Enhanced Desalination Performance of Capacitive Deionization Using Nanoporous Carbon Derived from ZIF-67 Metal Organic Frameworks and CNTs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-67@ CNT
2.3. Preparation of CDI Electrode
2.4. CDI Operation
2.5. Calculation of Desalination Parameters
2.6. Electrode Characterization
2.7. Electrochemical Characterization
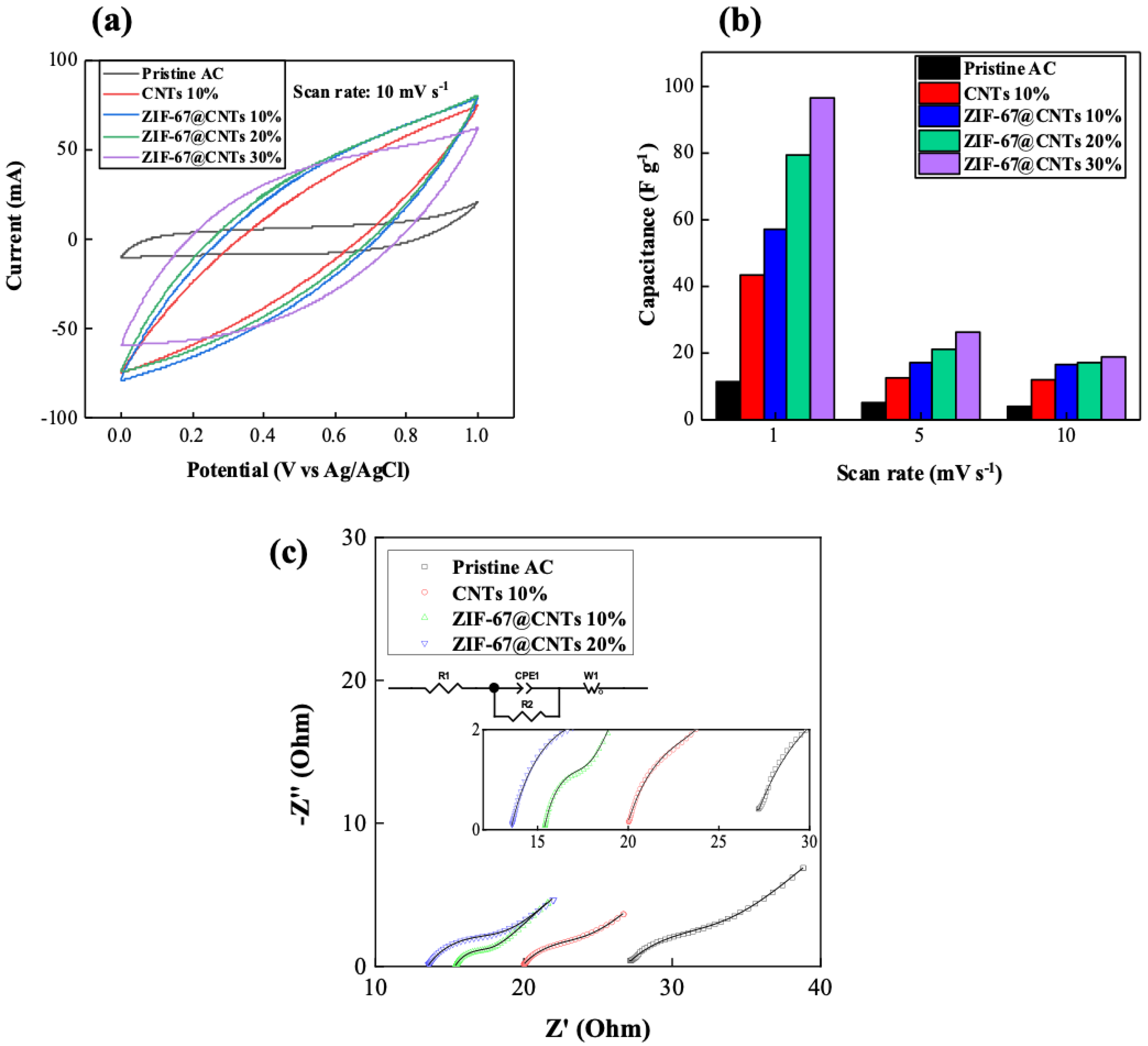
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cho, Y.; Lee, K.S.; Yang, S.; Choi, J.; Park, H.-R.; Kim, D.K. A novel three-dimensional desalination system utilizing honeycomb-shaped lattice structures for flow-electrode capacitive deionization. Energy Environ. Sci. 2017, 10, 1746–1750. [Google Scholar] [CrossRef]
- Ha, Y.; Bin Jung, H.; Lim, H.; Jo, P.S.; Yoon, H.; Yoo, C.-Y.; Pham, T.K.; Ahn, W.; Cho, Y. Continuous Lithium Extraction from Aqueous Solution Using Flow-Electrode Capacitive Deionization. Energies 2019, 12, 2913. [Google Scholar] [CrossRef]
- Ha, Y.; Lee, H.; Yoon, H.; Shin, D.; Ahn, W.; Cho, N.; Han, U.; Hong, J.; Tran, N.A.T.; Yoo, C.-Y.; et al. Enhanced salt removal performance of flow electrode capacitive deionization with high cell operational potential. Sep. Purif. Technol. 2020, 254, 117500. [Google Scholar] [CrossRef]
- Lim, H.; Ha, Y.; Bin Jung, H.; Jo, P.S.; Yoon, H.; Quyen, D.; Cho, N.; Yoo, C.-Y.; Cho, Y. Energy storage and generation through desalination using flow-electrodes capacitive deionization. J. Ind. Eng. Chem. 2020, 81, 317–322. [Google Scholar] [CrossRef]
- Jeon, S.-I.; Park, H.-R.; Yeo, J.-G.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Van Der Wal, A. Membrane capacitive deionization. J. Membr. Sci. 2010, 346, 256–262. [Google Scholar] [CrossRef]
- Ma, J.; He, C.; He, D.; Zhang, C.; Waite, T.D. Analysis of capacitive and electrodialytic contributions to water desalination by flow-electrode CDI. Water Res. 2018, 144, 296–303. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)—Problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Cho, Y.; Yoo, C.-Y.; Lee, S.W.; Yoon, H.; Lee, K.S.; Yang, S.; Kim, D.K. Flow-electrode capacitive deionization with highly enhanced salt removal performance utilizing high-aspect ratio functionalized carbon nanotubes. Water Res. 2019, 151, 252–259. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.-A.; Zhou, K.; Wu, P.-C.; Hou, C.-H. Enhanced desalination performance via mixed capacitive-Faradaic ion storage using RuO2-activated carbon composite electrodes. Electrochim. Acta 2019, 295, 769–777. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Y.; An, Z.; Xu, G.; Gadgil, A.J.; Ruan, G. The polymeric conformational effect on capacitive deionization performance of graphene oxide/polypyrrole composite electrode. Desalination 2020, 486, 114407. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Y.; Han, X.; Zhang, L.; Xu, S.; Wang, J. Optimization on electrode assemblies based on ion-doped polypyrrole/carbon nanotube composite in capacitive deionization process. J. Electroanal. Chem. 2016, 768, 72–80. [Google Scholar] [CrossRef]
- Evans, S.F.; Ivancevic, M.R.; Wilson, D.J.; Hood, Z.D.; Adhikari, S.P.; Naskar, A.K.; Tsouris, C.; Paranthaman, M.P. Carbon polyaniline capacitive deionization electrodes with stable cycle life. Desalination 2019, 464, 25–32. [Google Scholar] [CrossRef]
- El-Deen, A.G.; Barakat, N.A.; Kim, H.Y. Graphene wrapped MnO2-nanostructures as effective and stable electrode materials for capacitive deionization desalination technology. Desalination 2014, 344, 289–298. [Google Scholar] [CrossRef]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Li, F.; Zhu, W.; Cui, J.; Tao, C.-A.; Lin, C.; Hannam, P.M.; Li, G. Metal-Organic Frameworks with a Three-Dimensional Ordered Macroporous Structure: Dynamic Photonic Materials. Angew. Chem. Int. Ed. 2011, 50, 12518–12522. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Q.; Lin, Z.; Zhang, T.; Xu, J.; Tan, Y.; Tian, W.; Jiang, L. Constructing Free Standing Metal Organic Framework MIL-53 Membrane Based on Anodized Aluminum Oxide Precursor. Sci. Rep. 2014, 4, 4947. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, X.-J.; Xie, M.; Huang, Y.-L.; Luo, D.; Wang, T.; Zhao, Y.; Lu, W.; Li, D. Cage-Interconnected Metal–Organic Framework with Tailored Apertures for Efficient C2H6/C2H4 Separation under Humid Conditions. J. Am. Chem. Soc. 2019, 141, 20390–20396. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, Z.; Meng, J.; Chen, L.; Chen, H.; Qin, H. The cube-like porous ZnO/C composites derived from metal organic framework-5 as anodic material with high electrochemical performance for Ni–Zn rechargeable battery. J. Power Sources 2019, 438. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Lei, J.; Wang, S.; Ji, X.; Liu, H.; Yang, Q. Metal–Organic-Framework-Derived Carbon Nanostructures for Site-Specific Dual-Modality Photothermal/Photodynamic Thrombus Therapy. Adv. Sci. 2019, 6, 1901378. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Chen, H.; Bai, X.; Wang, S.; Li, L.; Shao, L.; He, W.; Li, Y.; Wang, T.-Q.; Zhang, X.; et al. Fabrication of 2D metal–organic framework nanosheet@fiber composites by spray technique. Chem. Commun. 2019, 55, 8293–8296. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Ding, Z.; Li, Y.; Lu, T.; Pan, L. Metal–organic-frameworks-derived NaTi2(PO4)3/carbon composites for efficient hybrid capacitive deionization. J. Mater. Chem. A 2019, 7, 12126–12133. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Liao, Y.-T.; Kang, T.-C.; Chen, J.E.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Wu, K.C.-W. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion. Green Chem. 2016, 18, 5957–5961. [Google Scholar] [CrossRef]
- Nishinaga, O.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Rapid fabrication of self-ordered porous alumina with 10-/sub-10-nm-scale nanostructures by selenic acid anodizing. Sci. Rep. 2013, 3, 2748. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Zhang, D.; Han, Y.; Sun, Z.; Qi, J.; Wei, F.; He, Y.; Meng, Q. Effects of Carbonization Temperature on Nature of Nanostructured Electrode Materials Derived from Fe-MOF for Supercapacitors. Electron. Mater. Lett. 2018, 14, 548–555. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Sheng, J.; Tang, F.; Zeng, K.; Wang, L.; Liang, K.; Hu, H.; Liu, Y.-N. Photostable core-shell CdS/ZIF-8 composite for enhanced photocatalytic reduction of CO2. Appl. Surf. Sci. 2019, 498, 143899. [Google Scholar] [CrossRef]
- López-Cabrelles, J.; Romero, J.; Abellán, G.; Giménez-Marqués, M.; Palomino, M.; Valencia, S.; Rey, F.; Espallargas, G.M. Solvent-Free Synthesis of ZIFs: A Route toward the Elusive Fe(II) Analogue of ZIF-8. J. Am. Chem. Soc. 2019, 141, 7173–7180. [Google Scholar] [CrossRef]
- Malkar, R.S.; Daly, H.; Hardacre, C.; Yadav, G.D. Aldol Condensation of 5-Hydroxymethylfurfural to Fuel Precursor over Novel Aluminum Exchanged-DTP@ZIF-8. ACS Sustain. Chem. Eng. 2019, 7, 16215–16224. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, M.; Tang, H.; Xu, B.; Tang, Y.; Pang, H. Amorphous Intermediate Derivative from ZIF-67 and Its Outstanding Electrocatalytic Activity. Small 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ji, D.; Zhang, Y.; Gao, X.; Xu, P.; Li, X.; Liu, C.-C.; Wen, W. Detection of Phenylketonuria Markers Using a ZIF-67 Encapsulated PtPd Alloy Nanoparticle (PtPd@ZIF-67)-Based Disposable Electrochemical Microsensor. ACS Appl. Mater. Interfaces 2019, 11, 20734–20742. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yan, T.; Shi, L.; Park, H.S.; Chen, X.; Zhao, Z.; Zhang, D. Graphene-based materials for capacitive deionization. J. Mater. Chem. A 2017, 5, 13907–13943. [Google Scholar] [CrossRef]
- Khan, Z.U.; Yan, T.; Shi, L.; Zhang, D. Improved capacitive deionization by using 3D intercalated graphene sheet–sphere nanocomposite architectures. Environ. Sci. Nano 2018, 5, 980–991. [Google Scholar] [CrossRef]
- Divyapriya, G.; Vijayakumar, K.K.; Nambi, I. Development of a novel graphene/Co3O4 composite for hybrid capacitive deionization system. Desalination 2019, 451, 102–110. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.; Wang, C.; Ji, L.; Kaneti, Y.V.; Huang, H.; Yang, T.; Wu, K.C.-W.; Yamauchi, Y. Three-Dimensional Nanoarchitecture of Carbon Nanotube-Interwoven Metal–Organic Frameworks for Capacitive Deionization of Saline Water. ACS Sustain. Chem. Eng. 2019, 7, 13949–13954. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Huang, M.; Ma, H.-L.; Zhang, Z.-Q.; Gao, J.-M.; Zhu, Y.-L.; Han, X.-J.; Guo, X.-Y. Preparation of a Carbon-Based Solid Acid Catalyst by Sulfonating Activated Carbon in a Chemical Reduction Process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef]
- Soleimani, H.; Baig, M.K.; Yahya, N.; Khodapanah, L.; Sabet, M.; Demiral, B.M.; Burda, M. Impact of carbon nanotubes based nanofluid on oil recovery efficiency using core flooding. Results Phys. 2018, 9, 39–48. [Google Scholar] [CrossRef]
- Khan, A.; Ali, M.; Ilyas, A.; Naik, P.; Vankelecom, I.F.; Gilani, M.A.; Bilad, M.R.; Sajjad, Z.; Khan, A.L. ZIF-67 filled PDMS mixed matrix membranes for recovery of ethanol via pervaporation. Sep. Purif. Technol. 2018, 206, 50–58. [Google Scholar] [CrossRef]
- Song, X.; Yu, J.; Wei, M.; Li, R.; Pan, X.; Yang, G.; Tang, H. Ionic Liquids-Functionalized Zeolitic Imidazolate Framework for Carbon Dioxide Adsorption. Materials 2019, 12, 2361. [Google Scholar] [CrossRef]
- Yu, G.; Sun, J.; Muhammad, F.; Wang, P.; Zhu, G. Cobalt-based metal organic framework as precursor to achieve superior catalytic activity for aerobic epoxidation of styrene. RSC Adv. 2014, 4, 38804–38811. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, J.; Han, J.; Yan, T.; Shi, L.; Zhang, D. N, P, S co-doped hollow carbon polyhedra derived from MOF-based core–shell nanocomposites for capacitive deionization. J. Mater. Chem. A 2018, 6, 15245–15252. [Google Scholar] [CrossRef]
- Yoo, J.; Park, J.H.; Lee, Y.A.; Cho, W.K.; Yoon, H.; Kim, H.-J.; Han, Y.-K. Pseudocapacitive slurry electrodes using redox-active quinone for high-performance flow capacitors: An atomic-level understanding of pore texture and capacitance enhancement. J. Mater. Chem. A 2015, 3, 23323–23332. [Google Scholar] [CrossRef]






| Samples | SBET (m2/g) | Vtotal (cm3/g) |
|---|---|---|
| CNTs | 110.7 | 0.55 |
| ZIF-67 | 169.6 | 0.45 |
| ZIF-67@CNTs | 239.9 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuoc, N.M.; Jung, E.; Tran, N.A.T.; Lee, Y.-W.; Yoo, C.-Y.; Kang, B.-G.; Cho, Y. Enhanced Desalination Performance of Capacitive Deionization Using Nanoporous Carbon Derived from ZIF-67 Metal Organic Frameworks and CNTs. Nanomaterials 2020, 10, 2091. https://doi.org/10.3390/nano10112091
Phuoc NM, Jung E, Tran NAT, Lee Y-W, Yoo C-Y, Kang B-G, Cho Y. Enhanced Desalination Performance of Capacitive Deionization Using Nanoporous Carbon Derived from ZIF-67 Metal Organic Frameworks and CNTs. Nanomaterials. 2020; 10(11):2091. https://doi.org/10.3390/nano10112091
Chicago/Turabian StylePhuoc, Ngo Minh, Euiyeon Jung, Nguyen Anh Thu Tran, Young-Woo Lee, Chung-Yul Yoo, Beom-Goo Kang, and Younghyun Cho. 2020. "Enhanced Desalination Performance of Capacitive Deionization Using Nanoporous Carbon Derived from ZIF-67 Metal Organic Frameworks and CNTs" Nanomaterials 10, no. 11: 2091. https://doi.org/10.3390/nano10112091
APA StylePhuoc, N. M., Jung, E., Tran, N. A. T., Lee, Y.-W., Yoo, C.-Y., Kang, B.-G., & Cho, Y. (2020). Enhanced Desalination Performance of Capacitive Deionization Using Nanoporous Carbon Derived from ZIF-67 Metal Organic Frameworks and CNTs. Nanomaterials, 10(11), 2091. https://doi.org/10.3390/nano10112091






