.; David, C.; Weiland, U.; Ballestrem, C.; Imhof, B.A.; Bastmeyer, M. Cell behaviour on micropatterned substrata: Limits of extracellular matrix geometry for spreading and adhesion. J. Cell Sci. 2004, 117, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Zhu, X.; Fan, H.; Xiao, Y.; Li, D.; Zhang, H.; Luxbacher, T.; Zhang, X. Effect of surface structure on protein adsorption to biphasic calcium-phosphate ceramics in vitro and in vivo. Acta Biomater. 2009, 5, 1311–1318. [Google Scholar] [CrossRef]
- Higgins, A.M.; Brown, J.L. Osteoinductive biomaterial geometries for bone regenerative engineering. Curr. Pharm. Des. 2013, 19, 3446–3455. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell Shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Lavenus, S.; Ricquier, J.-C.; Louarn, G.; Layrolle, P. Cell interaction with nanopatterned surface of implants. Nanomedicine 2010, 5, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, J.; Scheideler, L.; Altebaeumer, T.; Geis-Gerstorfer, J.; Kern, D. Cellular reactions of osteoblasts to micron-and submicron-scale porous structures of titanium surfaces. Cells Tissues Organs 2004, 178, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Limat, A.; Hunziker, T.; Breitkreutz, D.; Fusenig, N.E.; Braathen, L.R. Organotypic cocultures as models to study cell-cell and cell-matrix interactions of human hair follicle cells. Ski. Pharmacol. Physiol. 1994, 7, 47–54. [Google Scholar] [CrossRef]
- Giancotti, F.G. Integrin signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.E.; McMurray, R.J.; Biggs, M.J.P.; Kantawong, F.; Oreffo, R.O.; Dalby, M.J. Nanotopographical control of stem cell differentiation. J. Tissue Eng. 2010, 2010, 120623. [Google Scholar] [CrossRef]
- Harrison, R.G. On the stereotropism of embryonic cells. Science 1911, 34, 279–281. [Google Scholar] [CrossRef]
- Lozano-Calderón, S.A.; Swaim, S.O.; Federico, A.; Anderson, M.E.; Gebhardt, M.C. Predictors of soft-tissue complications and deep infection in allograft reconstruction of the proximal tibia. J. Surg. Oncol. 2016, 113, 811–817. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nat. Cell Biol. 2002, 418, 41–49. [Google Scholar] [CrossRef]
- Yang, W.; Han, W.; He, W.; Li, J.; Wang, J.; Feng, H.; Qian, Y. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater. Sci. Eng. C 2016, 60, 45–53. [Google Scholar] [CrossRef]
- Matsuzaka, K.; Yoshinari, M.; Shimono, M.; Inoue, T. Effects of multigrooved surfaces on osteoblast-like cellsin vitro: Scanning electron microscopic observation and mRNA expression of osteopontin and osteocalcin. J. Biomed. Mater. Res. 2003, 68, 227–234. [Google Scholar] [CrossRef]
- Faia-Torres, A.B.; Guimond-Lischer, S.; Rottmar, M.; Charnley, M.; Goren, T.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials 2014, 35, 9023–9032. [Google Scholar] [CrossRef] [PubMed]
- Hefti, T.; Frischherz, M.; Spencer, N.D.; Hall, H.; Schlottig, F. A comparison of osteoclast resorption pits on bone with titanium and zirconia surfaces. Biomaterials 2010, 31, 7321–7331. [Google Scholar] [CrossRef]
- Faia-Torres, A.B.; Charnley, M.; Goren, T.; Guimond-Lischer, S.; Rottmar, M.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Osteogenic differentiation of human mesenchymal stem cells in the absence of osteogenic supplements: A surface-roughness gradient study. Acta Biomater. 2015, 28, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-H.; Wilson, W.H.; Schroeder, D.C.; Allen, M.J.; Holden, M.T.G.; Parkhill, J.; Barrell, B.G.; Churcher, C.; Hamlin, N.; Mungall, K.; et al. TAZ, a Transcriptional modulator of mesenchymal stem cell differentiation. Science 2005, 309, 1074–1078. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nat. Cell Biol. 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Schantz, J.-T.; Lim, T.C.; Ning, C.; Teoh, S.H.; Tan, K.C.; Wang, S.C.; Hutmacher, D.W. Cranioplasty after trephination using a novel biodegradable burr hole cover: Technical case report. Oper. Neurosurg. 2006, 58. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xie, W.; Yu, L.; Camacho, L.C.; Nie, C.; Zhang, M.; Haag, R.; Wei, Q. Surface roughness gradients reveal topography-specific mechanosensitive responses in human mesenchymal stem cells. Small 2020, 16, e1905422. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.I.; Goriainov, V.; Kanczler, J.; Black, C.R.; Turner, L.-A.; Meek, R.M.; Burgess, K.; MacLaren, I.; Dalby, M.J.; Oreffo, R.O.; et al. Nanopatterned titanium implants accelerate bone formation in vivo. ACS Appl. Mater. Interfaces 2020, 12, 33541–33549. [Google Scholar] [CrossRef]
- Sjöström, T.; McNamara, L.E.; Dalby, M.J.; Meek, R.M.D.; Su, B. 2D and 3D Nanopatterning of titanium for enhancing osteoinduction of stem cells at implant surfaces. Adv. Heal. Mater. 2013, 2, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, K.; Tao, B.; Dai, L.; Yu, Y.; Mu, C.; Shen, X.; Hu, Y.; He, Y.; Cai, K. Multilayered coating of titanium implants promotes coupled osteogenesis and angiogenesis in vitro and in vivo. Acta Biomater. 2018, 74, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.L.; Cox, M.D.; Richter, G.T.; Dornhoffer, J.L. A comparative review of osseointegration failure between osseointegrated bone conduction device models in pediatric patients. Otol. Neurotol. 2016, 37, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, T.; Dalby, M.J.; Hart, A.; Tare, R.; Oreffo, R.O.; Su, B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009, 5, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Khang, D.; Choi, J.; Im, Y.-M.; Kim, Y.-J.; Jang, J.-H.; Kang, S.S.; Nam, T.-H.; Song, J.; Park, J.-W. Role of subnano-, nano- and submicron-surface features on osteoblast differentiation of bone marrow mesenchymal stem cells. Biomaterials 2012, 33, 5997–6007. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Bossert, J.; Jandt, K.D. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B Biointerfaces 2006, 49, 136–144. [Google Scholar] [CrossRef]
- Xu, K.; Chen, W.; Hu, Y.; Shen, X.; Xu, G.; Ran, Q.; Yu, Y.; Mu, C.; Cai, K. Influence of strontium ions incorporated into nanosheet-pore topographical titanium substrates on osteogenic differentiation of mesenchymal stem cells in vitro and on osseointegration in vivo. J. Mater. Chem. B 2016, 4, 4549–4564. [Google Scholar] [CrossRef]
- Xu, K.; Shen, X.; Chen, W.; Mu, C.; Jiang, C.; Zhao, Y.; Cai, K. Nanosheet-pore topographical titanium substrates: A biophysical regulator of the fate of mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 1797–1810. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Zhang, X.; Wang, W.; Zhang, Y.; Wu, Z.; Chu, P.K. The osteogenic activity of strontium loaded titania nanotube arrays on titanium substrates. Biomaterials 2013, 34, 19–29. [Google Scholar] [CrossRef]
- Huang, Y.; Zha, G.; Luo, Q.; Zhang, J.; Zhang, F.; Li, X.; Zhao, S.; Zhu, W.; Li, X. The construction of hierarchical structure on Ti substrate with superior osteogenic activity and intrinsic antibacterial capability. Sci. Rep. 2014, 4, 6172. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef]
- Lotz, E.M.; Berger, M.B.; Schwartz, Z.; Boyan, B.D. Regulation of osteoclasts by osteoblast lineage cells depends on titanium implant surface properties. Acta Biomater. 2018, 68, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, X.; Gao, L.; Jing, L.; Zhou, Q.; Chang, J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 73, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Xia, L.; Lin, K.; Jiang, X.; Xu, Y.; Zhang, M.; Chang, J.; Zhang, Z. Enhanced osteogenesis through nano-structured surface design of macroporous hydroxyapatite bioceramic scaffolds via activation of ERK and p38 MAPK signaling pathways. J. Mater. Chem. B 2013, 1, 5403–5416. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; De Boer, J.; Barradas, A.M.C.; De Ruiter, A.; Walsh, W.R.; Van Blitterswijk, C.A.; De Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, Z.; Han, Y. Formation and osteoblast behavior of HA nano-rod/fiber patterned coatings on tantalum in porous and compact forms. J. Mater. Chem. B 2015, 3, 5442–5454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, B.; Lu, S.; Zhang, L.; Han, Y. Regulation of osteoblast proliferation and differentiation by interrod spacing of Sr-HA nanorods on microporous titania coatings. ACS Appl. Mater. Interf. 2013, 5, 5358–5365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, B.; Han, Y.; Zhao, L. The osteogenic capacity of biomimetic hierarchical micropore/nanorod-patterned Sr-HA coatings with different interrod spacings. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, L.; Li, B.; Han, Y. Nanorod diameter modulated osteogenic activity of hierarchical micropore/nanorod-patterned coatings via a Wnt/β-catenin pathway. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1719–1731. [Google Scholar] [CrossRef]
- Loye, A.M.; Kinser, E.R.; Bensouda, S.; Shayan, M.; Davis, R.; Wang, R.; Chen, Z.; Schwarz, U.D.; Schroers, J.; Kyriakides, T.R. Regulation of mesenchymal stem cell differentiation by nanopatterning of bulk metallic glass. Sci. Rep. 2018, 8, 8758. [Google Scholar] [CrossRef]
- Kumar, G.; Tang, H.X.; Schroers, J. Nanomoulding with amorphous metals. Nat. Cell Biol. 2009, 457, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Staffier, P.A.; Blawzdziewicz, J.; Schwarz, U.D.; Schroers, J. Atomically smooth surfaces through thermoplastic forming of metallic glass. Appl. Phys. Lett. 2010, 97, 101907. [Google Scholar] [CrossRef]
- Kumar, G.; Blawzdziewicz, J.; Schroers, J. Controllable nanoimprinting of metallic glasses: Effect of pressure and interfacial properties. Nanotechnology 2013, 24, 105301. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, C.; Shek, C. Bulk metallic glasses. Mater. Sci. Eng. R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Lee, D.-K.; Yi, T.; Park, K.-E.; Lee, H.-J.; Cho, Y.-K.; Lee, S.J.; Lee, J.; Park, J.H.; Lee, M.-Y.; Song, S.U.; et al. Non-invasive characterization of the adipogenic differentiation of human bone marrow-derived mesenchymal stromal cells by HS-SPME/GC-MS. Sci. Rep. 2014, 4, 6550. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Zhai, D.; Zhang, N.; Liu, J.; Fang, B.; Chang, J.; Lin, K. Designing ordered micropatterned hydroxyapatite bioceramics to promote the growth and osteogenic differentiation of bone marrow stromal cells. J. Mater. Chem. B 2015, 3, 968–976. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, C.; Wang, X.; Shi, M.; Zhu, Y.; Jing, L.; Wu, C.; Chang, J. Stimulation of osteogenesis and angiogenesis by micro/nano hierarchical hydroxyapatite via macrophage immunomodulation. Nanoscale 2019, 11, 17699–17708. [Google Scholar] [CrossRef]
- Xia, L.; Lin, K.; Jiang, X.; Fang, B.; Xu, Y.; Liu, J.; Zeng, D.; Zhang, M.; Zhang, X.; Chang, J.; et al. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells. Biomaterials 2014, 35, 8514–8527. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Gan, J.; Zhang, Z.; Chen, H.; Jiang, X.; Chang, J. Tailoring the nanostructured surfaces of hydroxyapatite bioceramics to promote protein adsorption, osteoblast growth, and osteogenic differentiation. ACS Appl. Mater. Interf. 2013, 5, 8008–8017. [Google Scholar] [CrossRef]
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017, 122, 74–83. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Lee, C.Z.W.; Kozaki, T.; Ginhoux, F. Studying tissue macrophages in vitro: Are iPSC-derived cells the answer? Nat. Rev. Immunol. 2018, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Alaarg, A.; Pérez-Medina, C.; Metselaar, J.M.; Nahrendorf, M.; Fayad, Z.A.; Storm, G.; Mulder, W.J.M. Applying nanomedicine in maladaptive inflammation and angiogenesis. Adv. Drug Deliv. Rev. 2017, 119, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hwang, J.W.; Oh, J.-H.; Park, C.H.; Chung, S.H.; Lee, Y.-S.; Baek, J.-H.; Ryoo, H.-M.; Woo, K.M. Effects of the fibrous topography-mediated macrophage phenotype transition on the recruitment of mesenchymal stem cells: An in vivo study. Biomaterials 2017, 149, 77–87. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Rosamilia, A.; Kadam, V.; Truong, Y.B.; Werkmeister, J.A.; Gargett, C.E. Blended nanostructured degradable mesh with endometrial mesenchymal stem cells promotes tissue integration and anti-inflammatory response in vivo for pelvic floor application. Biomacromolecules 2018, 20, 454–468. [Google Scholar] [CrossRef]
- Li, K.; Hu, D.; Xie, Y.; Huang, L.; Zheng, X. Sr-doped nanowire modification of Ca–Si-based coatings for improved osteogenic activities and reduced inflammatory reactions. Nanotechnology 2018, 29, 084001. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Zheng, S.; Wang, Y.; Feng, M.; Shuai, Y.; Duan, B.; Fan, X.; Yang, M.; Mao, C. Air-plasma treatment promotes bone-like nano-hydroxylapatite formation on protein films for enhanced in vivo osteogenesis. Biomater. Sci. 2019, 7, 2326–2334. [Google Scholar] [CrossRef]
- Lin, K.-F.; He, S.; Song, Y.; Wang, C.-M.; Gao, Y.; Li, J.-Q.; Tang, P.; Wang, Z.; Bi, L.; Pei, G.-X. Low-temperature additive manufacturing of biomimic three-dimensional hydroxyapatite/collagen scaffolds for bone regeneration. ACS Appl. Mater. Interf. 2016, 8, 6905–6916. [Google Scholar] [CrossRef]
- Wagener, V.; Boccaccini, A.R.; Virtanen, S. Protein-adsorption and Ca-phosphate formation on chitosan-bioactive glass composite coatings. Appl. Surf. Sci. 2017, 416, 454–460. [Google Scholar] [CrossRef]
- Cardoso, G.B.C.; Maniglio, D.; Volpato, F.Z.F.Z.; Tondon, A.; Migliaresi, C.; Kaunas, R.; Zavaglia, C.A.C.C.A.C. Oleic acid surfactant in polycaprolactone/hydroxyapatite-composites for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 1076–1082. [Google Scholar] [CrossRef]
- Gogoi, D.; Choudhury, A.J.; Chutia, J.; Pal, A.R.; Khan, M.; Choudhury, M.; Pathak, P.; Das, G.; Patil, D.S. Development of advanced antimicrobial and sterilized plasma polypropylene grafted muga (antheraea assama) silk as suture biomaterial. Biopolymers 2014, 101, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hao, W.; Li, Y.; Yao, J.; Zheng, G.; Li, H.; Yang, J.; Chen, S. Erratum to: Hydroxyapatite/regenerated silk fibroin scaffold-enhanced osteoinductivity and osteoconductivity of bone marrow-derived mesenchymal stromal cells. Biotechnol. Lett. 2013, 35, 1349–1350. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.W.; Wang, Y.F.; Zhao, H.S.; Xiong, S.; Wu, Y.; Heng, B.C.; An, C.R.; Zhu, G.H.; Xie, D.H. Composite scaffolds of nano-hydroxyapatite and silk fibroin enhance mesenchymal stem cell-based bone regeneration via the interleukin 1 alpha autocrine/paracrine signaling loop. Biomaterials 2015, 49, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef]
- Qiu, J.; Li, J.; Wang, S.; Ma, B.; Zhang, S.; Guo, W.; Zhang, X.; Tang, W.; Sang, Y.; Liu, H. TiO2 nanorod array constructed nanotopography for regulation of mesenchymal stem cells fate and the realization of location-committed stem cell differentiation. Small 2016, 12, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials 2015, 39, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Trichet, V.; Le Chevalier, S.; Hoornaert, A.; Louarn, G.; Layrolle, P. Cell differentiation and osseointegration influenced by nanoscale anodized titanium surfaces. Nanomedicine 2012, 7, 967–980. [Google Scholar] [CrossRef]
- Oh, S.; Daraio, C.; Chen, L.-H.; Pisanic, T.R.; Fiñones, R.R.; Jin, S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. Part A 2006, 78, 97–103. [Google Scholar] [CrossRef]
- Hou, W.; Fu, H.; Liu, X.; Duan, K.; Lu, X.; Lu, M.; Sun, T.; Guo, T.; Weng, J. Cation Channel Transient Receptor Potential Vanilloid 4 Mediates Topography-Induced Osteoblastic Differentiation of Bone Marrow Stem Cells. ACS Biomater. Sci. Eng. 2019, 5, 6520–6529. [Google Scholar] [CrossRef]
- Lee, H.-P.; Stowers, R.; Chaudhuri, O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat. Commun. 2019, 10, 529. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, W.; Li, Z.; Xu, L.; Li, J.; Jin, Y.; Wang, G.; Liu, X.; Huang, Q. Effects of a hybrid micro/nanorod topography-modified titanium implant on adhesion and osteogenic differentiation in rat bone marrow mesenchymal stem cells. Int. J. Nanomed. 2013, 8, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.J.; Gadegaard, N.; Tsimbouri, P.M.; Burgess, K.V.; McNamara, L.E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R.O.C.; Dalby, M.J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 2011, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Abagnale, G.; Steger, M.; Nguyen, V.H.; Hersch, N.; Sechi, A.; Joussen, S.; Denecke, B.; Merkel, R.; Hoffmann, B.; Dreser, A.; et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 2015, 61, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Rubehn, B.; Stieglitz, T. In vitro evaluation of the long-term stability of polyimide as a material for neural implants. Biomaterials 2010, 31, 3449–3458. [Google Scholar] [CrossRef]
- Sun, Y.; Lacour, S.P.; Brooks, R.A.; Rushton, N.; Fawcett, J.W.; Cameron, R.E. Assessment of the biocompatibility of photosensitive polyimide for implantable medical device use. J. Biomed. Mater. Res. Part A 2009, 90, 648–655. [Google Scholar] [CrossRef]
- Prichard, H.L.; Reichert, W.M.; Klitzman, B. Adult adipose-derived stem cell attachment to biomaterials. Biomaterials 2007, 28, 936–946. [Google Scholar] [CrossRef][Green Version]
- Kolambkar, Y.M.; Bajin, M.; Wojtowicz, A.; Hutmacher, D.W.; Garcia, A.J.; Guldberg, R.E. Nanofiber orientation and surface functionalization modulate human mesenchymal stem cell behavior in vitro. Tissue Eng. Part A 2013, 20, 398–409. [Google Scholar] [CrossRef]
- Polini, A.; Pisignano, D.; Parodi, M.; Quarto, R.; Scaglione, S. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE 2011, 6, e26211. [Google Scholar] [CrossRef]
- Lü, L.-X.; Wang, Y.-Y.; Mao, X.; Xiao, Z.; Huang, N.-P. The effects of PHBV electrospun fibers with different diameters and orientations on growth behavior of bone-marrow-derived mesenchymal stem cells. Biomed. Mater. 2012, 7, 015002. [Google Scholar] [CrossRef]
- Lü, L.-X.; Zhang, X.-F.; Wang, Y.-Y.; Ortiz, L.; Mao, X.; Jiang, Z.-L.; Xiao, Z.-D.; Huang, N.-P. Effects of hydroxyapatite-containing composite nanofibers on osteogenesis of mesenchymal stem cells in vitro and bone regeneration in vivo. ACS Appl. Mater. Interf. 2013, 5, 319–330. [Google Scholar] [CrossRef]
- Zhang, N.; Xiao, Q.-R.; Man, X.-Y.; Liu, H.-X.; Lü, L.-X.; Huang, N.-P. Spontaneous osteogenic differentiation of mesenchymal stem cells on electrospun nanofibrous scaffolds. RSC Adv. 2016, 6, 22144–22152. [Google Scholar] [CrossRef]
- Liu, F.; Kohlmeier, S.; Wang, C.-Y. Wnt signaling and skeletal development. Cell. Signal. 2008, 20, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, J.; Guo, S.; Zhu, H.; Zhu, Z.; Li, H.; Wang, Y.; Zhang, C.; Chang, J. Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/β-catenin signaling pathway. Biomaterials 2015, 55, 1–11. [Google Scholar] [CrossRef]
- Nishimura, R.; Hata, K.; Harris, S.; Ikeda, F.; Yoneda, T. Core-binding factor α1 (Cbfa1) induces osteoblastic differentiation of C2C12 cells without interactions with Smad1 and Smad5. Bone 2002, 31, 303–312. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Q.; Zhang, Y.; Zhao, L. Involvement of ILK/ERK1/2 and ILK/p38 pathways in mediating the enhanced osteoblast differentiation by micro/nanotopography. Acta Biomater. 2014, 10, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Izadpanahi, M.; Seyedjafari, E.; Arefian, E.; Hamta, A.; Hosseinzadeh, S.; Kehtari, M.; Soleimani, M.; Kehtari, M. Nanotopographical cues of electrospun PLLA efficiently modulate non-coding RNA network to osteogenic differentiation of mesenchymal stem cells during BMP signaling pathway. Mater. Sci. Eng. C 2018, 93, 686–703. [Google Scholar] [CrossRef]
- Kaplan, L.; Fu, F. Clinical Applications of Orthopedic Tissue Engineering. Orthop. Tissue Eng. 2004, 261. [Google Scholar] [CrossRef]
- Teh, T.K.; Toh, S.-L.; Goh, J.C. Aligned fibrous scaffolds for enhanced mechanoresponse and tenogenesis of mesenchymal stem cells. Tissue Eng. Part A 2013, 19, 1360–1372. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Debski, R.E.; Zeminski, J.; Abramowitch, S.D.; Saw, S.; Fenwick, J.A. Injury and repair of ligaments and tendons. Annu. Rev. Biomed. Eng. 2000, 2, 83–118. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Vogrin, T.M.; Abramowitch, S.D. Healing and repair of ligament injuries in the knee. J. Am. Acad. Orthop. Surg. 2000, 8, 364–372. [Google Scholar] [CrossRef]
- Hogan, M.V.; Bagayoko, N.; James, R.; Starnes, T.; Katz, A.; Chhabra, B.A. Tissue engineering solutions for tendon repair. J. Am. Acad. Orthop. Surg. 2011, 19, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, S.K.; Tsai, T.-L.; Duenwald-Kuehl, S.E.; Vanderby, R.; Li, W.-J. Tenogenic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells dictated by properties of braided submicron fibrous scaffolds. Biomaterials 2014, 35, 6907–6917. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Chen, J.L.; Shen, W.-L.; Nguyen, T.M.H.; Gao, L.; Ouyang, H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 2010, 31, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.Y.; Shen, W.; Yeung, C.W.; Zhao, Y.; Cheng, S.H.; Chu, P.K.; Chan, D.; Chan, G.C.; Cheung, K.M.; Yeung, K.W.K.; et al. Functional replication of the tendon tissue microenvironment by a bioimprinted substrate and the support of tenocytic differentiation of mesenchymal stem cells. Biomaterials 2012, 33, 7686–7698. [Google Scholar] [CrossRef]
- Ker, E.D.; Nain, A.S.; Weiss, L.E.; Wang, J.; Suhan, J.; Amon, C.H.; Campbell, P.G. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 2011, 32, 8097–8107. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Mizuno, M.; Kuboki, Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J. Biochem. 2001, 129, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Teh, T.K.; Toh, S.-L.; Goh, J.C. Aligned hybrid silk scaffold for enhanced differentiation of mesenchymal stem cells into ligament fibroblasts. Tissue Eng. Part C Methods 2011, 17, 687–703. [Google Scholar] [CrossRef]
- Banes, A.J.; Lee, G.; Graff, R.; Otey, C.; Archambault, J.; Tsuzaki, M.; Elfervig, M.; Qi, J. Mechanical forces and signaling in connective tissue cells: Cellular mechanisms of detection, transduction, and responses to mechanical deformation. Curr. Opin. Orthop. 2001, 12, 389–396. [Google Scholar] [CrossRef]
- Hung, C.T.; Allen, F.D.; Pollack, S.R.; Attia, E.T.; Hannafin, J.A.; Torzilli, P.A. Intracellular calcium response of ACL and MCL ligament fibroblasts to fluid-induced shear stress. Cell. Signal. 1997, 9, 587–594. [Google Scholar] [CrossRef]
- Tsuzaki, M.; Bynum, D.; Almekinders, L.; Yang, X.; Faber, J.; Banes, A. ATP modulates load-inducible IL-1, COX 2, and MMP-3 gene expression in human tendon cells. J. Cell. Biochem. 2003, 89, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, M.; Gelman, L.; Lutz, R.; Maier, S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.G.; Handorf, A.M.; Allee, T.J.; Li, W.-J. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng. Part A 2013, 19, 1265–1274. [Google Scholar] [CrossRef]
- Li, W.-J.; Jiang, Y.J.; Tuan, R.S. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006, 12, 1775–1785. [Google Scholar] [CrossRef]
- Ralphs, J.; Waggett, A.D.; Benjamin, M. Actin stress fibres and cell-cell adhesion molecules in tendons: Organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002, 21, 67–74. [Google Scholar] [CrossRef]
- Diekman, B.O.; Christoforou, N.; Willard, V.P.; Sun, H.; Sanchez-Adams, J.; Leong, K.W.; Guilak, F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 19172–19177. [Google Scholar] [CrossRef]
- Li, W.-J.; Cooper, J.A.; Mauck, R.L.; Tuan, R.S. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Lauro, B.B.; Yang, G.; Debski, R.E.; Musahl, V.; Tuan, R.S. Braided and stacked electrospun nanofibrous scaffolds for tendon and ligament tissue engineering. Tissue Eng. Part A 2017, 23, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.B.; Chainani, A.; Hippensteel, K.; Kishan, A.; Gilchrist, C.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef]
- Vuornos, K.; Björninen, M.; Talvitie, E.; Paakinaho, K.; Kellomäki, M.; Huhtala, H.; Miettinen, S.; Seppänen-Kaijansinkko, R.; Haimi, S. Human adipose stem cells differentiated on braided polylactide scaffolds is a potential approach for tendon tissue engineering. Tissue Eng. Part A 2016, 22, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Song, H.-X.; Hu, J.-J.; Tang, Q.-M.; Zhu, T.; Shen, W.-L.; Chen, J.-L.; Liu, H.; Heng, B.C.; et al. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials 2015, 44, 173–185. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Cao, Y. Recent Progress in Cartilage Tissue Engineering—Our Experience and Future Directions. Engineering 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical Technique and Rehabilitation to Treat Chondral Defects. Clin. Orthop. Relat. Res. 2001, 391, S362–S369. [Google Scholar] [CrossRef]
- Hangody, L.; Füles, P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints. J. Bone Jt. Surg. Am. Vol. 2003, 85, 25–32. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.J.; Pascual-Garrido, C.; Chubinskaya, S.; Potter, H.G.; Warren, R.F.; Cole, B.J.; Rodeo, S.A. Restoration of articular cartilage. J. Bone Jt. Surg. Am. Vol. 2014, 96, 336–344. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, G.J.V.M.; Brittberg, M.; Dennis, J.; Bastiaansen-Jenniskens, Y.; Erben, R.; Konttinen, Y.; Luyten, F. Cartilage repair: Past and future-lessons for regenerative medicine. J. Cell. Mol. Med. 2009, 13, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, C.; Gordeladze, J.; Noel, D. Tissue engineering through autologous mesenchymal stem cells. Curr. Opin. Biotechnol. 2004, 15, 406–410. [Google Scholar] [CrossRef]
- Caplan, A.I. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Baker, B.M.; Sen, S.; Wible, E.E.; Elliott, D.M.; Mauck, R.L. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat. Mater. 2009, 8, 986–992. [Google Scholar] [CrossRef]
- Baker, B.M.; Mauck, R.L. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 2007, 28, 1967–1977. [Google Scholar] [CrossRef]
- Baker, B.M.; Nathan, A.S.; Gee, A.O.; Mauck, R.L. The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials 2010, 31, 6190–6200. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Z.; Law, J.B.K.; He, A.Y.; Abbas, A.A.; Denslin, V.; Kamarul, T.; Hui, J.; Lee, E.H. The Combined effect of substrate stiffness and surface topography on chondrogenic differentiation of mesenchymal stem cells. Tissue Eng. Part A 2017, 23, 43–54. [Google Scholar] [CrossRef]
- Jurvelin, J.; Müller, D.J.; Wong, M.; Studer, D.; Engel, A.; Hunziker, E. Surface and subsurface morphology of bovine humeral articular cartilage as assessed by atomic force and transmission electron microscopy. J. Struct. Biol. 1996, 117, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-N.; Yang, Z.; Hui, J.H.; Ouyang, H.-W.; Lee, E.H. Cartilaginous ECM component-modification of the micro-bead culture system for chondrogenic differentiation of mesenchymal stem cells. Biomaterials 2007, 28, 4056–4067. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Hwang, N.S.; Canver, A.C.; Theprungsirikul, P.; Lin, D.W.; Elisseeff, J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008, 27, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.J.; Malda, J.; Sah, R.L.; Hutmacher, D.W. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng. Part B Rev. 2009, 15, 143–157. [Google Scholar] [CrossRef]
- Hayes, A.J.; Hall, A.; Brown, L.; Tubo, R.; Caterson, B. Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J. Histochem. Cytochem. 2007, 55, 853–866. [Google Scholar] [CrossRef]
- McMahon, R.E.; Wang, L.; Skoracki, R.; Mathur, A.B. Development of nanomaterials for bone repair and regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 101, 387–397. [Google Scholar] [CrossRef]
- Walpole, A.R.; Xia, Z.; Wilson, C.W.; Triffitt, J.T.; Wilshaw, P.R. A novel nano-porous alumina biomaterial with potential for loading with bioactive materials. J. Biomed. Mater. Res. Part A 2009, 90, 46–54. [Google Scholar] [CrossRef]
- Zhang, B.; Pei, X.; Song, P.; Sun, H.; Li, H.; Fan, Y.; Jiang, Q.; Zhou, C.; Zhang, X. Porous bioceramics produced by inkjet 3D printing: Effect of printing ink formulation on the ceramic macro and micro porous architectures control. Compos. Part B Eng. 2018, 155, 112–121. [Google Scholar] [CrossRef]
- Schumacher, M.; Deisinger, U.; Detsch, R.; Ziegler, G. Indirect rapid prototyping of biphasic calcium phosphate scaffolds as bone substitutes: Influence of phase composition, macroporosity and pore geometry on mechanical properties. J. Mater. Sci. Mater. Electron. 2010, 21, 3119–3127. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Zreiqat, H. Porous diopside (CaMgSi2O6) scaffold: A promising bioactive material for bone tissue engineering. Acta Biomater. 2010, 6, 2237–2245. [Google Scholar] [CrossRef]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 045008. [Google Scholar] [CrossRef]
- Daugela, P.; Pranskunas, M.; Juodzbalys, G.; Liesiene, J.; Baniukaitiene, O.; Afonso, A.; Gomes, P.S. Novel cellulose/hydroxyapatite scaffolds for bone tissue regeneration: In vitro and in vivo study. J. Tissue Eng. Regen. Med. 2018, 12, 1195–1208. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Multi-layered polydopamine coatings for the immobilization of growth factors onto highly-interconnected and bimodal PCL/HA-based scaffolds. Mater. Sci. Eng. C 2020, 117, 111245. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Immobilization of BMP-2 and VEGF within multilayered polydopamine-coated scaffolds and the resulting osteogenic and angiogenic synergy of co-cultured human mesenchymal stem cells and human endothelial progenitor cells. Int. J. Mol. Sci. 2020, 21, 6418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Zhao, F.; Mc Garrigle, M.J.; Vaughan, T.J.; McNamara, L.M. In silico study of bone tissue regeneration in an idealised porous hydrogel scaffold using a mechano-regulation algorithm. Biomech. Model. Mechanobiol. 2017, 17, 5–18. [Google Scholar] [CrossRef]
- Raafat, A.I.; Abd-Allah, W.M. In vitro apatite forming ability and ketoprofen release of radiation synthesized (gelatin-polyvinyl alcohol)/bioglass composite scaffolds for bone tissue regeneration. Polym. Compos. 2016, 39, 606–615. [Google Scholar] [CrossRef]
- Shim, K.-S.; Kim, H.J.; Yun, Y.-P.; Jeon, D.I.; Kim, H.J.; Park, K.; Song, H.-R. Surface immobilization of biphasic calcium phosphate nanoparticles on 3D printed poly (caprolactone) scaffolds enhances osteogenesis and bone tissue regeneration. J. Ind. Eng. Chem. 2017, 55, 101–109. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2015, 68, 355–369. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Ashman, A.; Moss, M.L. Implantation of porous polymethylmethacrylate resin for tooth and bone replacement. J. Prosthet. Dent. 1977, 37, 657–665. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Young, G.; Shortkroff, S.; Louie, L.K.; Sledge, C.B.; Yannas, I.V.; Spector, M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials 1997, 18, 769–776. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.W.; Lee, Y.M.; Lee, H.B.; Khang, G. Macroporous biodegradable natural/synthetic hybrid scaffolds as small intestine submucosa impregnated poly(D,L-lactide-co-glycolide) for tissue-engineered bone. J. Biomater. Sci. Polym. Ed. 2004, 15, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Davies, J.E.; Kim, S. Three-dimensional matrices of calcium polyphosphates support bone growth in vitro and in vivo. J. Mater. Sci. Mater. Electron. 1998, 9, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.J.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Polymer concepts in tissue engineering. J. Biomed. Mater. Res. 1998, 43. [Google Scholar] [CrossRef]
- Ishaug, S.L.; Crane, G.M.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997, 36. [Google Scholar] [CrossRef]
- Akay, G.; Birch, M.; Bokhari, M. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 2004, 25, 3991–4000. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Delivery Systems for the BMPs Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. BMPS 2001, 83, 105–115. [Google Scholar]
- Van Tienen, T.G.; Heijkants, R.G.J.C.; De Groot, J.H.; Pennings, A.J.; Schouten, A.J.; Veth, R.P.H.; Buma, P. Replacement of the Knee Meniscus by a Porous Polymer Implant. Am. J. Sports Med. 2006, 34, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Sha’Ban, M.; Kim, S.H.; Ruszymah, B.; Khang, G. Fibrin and poly(lactic-co-glycolic acid) hybrid scaffold promotes early chondrogenesis of articular chondrocytes: An in vitro study. J. Orthop. Surg. Res. 2008, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nie, H.; Xu, Z.; Niu, X.; Guo, S.; Yin, J.; Guo, F.; Li, G.; Wang, Y.; Zhang, C. The effect of 3D nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PLoS ONE 2014, 9, e111566. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H. Titanium alloys in total joint replacement-A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Itälä, A.I.; Ylänen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef]
- Braem, A.; Chaudhari, A.; Cardoso, M.V.; Schrooten, J.; Duyck, J.; Vleugels, J. Peri- and intra-implant bone response to microporous Ti coatings with surface modification. Acta Biomater. 2014, 10, 986–995. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Chan, O.; Coathup, M.; Nesbitt, A.; Ho, C.-Y.; Hing, K.; Buckland, T.; Campion, C.; Blunn, G.W. The effects of microporosity on osteoinduction of calcium phosphate bone graft substitute biomaterials. Acta Biomater. 2012, 8, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Campion, C.R.; Chander, C.; Buckland, T.; Hing, K. Increasing strut porosity in silicate-substituted calcium-phosphate bone graft substitutes enhances osteogenesis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Aarvold, A.; Smith, J.O.; Tayton, E.R.; Lanham, S.; Chaudhuri, J.B.; Turner, I.G.; Oreffo, R.O. The effect of porosity of a biphasic ceramic scaffold on human skeletal stem cell growth and differentiationin vivo. J. Biomed. Mater. Res. Part A 2013, 101, 3431–3437. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Story, B.J.; Wagner, W.R.; Gaisser, D.M.; Cook, S.D.; Rust-Dawicki, A.M. In vivo performance of a modified CSTi dental implant coating. Int. J. Oral Maxillofac. Implant. 1998, 13, 749–757. [Google Scholar]
- Eqtesadi, S.; Motealleh, A.; Pajares, A.; Miranda, P. Effect of milling media on processing and performance of 13-93 bioactive glass scaffolds fabricated by robocasting. Ceram. Int. 2015, 41, 1379–1389. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cun, X.; Hosta-Rigau, L. Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications. Nanomaterials 2020, 10, 2070. https://doi.org/10.3390/nano10102070
Cun X, Hosta-Rigau L. Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications. Nanomaterials. 2020; 10(10):2070. https://doi.org/10.3390/nano10102070
Chicago/Turabian StyleCun, Xingli, and Leticia Hosta-Rigau. 2020. "Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications" Nanomaterials 10, no. 10: 2070. https://doi.org/10.3390/nano10102070
APA StyleCun, X., & Hosta-Rigau, L. (2020). Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications. Nanomaterials, 10(10), 2070. https://doi.org/10.3390/nano10102070





