Hydrothermal Synthesis of Polyhedral Nickel Sulfide by Dual Sulfur Source for Highly-Efficient Hydrogen Evolution Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of NiS2 (MS) with Dual Sulfur Source
2.3. Synthesis of NiS2 with MPS
2.4. Synthesis of NiS2 with S
2.5. Materials Characterization
2.6. Electrochemical Measurements
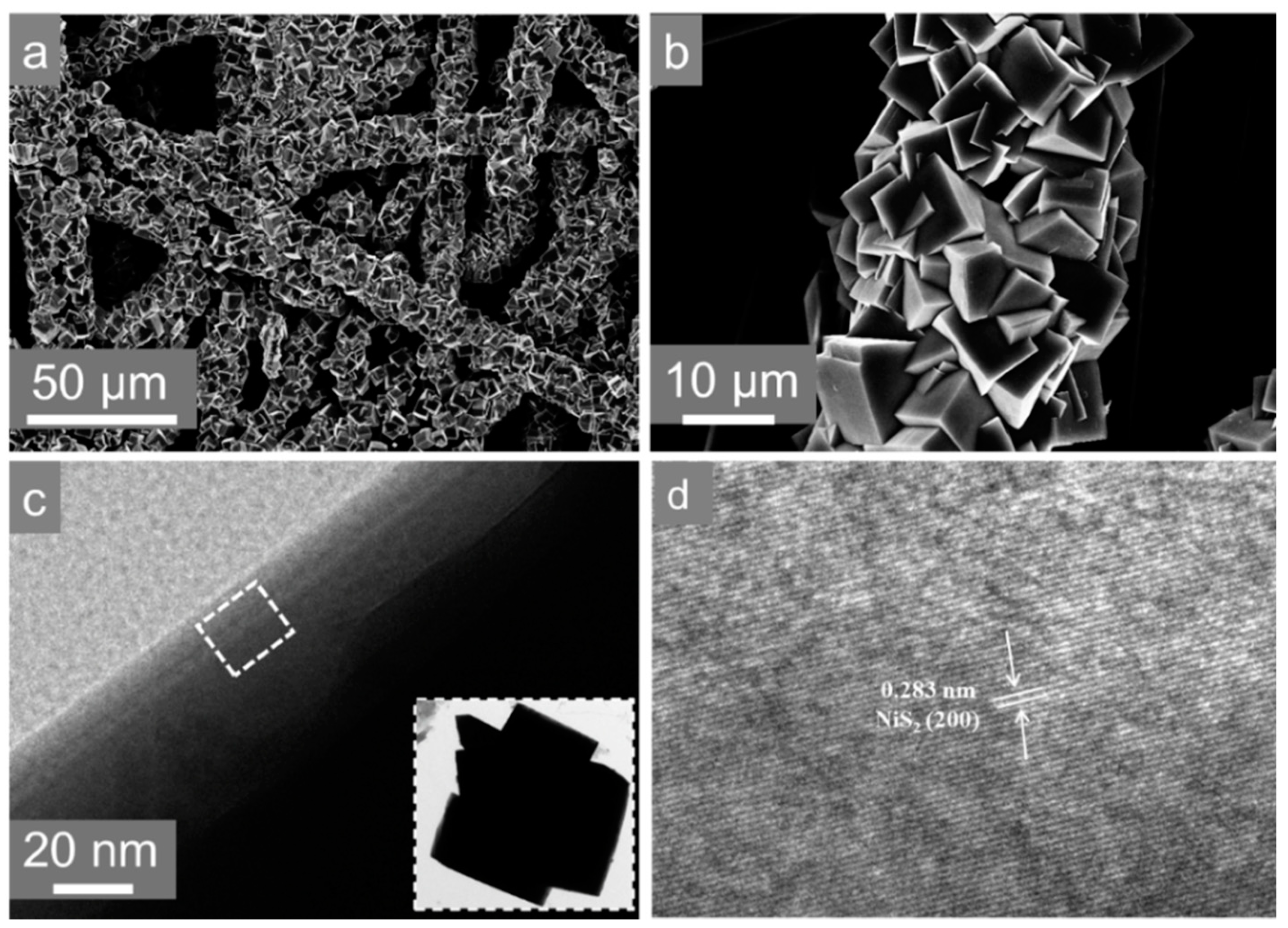
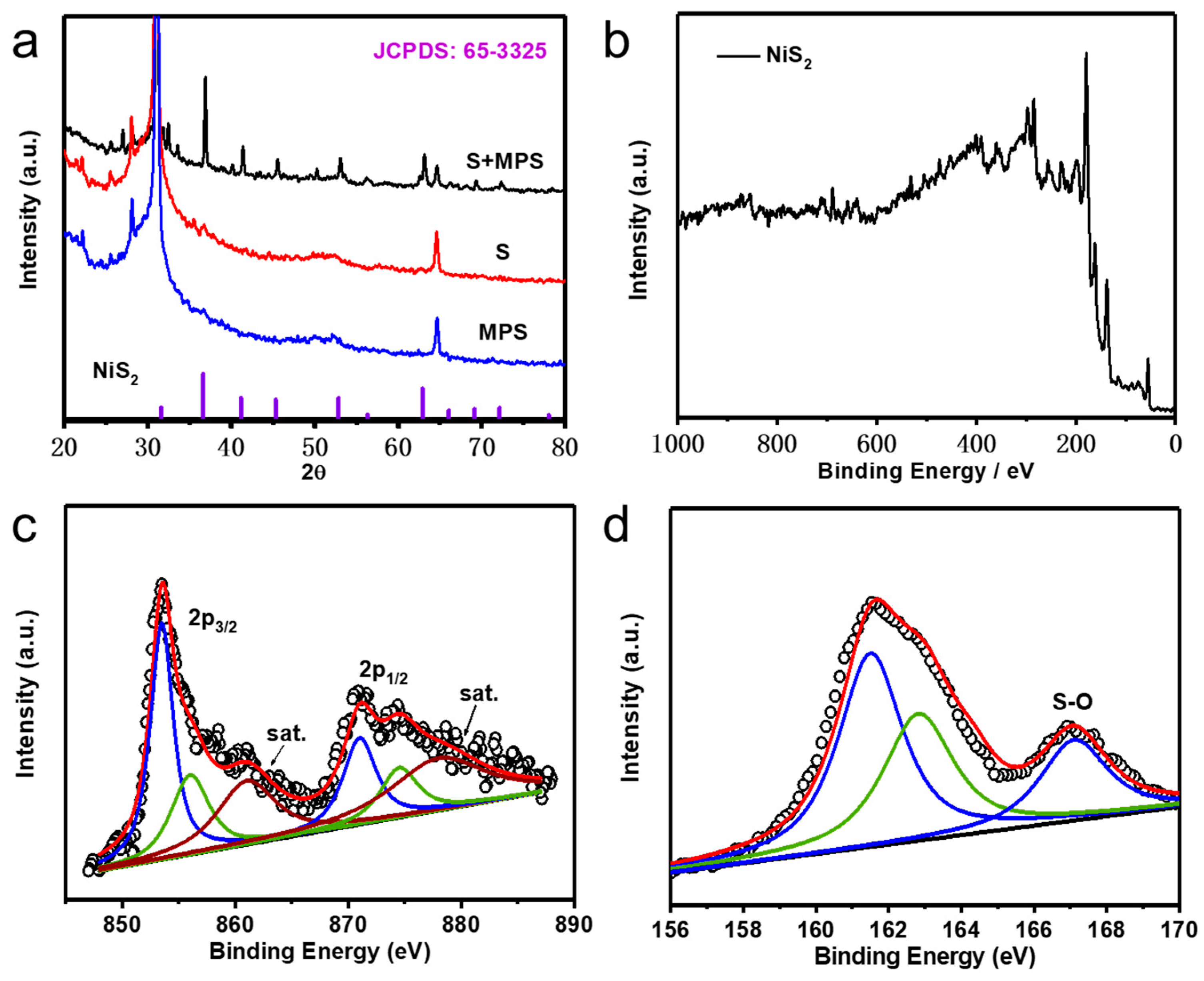
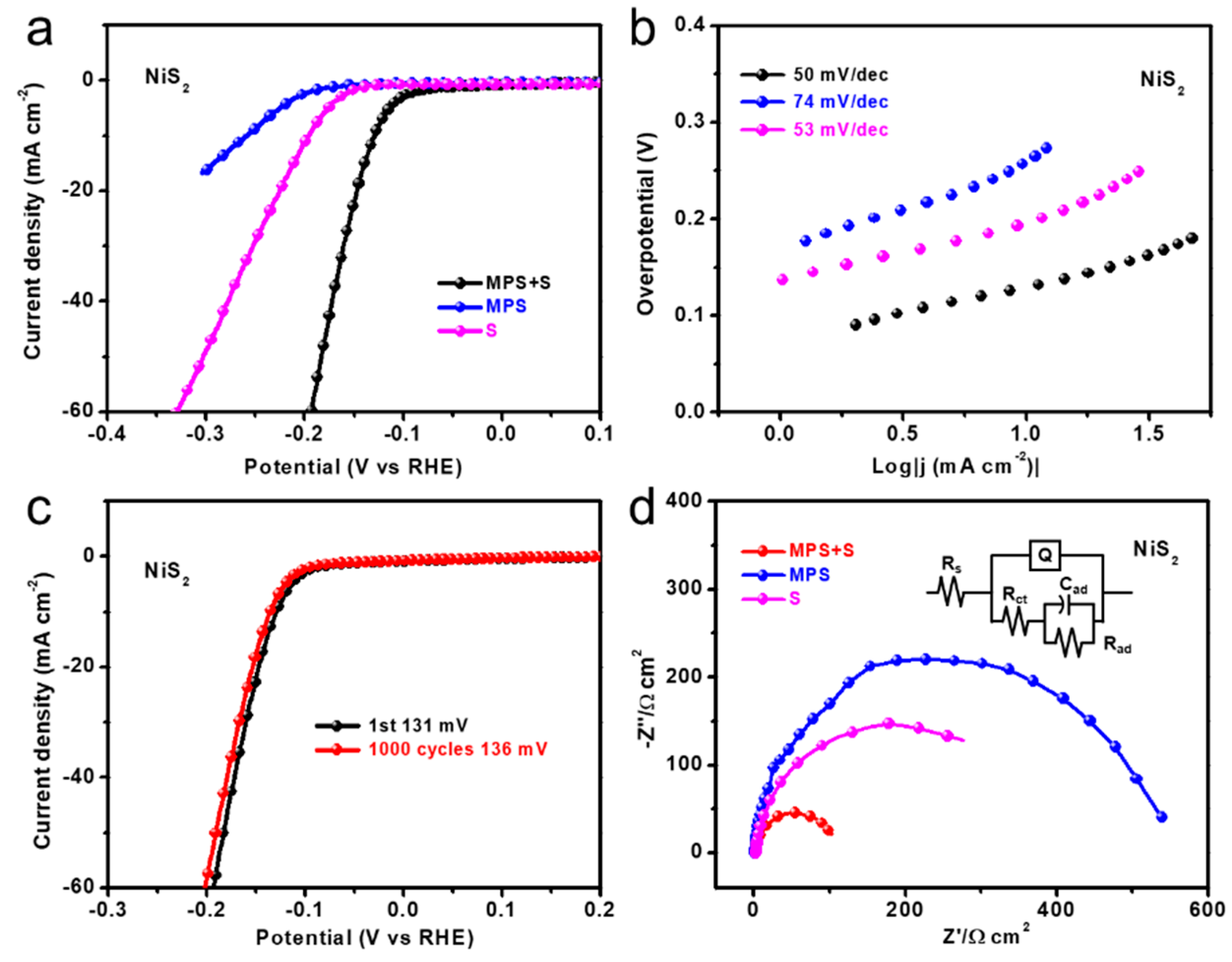
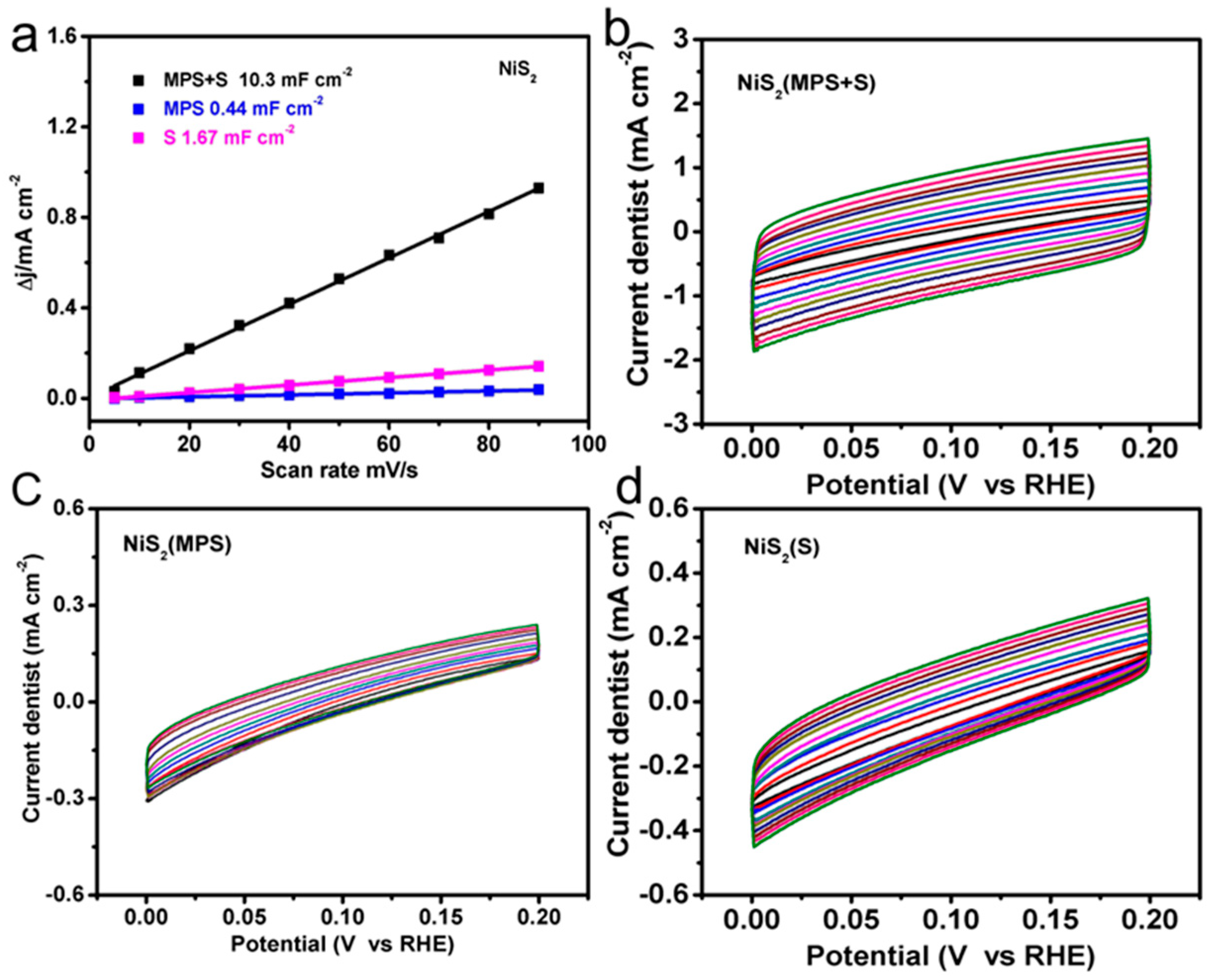
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tang, C.; Pu, Z.; Liu, Q.; Asiri, A.M.; Jiang, P. NiS2 nanosheets array grown on carbon cloth as an efficient 3D hydrogen evolution cathode. Electrochim. Acta 2015, 153, 508–514. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Yu, F.; Huang, Y.; Sun, J.; Zhu, Z.; Nielsen, R.J.; He, R.; Bao, J.; Iii, W.A.G.; Chen, S.; et al. Efficient hydrogen evolution by ternary molybdenum sulfoselenide particles on self-standing porous nickel diselenide foam. Nat. Commun. 2016, 7, 12765. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhang, R.; Lu, W.; Wang, Z.; Liu, D.; Hao, S.; Du, G.; Asiri, A.M.; Sun, X. Energy-Saving Electrolytic Hydrogen Generation: Ni2P Nanoarray as a High-Performance Non-Noble-Metal Electrocatalyst. Angew. Chem. Int. Ed. 2016, 56, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Dai, Q.; Wang, M.; Qu, L.; Dai, L. Earth-abundant carbon catalysts for renewable generation of clean energy from sunlight and water. Nano Energy 2017, 41, 367–376. [Google Scholar] [CrossRef]
- Ying, Z.; Zheng, X.; Cui, G. Detailed kinetic study of the electrochemical Bunsen reaction in the sulfur–iodine cycle for hydrogen production. Energy Convers. Manag. 2016, 115, 26–31. [Google Scholar] [CrossRef]
- Hu, S.; Xu, L.; Wang, L.; Li, D.; Zhang, P.; Chen, S. Activity and stability of monometallic and bimetallic catalysts for high-temperature catalytic HI decomposition in the iodine–sulfur hydrogen production cycle. Int. J. Hydrogen Energy 2016, 41, 773–783. [Google Scholar] [CrossRef]
- Wu, A.; Xie, Y.; Ma, H.; Tian, C.; Gu, Y.; Yan, H.; Zhang, X.; Yang, G.; Fu, H. Integrating the active OER and HER components as the heterostructures for the efficient overall water splitting. Nano Energy 2018, 44, 353–363. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, B.; Zhao, D.; Wang, H.; Plebanski, M. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bhattacharyya, S. Porous NiFe-Oxide Nanocubes as Bifunctional Electrocatalysts for Efficient Water-Splitting. ACS Appl. Mater. Interfaces 2017, 9, 41906–41915. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Yang, S.; Lin, H.; Hu, C.; Long, X.; Yang, S. Hydrogen evolution electrocatalysis with binary-nonmetal transition metal compounds. J. Mater. Chem. A 2017, 5, 5995–6012. [Google Scholar] [CrossRef]
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-Abundant Metal Pyrites (FeS2, CoS2, NiS2, and Their Alloys) for Highly Efficient Hydrogen Evolution and Polysulfide Reduction Electrocatalysis. J. Phys. Chem. C 2014, 118, 21347–21356. [Google Scholar] [CrossRef] [Green Version]
- An, T.; Wang, Y.; Tang, J.; Wei, W.; Cui, X.; Alenizi, A.M.; Zhang, L.; Zheng, G. Interlaced NiS2–MoS2 nanoflake-nanowires as efficient hydrogen evolution electrocatalysts in basic solutions. J. Mater. Chem. A 2016, 4, 13439–13443. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.X.; Lv, F.; Lu, M.; Sun, K.; Wang, W. Oxygen vacancies dominated NiS2/CoS2 interface porous nanowires for portable Zn–air batteries driven water splitting devices. Adv. Mater. 2017, 29, 1704681. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Huang, L.; Ai, L.; Jiang, J. Surface anion-rich NiS2 hollow microspheres derived from metal–organic frameworks as a robust electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 20985–20992. [Google Scholar] [CrossRef]
- Wu, X.; Yang, B.; Li, Z.; Lei, L.; Zhang, X. Synthesis of supported vertical NiS2 nanosheets for hydrogen evolution reaction in acidic and alkaline solution. RSC Adv. 2015, 5, 32976–32982. [Google Scholar] [CrossRef]
- Lin, J.H.; Wang, P.C.; Wang, H.H.; Li, C. Defect-Rich Heterogeneous MoS2/NiS2 Nanosheets Electrocatalysts for Efficient Overall Water Splitting. Adv. Sci. 2019, 6, 1900246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; He, Q.; Jiang, H.; Lin, Y.; Zhang, Y.; Habib, M.; Chen, S.; Song, L. Electronic Structure Reconfiguration toward Pyrite NiS2 via Engineered Heteroatom Defect Boosting Overall Water Splitting. ACS Nano 2017, 11, 11574–11583. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, W.-J.; Lian, Y.; Li, H.; Hong, S.; Xu, S.; Yan, H.; Hu, J.-S. NiS2 nanodotted carnation-like CoS2 for enhanced electrocatalytic water splitting. Chem. Commun. 2019, 55, 3781–3784. [Google Scholar] [CrossRef]
- Hao, J.; Yang, W.; Hou, J.; Mao, B.; Huang, Z.; Shi, W. Nitrogen doped NiS2 nanoarrays with enhanced electrocatalytic activity for water oxidation. J. Mater. Chem. A 2017, 5, 17811–17816. [Google Scholar] [CrossRef]
- Huang, T.; Fang, H.; Xu, L.; Wang, Z.; Xin, Y.; Yu, J.; Yao, S.; Zhang, Z. Electrocatalytic performance of cubic NiS2 and hexagonal NiS for oxygen reduction reaction. J. Catal. 2018, 359, 223–232. [Google Scholar] [CrossRef]
- Kuang, P.Y.; He, M.; Zhu, B.C.; Yu, J.G.; Fan, K. 0D/2D NiS2/V-MXene composite for electrocatalytic H2 evolution. J. Catal. 2019, 375, 8–20. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Lu, Y.; Xiang, B. Facile synthesis of NiS2 nanowires and its efficient electrocatalytic performance for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 72–77. [Google Scholar] [CrossRef]
- Wei, W.; Mi, L.; Gao, Y.; Zheng, Z.; Chen, W.; Guan, X. Partial Ion-Exchange of Nickel-Sulfide-Derived Electrodes for High Performance Supercapacitors. Chem. Mater. 2014, 26, 3418–3426. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Qi, F.; Zheng, B.; He, J.; Lin, J.; Zhang, W.; Li, Y.; Chen, Y. Self-Assembled Coral-like Hierarchical Architecture Constructed by NiSe2 Nanocrystals with Comparable Hydrogen-Evolution Performance of Precious Platinum Catalyst. ACS Appl. Mater. Interfaces 2017, 9, 7154–7159. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, X.; Luo, S.-L.; Xu, S.-Q.; Yuan, N.-Y.; Ding, J.-N. High performance Li–S battery based on amorphous NiS2 as the host material for the S cathode. J. Mater. Chem. A 2016, 4, 13395–13399. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Wang, S.-W.; Wang, G.-S.; Li, Z.; Guo, A.-P.; Zhu, J.-Q.; Liu, D.-P.; Yin, P.-G. Facile synthesis of NiS2 @MoS2 core–shell nanospheres for effective enhancement in microwave absorption. RSC Adv. 2017, 7, 22454–22460. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, K.; Lin, Z.; Song, H.; Duan, X.; Peng, Z.; Yan, S. Hydrothermal Synthesis of Polyhedral Nickel Sulfide by Dual Sulfur Source for Highly-Efficient Hydrogen Evolution Catalysis. Nanomaterials 2020, 10, 2115. https://doi.org/10.3390/nano10112115
Gao Y, Wang K, Lin Z, Song H, Duan X, Peng Z, Yan S. Hydrothermal Synthesis of Polyhedral Nickel Sulfide by Dual Sulfur Source for Highly-Efficient Hydrogen Evolution Catalysis. Nanomaterials. 2020; 10(11):2115. https://doi.org/10.3390/nano10112115
Chicago/Turabian StyleGao, Yuan, Ka Wang, Zixia Lin, Haizeng Song, Xiaomeng Duan, Zehui Peng, and Shancheng Yan. 2020. "Hydrothermal Synthesis of Polyhedral Nickel Sulfide by Dual Sulfur Source for Highly-Efficient Hydrogen Evolution Catalysis" Nanomaterials 10, no. 11: 2115. https://doi.org/10.3390/nano10112115
APA StyleGao, Y., Wang, K., Lin, Z., Song, H., Duan, X., Peng, Z., & Yan, S. (2020). Hydrothermal Synthesis of Polyhedral Nickel Sulfide by Dual Sulfur Source for Highly-Efficient Hydrogen Evolution Catalysis. Nanomaterials, 10(11), 2115. https://doi.org/10.3390/nano10112115




