Experimental Studies on the Dynamic Memcapacitance Modulation of the ReO3@ReS2 Composite Material-Based Diode
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Physicochemical Characterization of the ReO3@ReS2
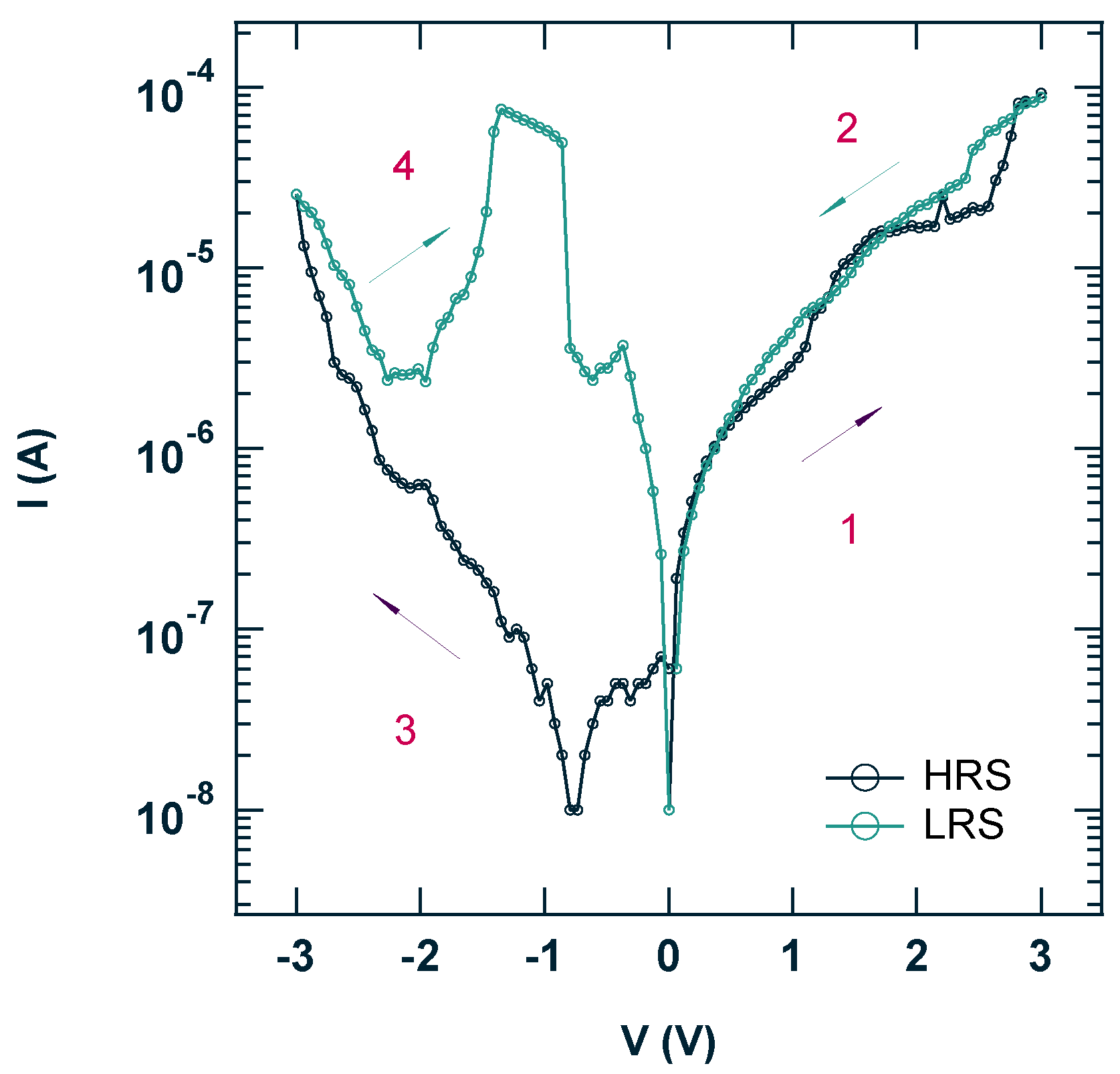
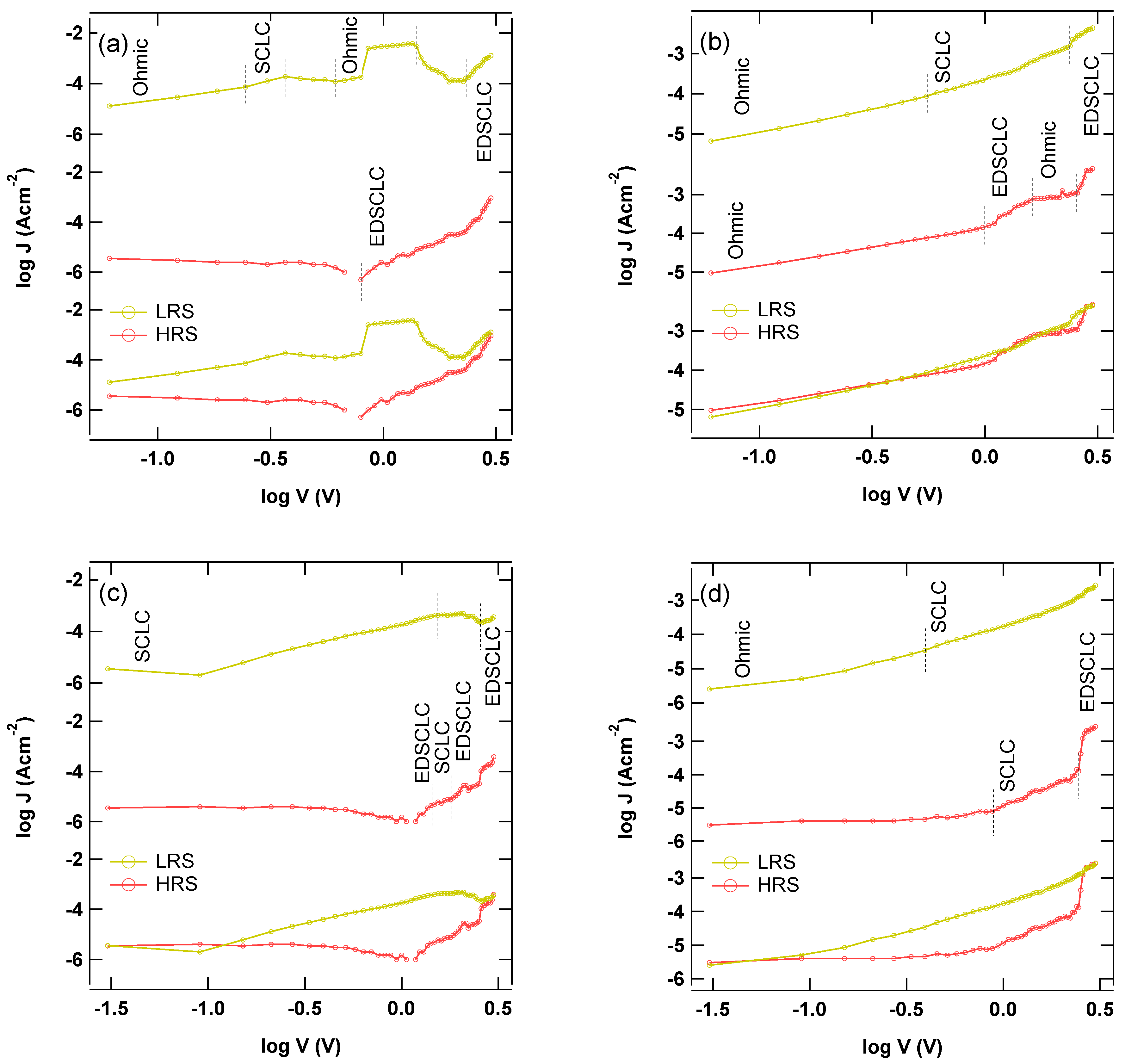
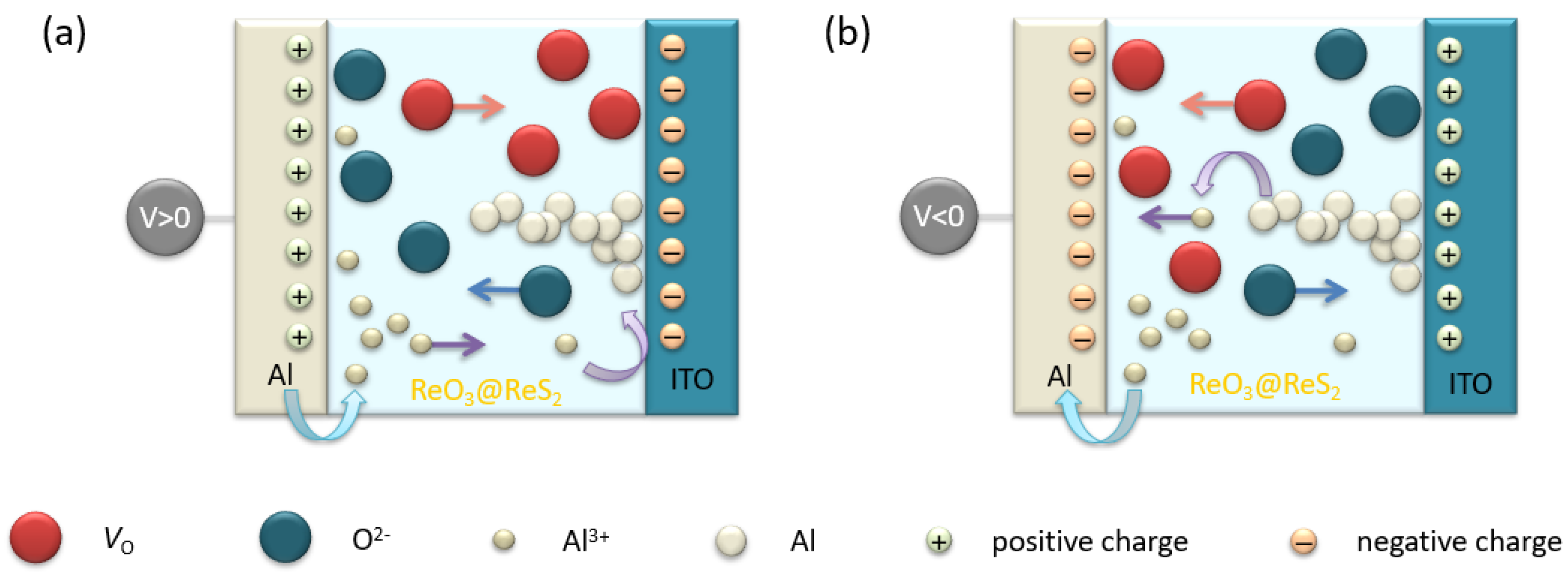
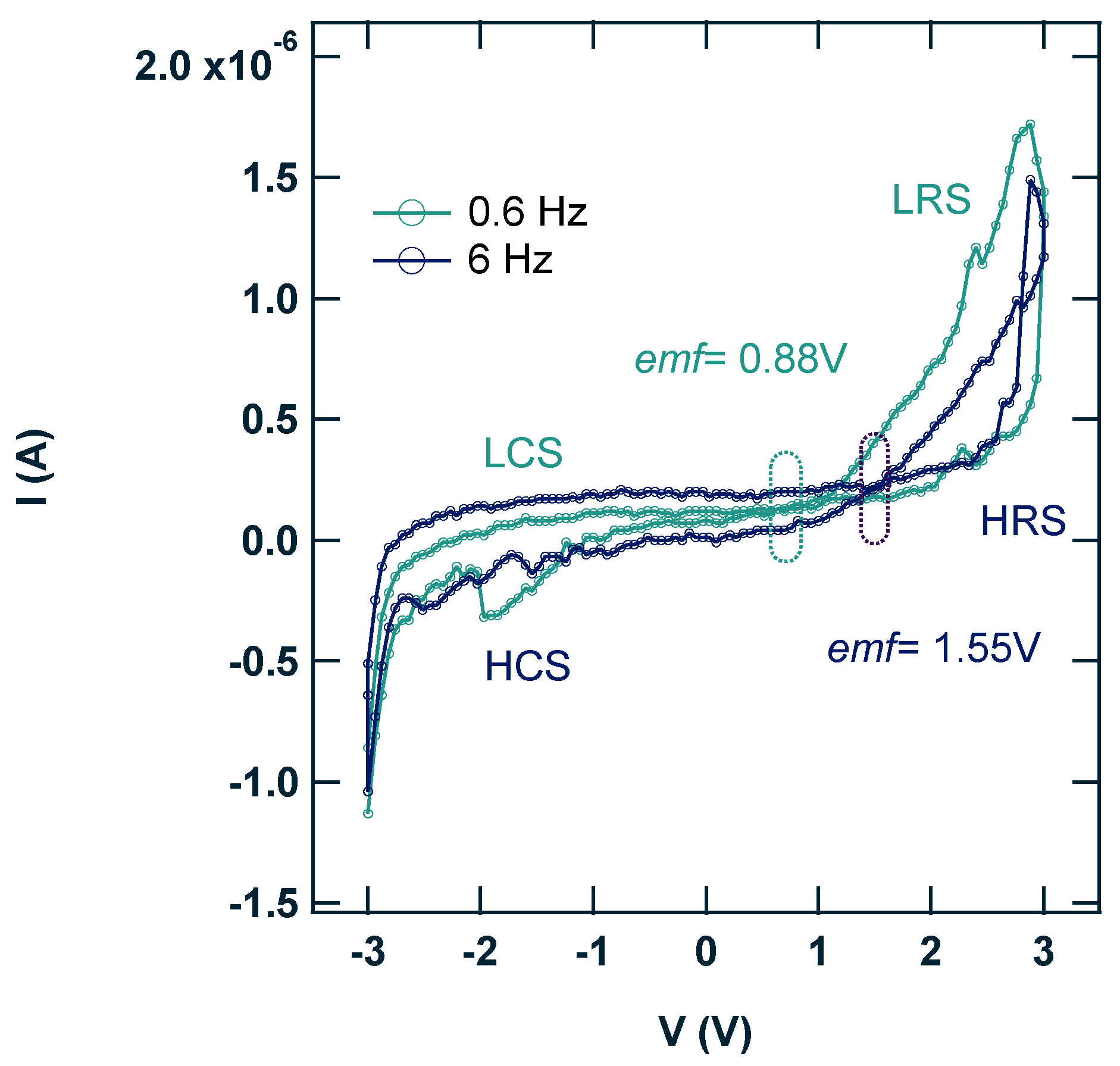
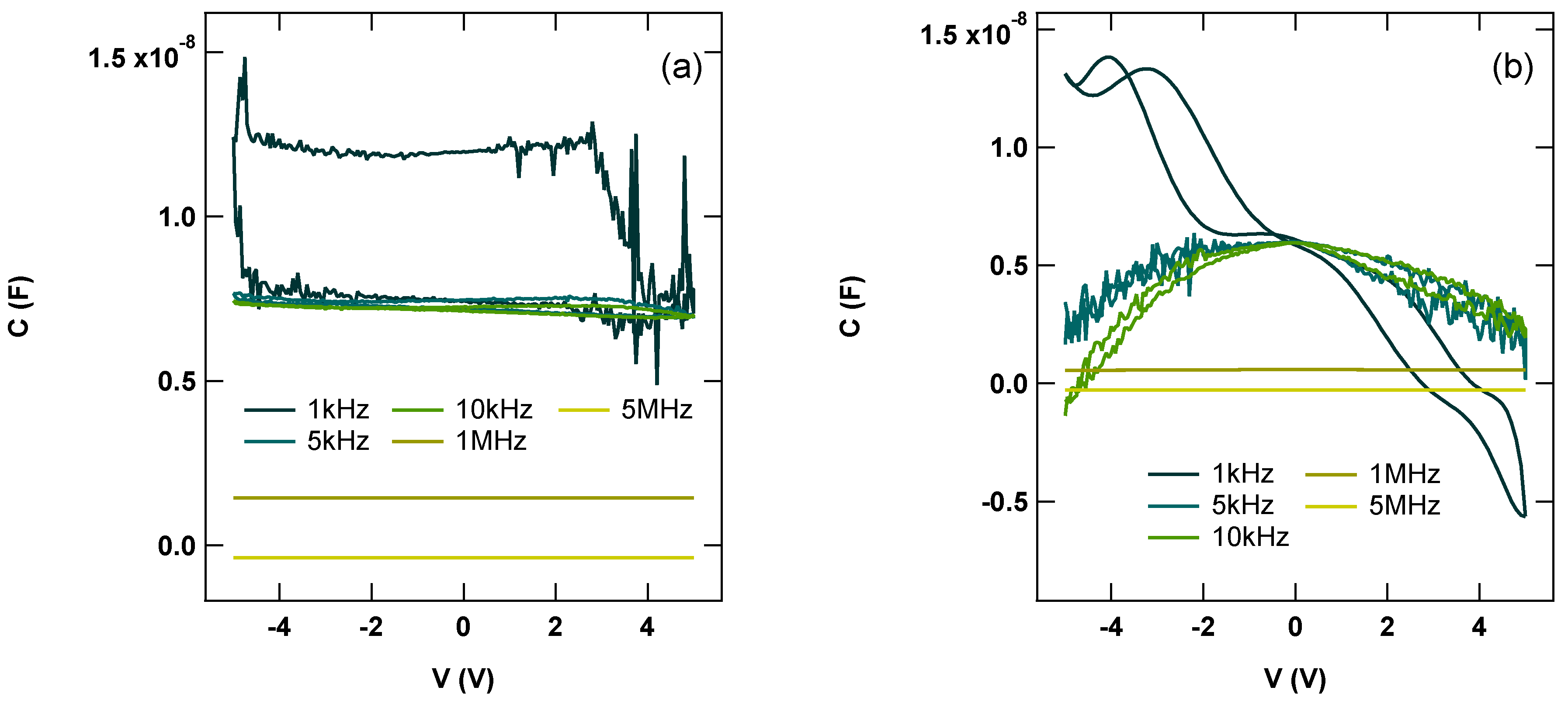
3.2. Electronic Characterization of the ITO/ReO3@ReS2/Al Diode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Di Ventra, M.; Pershin, Y.V.; Chua, L.O. Circuit elements with memory: Memristors, memcapacitors and meminductors. Proc. IEEE 2009, 97, 1717. [Google Scholar] [CrossRef]
- Chua, L.O. Meristor—The missing circuit element. IEEE Trans. Circuit Theory 1971, 18, 507. [Google Scholar] [CrossRef]
- Zidan, M.A.; Strachan, J.P.; Lu, W.D. The future of electronics based on memristive systems. Nat. Electron. 2018, 1, 22. [Google Scholar] [CrossRef]
- Backus, J. Can programming be liberated from the von Neumann style? A functional style and its algebra of programs. Commun. ACM 1978, 21, 21613. [Google Scholar] [CrossRef]
- Rahmani, M.K.; Kim, M.H.; Hussain, F.; Abbas, Y.; Ismail, M.; Hong, K.; Mahata, C.; Choi, C.; Park, B.G.; Kim, S. Memristive and synaptic characteristics of nitride-based heterostructures on Si substrate. Nanomaterials 2020, 10, 994. [Google Scholar] [CrossRef]
- Van de Burgt, Y.; Lubberman, E.; Fuller, E.J.; Keene, S.T.; Faria, G.C.; Agarwal, S.; Marinella, M.J.; Talin, A.A.; Salleo, A. A non-volatile organic chemical devices as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 2017, 16, 414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rao, M.; Han, J.W.; Zhang, J.; Lin, P.; Li, Y.; Li, C.; Song, W.; Asapu, S.; Midya, R.; et al. Capacitive neural network with neuro-transistors. Nat. Commun. 2018, 9, 3208. [Google Scholar] [CrossRef]
- Ryu, H.; Kim, S. Pseudo-interface switching of a two-terminal TaOx/HfO2 synaptic device for neuromorphic applications. Nanomaterials 2020, 10, 1550. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, S.; Savelev, S.; Song, W.; Midya, R.; Li, Y.; Rao, M.; Asapu, S.; Zhuo, Y.; Jiang, H.; et al. Fully memristive neural networks for pattern classification with unsupervised learning. Nat. Electron. 2018, 1, 137. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Yu, Y.; Fernández Díez, F.J. Research on low pass filter based on memristor and memcapacitor. In Proceedings of the 36th Chinese Control Conference, Dalian, China, 26–27 July 2017; Volume 7. [Google Scholar]
- Bao, B.C.; Wang, N.; Xu, Q.; Wu, H.G.; Hu, Y.H. A simple third-order memristive band pass filter chaotic circuit. IEEE Trans. Circuits Syst. II Exp. Briefs 2016, 64, 977. [Google Scholar] [CrossRef]
- Wang, C.H.; Zhou, L.; Wu, R.P. The design and realization of a hyper-chaotic circuit based on a flux-controlled memristor with linear memductance. J. Circuit Syst. Comp. 2018, 27, 1850038. [Google Scholar] [CrossRef]
- Jeong, D.S.; Thomas, R.; Katiyar, R.S.; Scott, J.F.; Kohlstedt, H.; Petraru, A.; Hwang, C.S. Emerging memories: Resistive switching mechanisms and current status. Rep. Prog. Phys. 2012, 75, 076502. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fischer, A.; Sawatzki, M.; Hai, D.; Matthias, D.; Glitzky, L.A.; Reineke, S.; Mannsfeld, S.C.B. Introducing pinMOS memory: A novel, nonvolatile organic memory device. Adv. Funct. Mater. 2020, 30, 1907119. [Google Scholar] [CrossRef]
- Cohen, G.Z.; Pershin, Y.V.; Di Ventra, M. Lagrange formalism of memory circuit elements: Classical and quantum formulations. Phys. Rev. B 2012, 85, 165428. [Google Scholar] [CrossRef]
- Dubi, Y.; Di Ventra, M. Colloquium: Heat flow and thermoelectricity in atomic and molecular junctions. Rev. Mod. Phys. 2011, 83, 131. [Google Scholar] [CrossRef]
- Khan, A.K.; Lee, B.H. Monolayer MoS2 metal insulator transition based memcapacitor modeling with extension to a ternary device. AIP Adv. 2016, 6, 095022. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, C.H.; Zhang, X. A universal emulator for memristor, memcapacitor, and meminductor and its chaotic circuit. Chaos 2019, 29, 013141. [Google Scholar] [CrossRef]
- Chu, H.L.; Lai, J.J.; Wu, L.Y.; Chang, S.L.; Liu, C.M.; Jian, W.B.; Chen, Y.C.; Yuan, C.J.; Wu, T.S.; Soo, Y.L.; et al. Exploration and characterization of the memcapacitor and memristor properties of Ni–DNA nanowire devices. NPG Asia Mater. 2017, 9, e430. [Google Scholar] [CrossRef][Green Version]
- Park, D.; Yang, P.; Kim, H.J.; Beom, K.; Lee, H.H.; Kang, C.J.; Yoon, T.S. Analog reversible nonvolatile memcapacitance in metal-oxide-semiconductor memcapacitor with ITO/HfOx/Si structure. Appl. Phys. Lett. 2018, 113, 162102. [Google Scholar] [CrossRef]
- Yang, P.; Noh, Y.J.; Baek, Y.J.; Zheng, H.; Kang, C.J.; Lee, H.H.; Yoon, T.S. Memcapacitive characteristics in reactive-metal (Mo, Al) HfOx/n-Si structures through migration of oxygen by applied voltage. Appl. Phys. Lett. 2016, 108, 052108. [Google Scholar] [CrossRef]
- Noh, Y.J.; Baek, Y.J.; Hu, Q.; Kang, C.J.; Choi, Y.J.; Lee, H.H.; Yoon, T.S. Analog memristive and memcapacitive characteristics of Pt-Re2O3 core-shell nanoparticles assembly on p+-Si substrate. IEEE Trans. Nanotechnol. 2015, 14, 798. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, Z.; Cheng, S.; Hong, L.; Li, Y.; Tian, G.; Chen, D.; Hou, Z.; Qin, M.; Zeng, M.; et al. An artificial optoelectronic synapse based on a photoelectric memcapacitor. Adv. Electron. Mater. 2020, 6, 1900858. [Google Scholar] [CrossRef]
- Waser, R.; Dittmann, R.; Staikov, G.; Szot, K. Redox-based resistive switching memories-nanoionic mechanisms, prospects, and challenges. Adv. Mater. 2009, 21, 2632. [Google Scholar] [CrossRef]
- Akinaga, H.; Shima, H. Resistive random access memory (ReRAM) based on metal oxides. Proc. IEEE 2010, 98, 2237. [Google Scholar] [CrossRef]
- Sawa, A. Resistive switching in transition metal oxides. Mater. Today 2008, 11, 28. [Google Scholar] [CrossRef]
- Waser, R.; Aono, M. Nanoionics-based resistive switching memories. Nat. Mater. 2007, 6, 833. [Google Scholar] [CrossRef]
- Borowiec, J.; Liang, W.; Boi, F.S.; He, Y.; Wang, S.L.; Gillin, W.P. Aluminium promoted sulfidation of ammonium perrhenate: Presence of nanobattery in the ReS2 composite material based memcapacitor. Chem. Eng. J. 2019, 392, 123745. [Google Scholar] [CrossRef]
- Corbet, C.M.; McClellan, C.; Rai, A.; Sonde, S.S.; Tutuc, E.; Banerjee, S.K. Field effect transistors with current saturation and voltage gain in ultrathin ReS2. ACS Nano 2015, 9, 363. [Google Scholar] [CrossRef]
- Zhang, E.; Jin, Y.; Yuan, X.; Wang, W.; Zhang, C.; Tang, L.; Liu, S.; Zhou, P.; Hu, W.; Xiu, F. ReS2-based field-effect transistors and photodetectors. Adv. Funct. Mater. 2015, 25, 4076. [Google Scholar] [CrossRef]
- Liu, F.; Zheng, S.; He, X.; Chaturvedi, A.; He, J.; Chow, W.L.; Mion, T.R.; Wang, X.; Zhou, J.; Fu, Q.; et al. Highly sensitive detection of polarized light using anisotropic 2D ReS2. Adv. Funct. Mater. 2016, 26, 1169. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, S.; Mendes, R.G.; Sun, Z.; Chen, Y.; Kong, X.; Xue, Y.; Rummeli, M.H.; Wu, X.; Chen, S.; et al. Extremely weak van der Waals coupling in vertical ReS2 nanowalls for high-current-density lithium-ion batteries. Adv. Mater. 2016, 28, 2616. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Oh, S.; Kang, D.H.; Jo, S.H.; Ali, M.H.; Choi, W.Y.; Heo, K.; Jeon, J.; Lee, S.; Kim, M.; et al. Phosphorene/rhenium disulfide heterojunction-based negative differential resistance device for multi-valued logic. Nat. Commun. 2016, 7, 13413. [Google Scholar] [CrossRef] [PubMed]
- Cazzanelli, E.; Castriota, M.; Marino, S.; Scaramuzza, N.; Purans, J.; Kuzmin, A.; Kalendarev, R.; Mariotto, G.; Das, G. Characterization of rhenium oxide films and their application to liquid crystal cells. J. Appl. Phys. 2009, 105, 114904. [Google Scholar] [CrossRef]
- Xu, H.; Yang, L.Y.; Tian, H.; Yin, S.G.; Zhang, F.L. Rhenium oxide as the interfacial buffer layer for polymer photovoltaic cells. Optoelectron. Lett. 2010, 6, 176. [Google Scholar] [CrossRef]
- Pearsall, T.P.; Lee, C.A. Electronic transport in ReO3: Dc conductivity and Hall effect. Phys. Rev. B 1974, 10, 2190. [Google Scholar] [CrossRef]
- Kroeger, F.A. The Chemistry of Imperfect Crystals; North-Holland Publishing Company: Amsterdam, The Netherland, 1973. [Google Scholar]
- Kozicki, M.N.; Mitkova, M.; Valov, I. Resistive Switching: From fundamentals of nanoionic redox processes to memristive device applications, Electrochemical metallization memories. In Resistive Switching: From Fundamentals of Nanoionic Redox Processes to Memristive Device Applications; Wiley-VCH: Weinheim, Germany, 2016; Chapter 17. [Google Scholar]
- Sze, S.M. Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 1981. [Google Scholar]
- Silinsh, E.A. On the physical nature of traps in molecular crystals. Phys. Stat. Sol. A 1970, 3, 817. [Google Scholar] [CrossRef]
- Sim, H.; Choi, H.; Lee, D.; Chang, M.; Choi, D.; Son, Y.; Lee, E.H.; Kim, W.; Park, Y.D.; Yoo, I.K.; et al. Resistance-switching characteristics of polycrystalline Nb2O5 for nonvolatile memory application. Electron Devices Meeting IEDM Tech. Dig. 2005, 26, 292. [Google Scholar]
- Jeong, D.S.; Schroeder, H.; Breuer, U.; Waser, R. Characteristic electroforming behavior in Pt/TiO2/Pt resistive switching cells depending on atmosphere. J. Appl. Phys. 2008, 104, 123716. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Qin, L.; Zheng, Q.; Qiu, X.; Huang, R. Probing nanoscale oxygen ion motion in memristive systems. Nat. Commun. 2017, 8, 15173. [Google Scholar] [CrossRef] [PubMed]
- Serway, R.A. Principles of Physics; Saunders College Publishing: Texas, TX, USA, 1998. [Google Scholar]
- Liu, M.; Borowiec, J.; Sun, L.J.J.; Konop, M.; Rahman, M.M.; Taallah, A.; Boi, F.S.; Gillin, W.P. Experimental studies on the conduction mechanism and electrical properties of the inverted Ba doped ZnO nanoparticles based memristor. Appl. Phys. Lett. 2019, 115, 073505. [Google Scholar] [CrossRef]
- O’Dwyer, J.J. The Theory of Electrical Conduction and Breakdown in Solid Dielectrics; Clarendon: Oxford, UK, 1973. [Google Scholar]
- Chua, L.O. Resistance switching memories are memristors. Appl. Phys. A Mater. Sci. Process 2011, 102, 765. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Terabe, K.; Hasegawa, T.; Valov, I.; Waser, R.; Aono, M. Effects of moisture on the switching characteristics of oxide-based, gapless-type atomic switches. Adv. Funct. Mater. 2012, 22, 70. [Google Scholar] [CrossRef]
- Corinto, F.; Civalleri, P.P.; Chua, L.O. A Theoretical Approach to Memristor Devices. IEEE J. Emerg. Sel. Topics Circuits Syst. 2015, 5, 123. [Google Scholar] [CrossRef]
- Valov, I.; Linn, E.; Tappertzhofen, S.; Schmelzer, S.; van den Hurk, J.; Lentz, F.; Wasser, R. Nanobatteries in redox-based resistive switches require extension of memristor theory. Nat. Commun. 2013, 4, 1771. [Google Scholar] [CrossRef] [PubMed]
- Kasap, S.O. Principles of Electronic Materials and Devices. McGraw-Hill, Co.: Boston, MA, USA, 2006; Chapter 7. [Google Scholar]
- Lai, Q.; Zhang, L.; Li, Z.; Stickle, W.F.; Williams, R.S.; Chen, Y. Analog memory capacitor based on field-configurable ion-doped polymers. Appl. Phys. Lett. 2009, 95, 213503. [Google Scholar] [CrossRef]
- Lee, E.; Gwon, M.; Kim, D.W.; Kim, H. Resistance state-dependent barrier inhomogeneity and transport mechanism in resistive-switching Pt/SrTiO3 junctions. Appl. Phys. Lett. 2011, 98, 132905. [Google Scholar] [CrossRef]
- Wu, S.X.; Xu, L.M.; Xing, X.J.; Chen, S.M.; Yuan, Y.B.; Liu, Y.J.; Yu, Y.P.; Li, X.Y.; Li, S.W. Reverse-bias-induced bipolar resistance switching in Pt/TiO2/SrTi0.99Nb0.01O3/Pt devices. Appl. Phys. Lett. 2008, 93, 043502. [Google Scholar] [CrossRef]
- Sawa, A.; Fujii, T.; Kawasaki, M.; Tokura, Y. Interface transport properties and resistance switching in perovskite-oxide heterojunctions, Strongly correlated electron materials: Physics and nanoengineering. Proc. SPIE 2005, 5932, 59322C. [Google Scholar]
- Sawa, A.; Fujii, T.; Kawasaki, T. Interface transport properties and resistance switching in perovskite-oxide heterojunctions. Appl. Phys. Lett. 2006, 88, 232112. [Google Scholar] [CrossRef]
- Nicollian, E.H.; Brews, J.R. Metal Oxide Semiconductor (MOS) Physics and Technology; John Wiley and Sons: Hoboken, NJ, USA, 1982. [Google Scholar]
- Bonafos, C.; Spiegel, Y.; Normand, P.; Ben-Assayag, G.; Groenen, J.; Carrada, M.; Dimitrakis, P.; Kapetanakis, E.; Sahu, B.S.; Slaoui, A.; et al. Controlled fabrication of Si nanocrystal delta-layers in thin SiO2 layers by plasma immersion ion implantation for nonvolatile memories. Appl. Phys. Lett. 2013, 103, 253118. [Google Scholar] [CrossRef]
- Misawa, T. Impedance of bulk semiconductor in junction diode. J. Phys. Soc. Jpn. 1957, 12, 882. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Tang, H.; Li, X. Anomalous capacitance in temperature and frequency characteristics of a TiW/P-InP Schottky barrier. Semicond. Sci. Technol. 2016, 31, 065023. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowiec, J.; Liu, M.; Liang, W.; Kreouzis, T.; Bevan, A.J.; He, Y.; Ma, Y.; Gillin, W.P. Experimental Studies on the Dynamic Memcapacitance Modulation of the ReO3@ReS2 Composite Material-Based Diode. Nanomaterials 2020, 10, 2103. https://doi.org/10.3390/nano10112103
Borowiec J, Liu M, Liang W, Kreouzis T, Bevan AJ, He Y, Ma Y, Gillin WP. Experimental Studies on the Dynamic Memcapacitance Modulation of the ReO3@ReS2 Composite Material-Based Diode. Nanomaterials. 2020; 10(11):2103. https://doi.org/10.3390/nano10112103
Chicago/Turabian StyleBorowiec, Joanna, Mengren Liu, Weizheng Liang, Theo Kreouzis, Adrian J. Bevan, Yi He, Yao Ma, and William P. Gillin. 2020. "Experimental Studies on the Dynamic Memcapacitance Modulation of the ReO3@ReS2 Composite Material-Based Diode" Nanomaterials 10, no. 11: 2103. https://doi.org/10.3390/nano10112103
APA StyleBorowiec, J., Liu, M., Liang, W., Kreouzis, T., Bevan, A. J., He, Y., Ma, Y., & Gillin, W. P. (2020). Experimental Studies on the Dynamic Memcapacitance Modulation of the ReO3@ReS2 Composite Material-Based Diode. Nanomaterials, 10(11), 2103. https://doi.org/10.3390/nano10112103






