Effect of the Morphology and Electrical Property of Metal-Deposited ZnO Nanostructures on CO Gas Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Nanostructures
2.2. Photodeposition of Metals on ZnO Nanostructures
2.3. Surface Characterization
2.4. Measurement of CO Gas Sensitivity
3. Results and Discussion
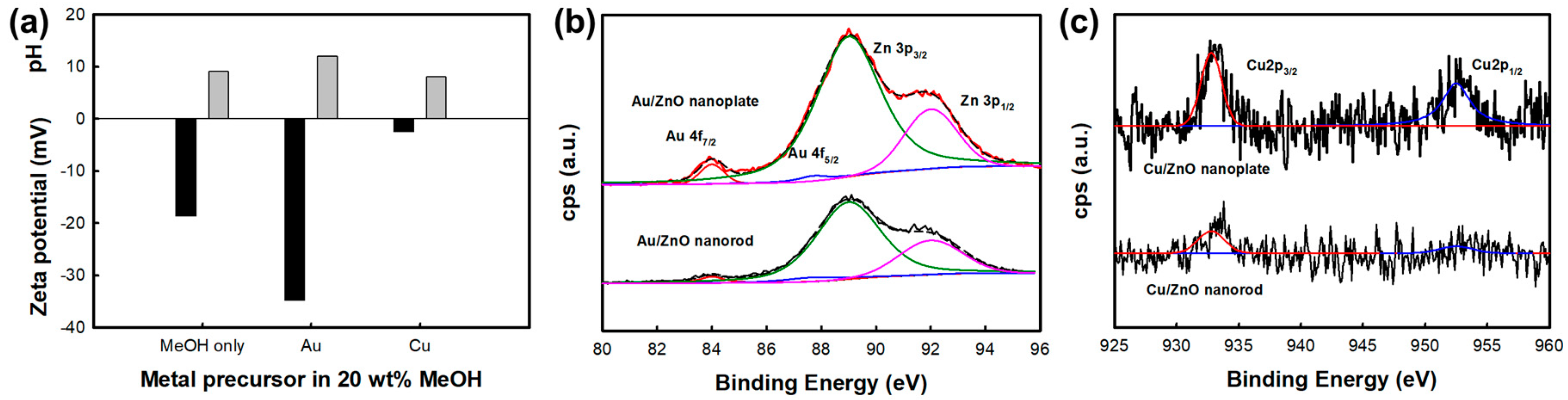
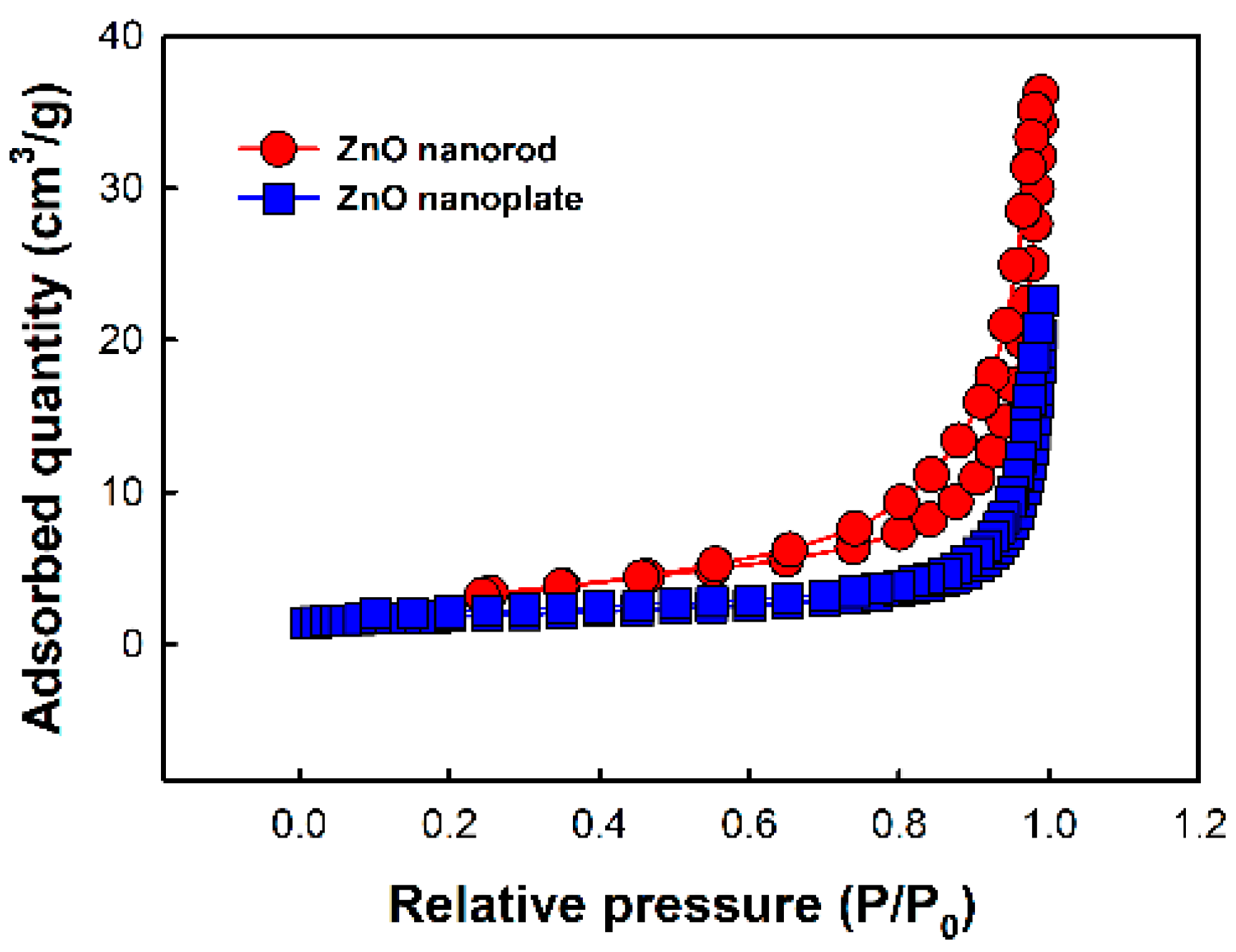
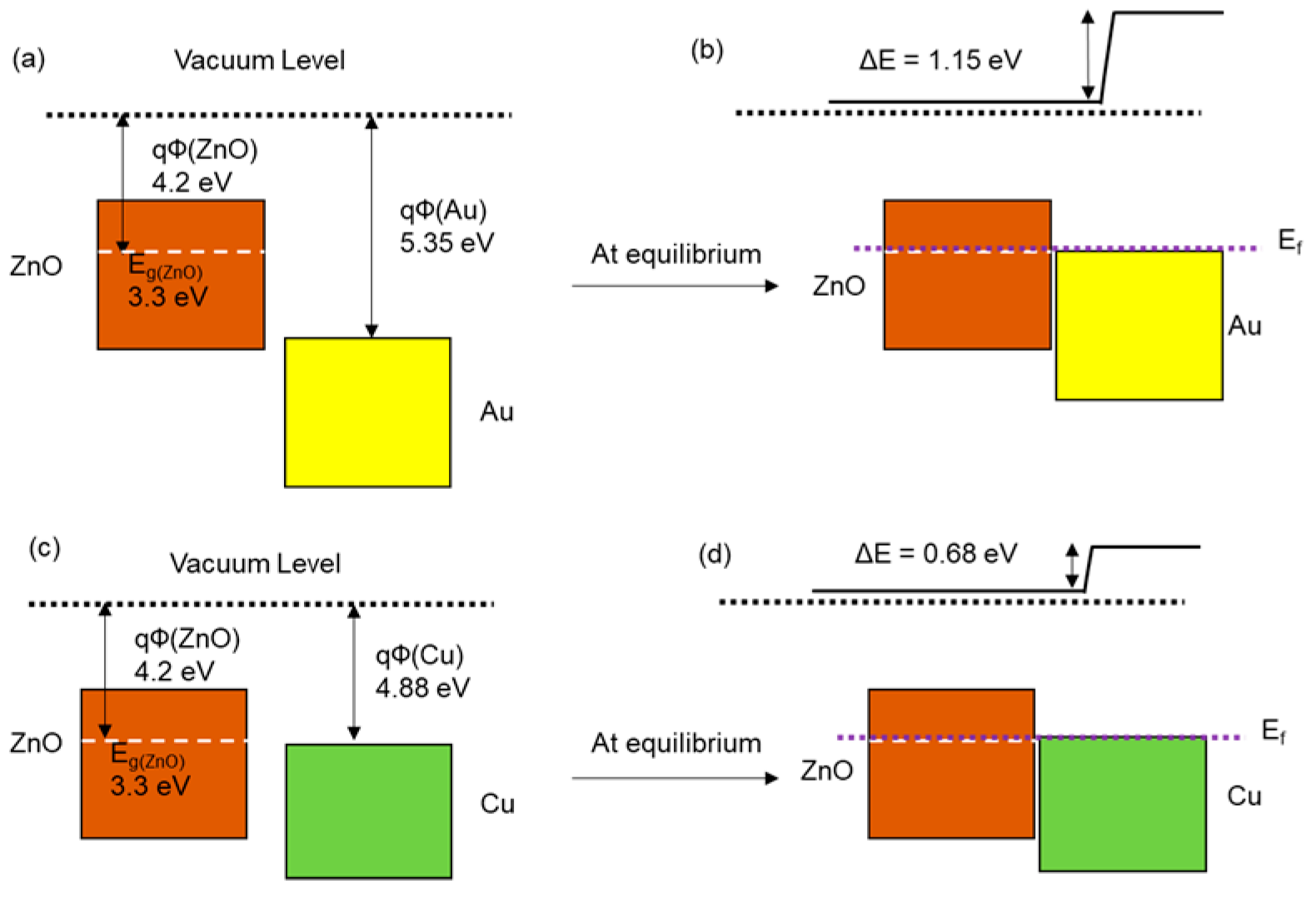
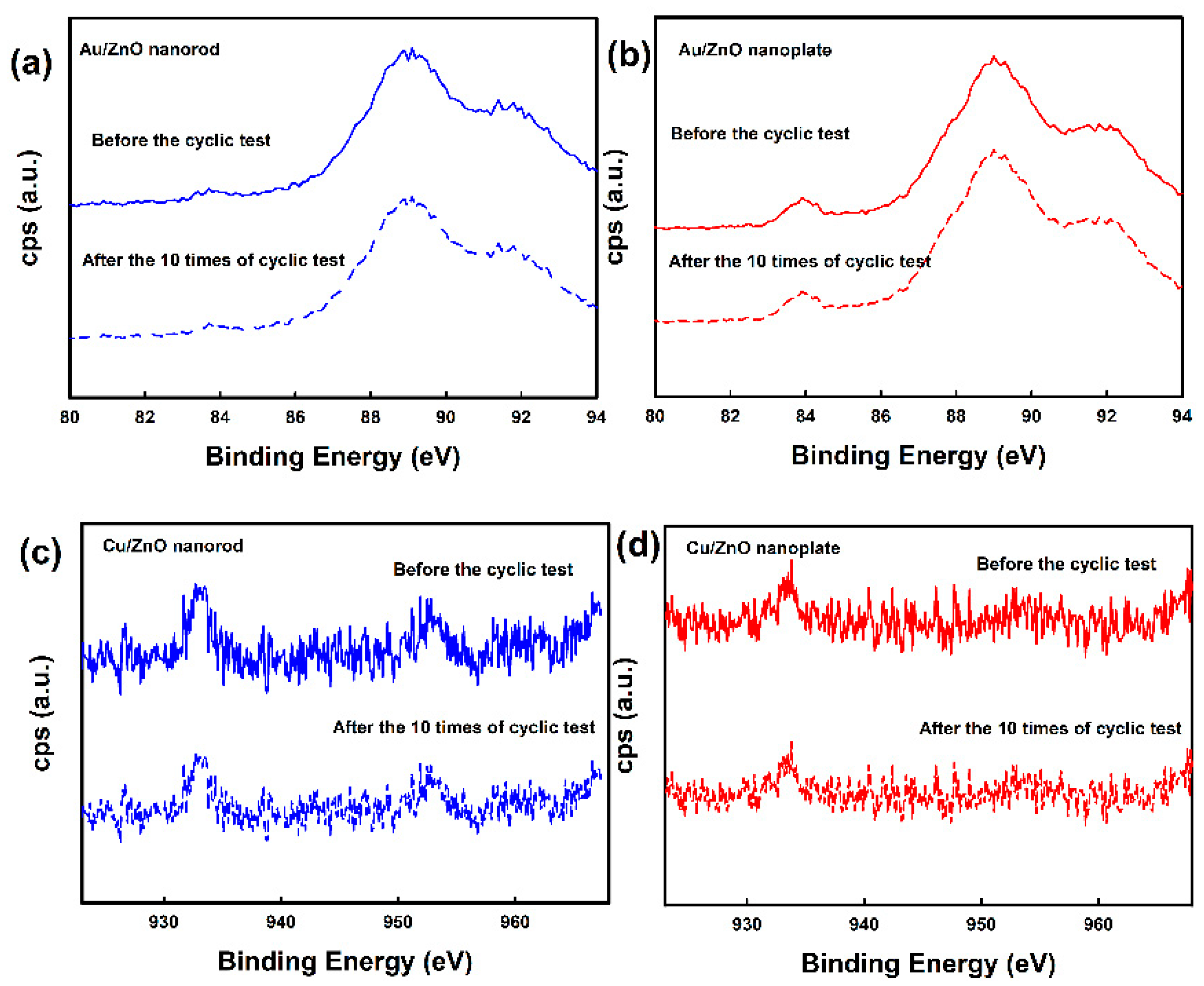
3.1. Surface Characterization of ZnO Nanostructures and Metal-Deposited ZnO Nanostructures
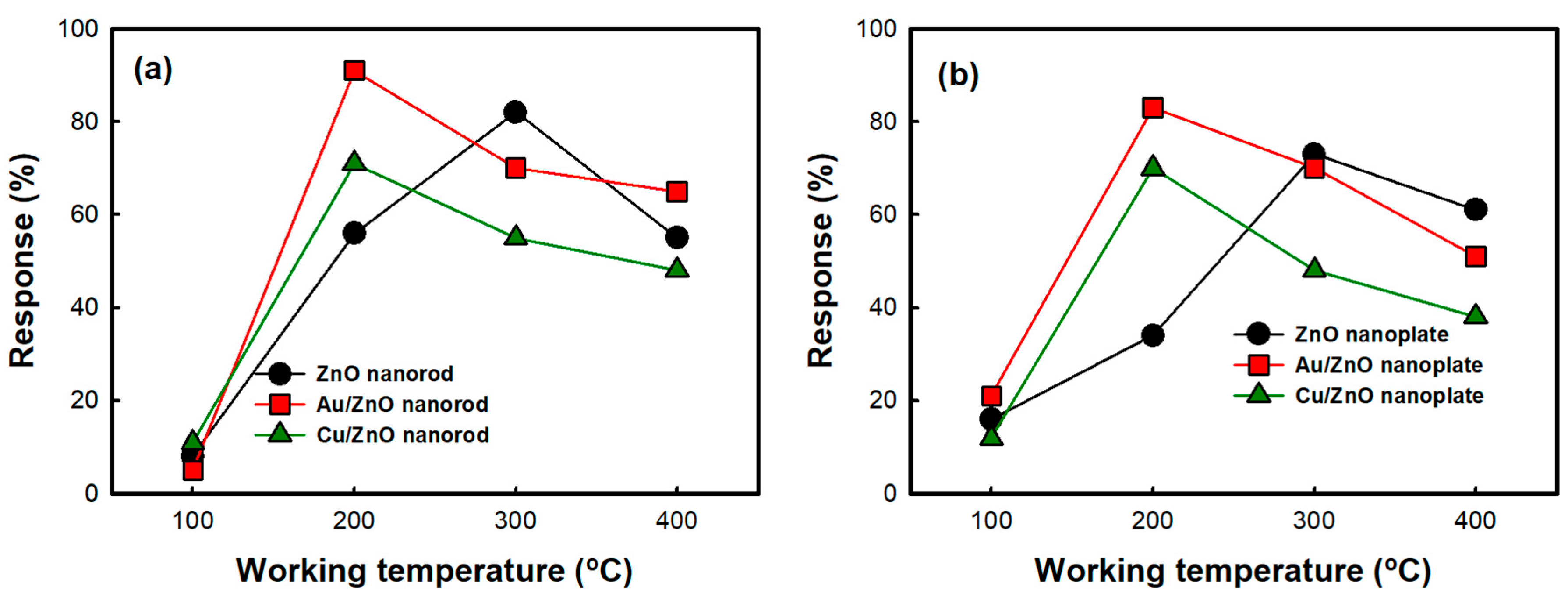
3.2. CO Gas Sensitivity of ZnO Nanostructures and Metal-Deposited ZnO Nanostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bleecker, M.L. Chapter 12—Carbon monoxide intoxication. In Handbook of Clinical Neurology; Lotti, M., Bleecker, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131, pp. 191–203. [Google Scholar]
- World Health Organization; Regional Office for Europe. WHO Guidelines for Indoor air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2010. [Google Scholar]
- Choi, K.-W.; Lee, J.-S.; Seo, M.-H.; Jo, M.-S.; Yoo, J.-Y.; Sim, G.S.; Yoon, J.-B. Batch-fabricated CO gas sensor in large-area (8-inch) with sub-10 mW power operation. Sens. Actuators B 2019, 289, 153–159. [Google Scholar] [CrossRef]
- Vallejos, S.; Gràcia, I.; Pizúrová, N.; Figueras, E.; Čechal, J.; Hubálek, J.; Cané, C. Gas sensitive ZnO structures with reduced humidity-interference. Sens. Actuators B 2019, 301, 127054. [Google Scholar] [CrossRef]
- Naberezhnyi, D.; Rumyantseva, M.; Filatova, D.; Batuk, M.; Hadermann, J.; Baranchikov, A.; Khmelevsky, N.; Aksenenko, A.; Konstantinova, E.; Gaskov, A. Effects of Ag additive in low temperature CO detection with In2O3 based gas sensors. Nanomaterials 2018, 8, 801. [Google Scholar] [CrossRef]
- Hsu, K.C.; Fang, T.H.; Tang, I.T.; Hsiao, Y.J.; Chen, C.Y. Mechanism and characteristics of Au-functionalized SnO2/In2O3 nanofibers for highly sensitive CO detection. J. Alloys Compd. 2020, 822, 9. [Google Scholar] [CrossRef]
- Punetha, D.; Pandey, S.K. CO gas sensor based on E-beam evaporated ZnO, MgZnO, and CdZnO thin films: A comparative study. IEEE Sens. J. 2019, 19, 2450–2457. [Google Scholar] [CrossRef]
- Giancaterini, L.; Cantalini, C.; Cittadini, M.; Sturaro, M.; Guglielmi, M.; Martucci, A.; Resmini, A.; Anselmi-Tamburini, U. Au and Pt nanoparticles effects on the optical and electrical gas sensing properties of sol–gel-based ZnO thin-film sensors. IEEE Sens. J. 2015, 15, 1068–1076. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—Nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; El Mir, L.; Alamri, H.; Bonavita, A.; Iannazzo, D.; Leonardi, S.G.; Neri, G. CO sensing characteristics of In-doped ZnO semiconductor nanoparticles. J. Sci. Adv. Mater. Devices 2017, 2, 34–40. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.-n.; Cao, J.-l. Rapid detection of low concentration CO using Pt-loaded ZnO nanosheets. J. Hazard. Mater. 2020, 381, 120944. [Google Scholar] [CrossRef]
- Arunkumar, S.; Hou, T.; Kim, Y.-B.; Choi, B.; Park, S.H.; Jung, S.; Lee, D.-W. Au Decorated ZnO hierarchical architectures: Facile synthesis, tunable morphology and enhanced CO detection at room temperature. Sens. Actuators B 2017, 243, 990–1001. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Gong, H.; Hu, J.Q.; Wang, J.H.; Ong, C.H.; Zhu, F.R. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B 2006, 115, 247–251. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, R.K.; Lee, P.S. Gold-Nanoparticle-Functionalized In2O3 Nanowires as CO Gas Sensors with a Significant Enhancement in Response. ACS Appl. Mater. Interfaces 2011, 3, 2246–2252. [Google Scholar] [CrossRef]
- Jeong, H.W.; Choi, S.-Y.; Hong, S.H.; Lim, S.K.; Han, D.S.; Abdel-Wahab, A.; Park, H. Shape-dependent charge transfers in crystalline ZnO photocatalysts: Rods versus plates. J. Phys. Chem. C 2014, 118, 21331–21338. [Google Scholar] [CrossRef]
- Kim, Y.K.; Seo, H.-J.; Kim, S.; Hwang, S.-H.; Park, H.; Lim, S.K. Effect of ZnO electrodeposited on carbon film and decorated with metal nanoparticles for solar hydrogen production. J. Mater. Sci. Technol. 2016, 32, 1059–1065. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Kim, Y.K.; Hong, S.H.; Lim, S.K. Cu/CuO@ZnO hollow nanofiber gas sensor: Effect of hollow nanofiber structure and P–N junction on operating temperature and sensitivity. Sensors 2019, 19, 3151. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Hwang, S.-H.; Kim, S.; Park, H. Preparation of ZnO nanorods by microemulsion synthesis and their application as a CO gas sensor. Sens. Actuators B 2011, 160, 94–98. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, S.-H.; Jeong, S.M.; Son, K.Y.; Lim, S.K. Colorimetric hydrogen gas sensor based on PdO/metal oxides hybrid nanoparticles. Talanta 2018, 188, 356–364. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Machado, B.F.; Bacsa, R.R.; Serp, P.; Dražić, G.; Faria, J.L.; Figueiredo, J.L. Catalytic performance of Au/ZnO nanocatalysts for CO oxidation. J. Catal. 2010, 273, 191–198. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, H.; Tang, Y.; Chang, Z.; Yin, S.; Liu, Z. Bio-inspired hierarchical assembly of Au/ZnO decorated carbonized spinach leaves with enhanced photocatalysis performance. J. Alloy. Compd. 2020, 829, 154393. [Google Scholar] [CrossRef]
- Jiang, J.; Mu, Z.; Xing, H.; Wu, Q.; Yue, X.; Lin, Y. Insights into the synergetic effect for enhanced UV/visible-light activated photodegradation activity via Cu-ZnO photocatalyst. Appl. Surf. Sci. 2019, 478, 1037–1045. [Google Scholar] [CrossRef]
- Peng, S.; Hong, P.; Li, Y.; Xing, X.; Yang, Y.; Wang, Z.; Zou, T.; Wang, Y. Pt decorated SnO2 nanoparticles for high response CO gas sensor under the low operating temperature. J. Mater. Sci. Mater. Electron. 2019, 30, 3921–3932. [Google Scholar] [CrossRef]
- Lin, T.T.; Lv, X.; Li, S.; Wang, Q.J. The Morphologies of the semiconductor oxides and their gas-sensing properties. Sensors 2017, 17, 2779. [Google Scholar] [CrossRef]
- Caglar, M.; Ilican, S.; Caglar, Y.; Yakuphanoglu, F. Electrical conductivity and optical properties of ZnO nanostructured thin film. Appl. Surf. Sci. 2009, 255, 4491–4496. [Google Scholar] [CrossRef]
- Nagarjuna, Y.; Hsiao, Y.-J. Au doping ZnO nanosheets sensing properties of ethanol gas prepared on MEMS device. Coatings 2020, 10, 945. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low power-consumption CO gas sensors based on Au-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Karami Horastani, Z.; Sayedi, S.M.; Sheikhi, M.H.; Rahimi, E. Effect of silver additive on electrical conductivity and methane sensitivity of SnO2. Mater. Sci. Semicond. Process. 2015, 35, 38–44. [Google Scholar] [CrossRef]
- Chang, J.; Ahmad, M.Z.; Wlodarski, W.; Waclawik, E.R. Self-Assembled 3D ZnO Porous Structures with Exposed Reactive {0001} Facets and Their Enhanced Gas Sensitivity. Sensors 2013, 13, 8445–8460. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Yu, Y.H.; Pei, Z.L.; Bai, X.D.; Sun, C.; Huang, R.F.; Wen, L.S. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Appl. Surf. Sci. 2000, 158, 134–140. [Google Scholar] [CrossRef]
- Betty, C.A.; Choudhury, S.; Girija, K.G. Reliability studies of highly sensitive and specific multi-gas sensor based on nanocrystalline SnO2 film. Sens. Actuators B 2014, 193, 484–491. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.-H.; Chang, D.; Kim, S. Preparation of mesoporous In2O3 nanofibers by electrospinning and their application as a CO gas sensor. Sens. Actuators B 2010, 149, 28–33. [Google Scholar] [CrossRef]



| Nano Particles | Morphology (ZnO) | Synthesis Method | Operating Temperature (°C) | Concentration of CO (ppm) | Sensitivity (Ra/Rg) | Ref. |
|---|---|---|---|---|---|---|
| In | Nanoparticle | Sol–gel | 300 | 100 | 5 | [10] |
| Au | Nanostar | Hydrothermal | RT | 100 | 15 | [12] |
| Pt | Nanosheet | Hydrothermal/calcination | 180 | 100 | 3.5 | [11] |
| Au | Nanorods | Hydrothermal | 150 | 1000 | 12 | [13] |
| Pd | Nanowires | VLS growth | RT | 0.1 | 1.02 | [13] |
| Sample | Metal (mM) | Zn (mM) | Metal/Zn 1 | Electrical Conductivity (S/m) |
|---|---|---|---|---|
| ZnO nanorod | - | 3.313 | - | 7.345 × 10−7 |
| Au/ZnO nanorod | 1.040 | 3.186 | 0.327 | 6.327 × 10−6 |
| Cu/ZnO nanorod | 0.185 | 3.048 | 0.061 | 1.057 × 10−6 |
| ZnO nanoplate | - | 3.061 | - | 6.148 × 10−7 |
| Au/ZnO nanoplate | 1.501 | 3.019 | 0.497 | 5.113 × 10−6 |
| Cu/ZnO nanoplate | 0.254 | 3.030 | 0.084 | 1.044 × 10−6 |
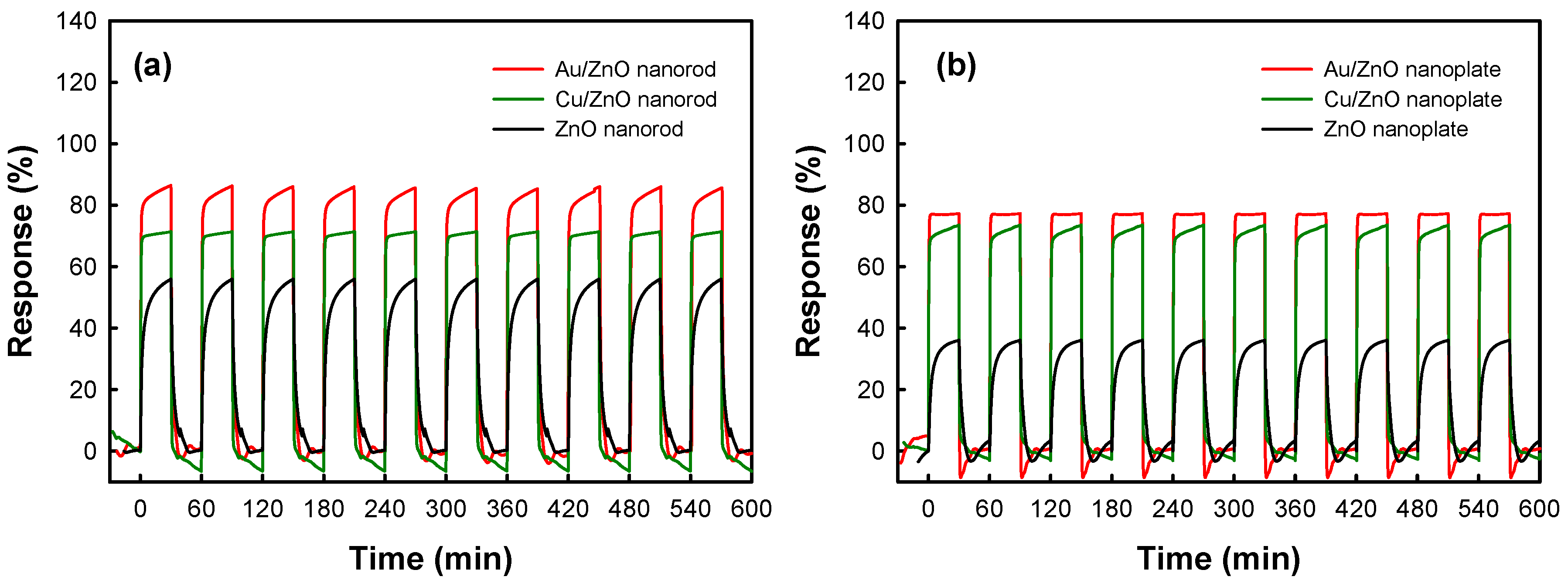
| Sample | 1 Gas Response (%) | 2 Response Time τ1 (sec) | 3 Recovery Time τ2 (sec) |
|---|---|---|---|
| ZnO nanorod | 55.96 | 151.519 | 157.970 |
| Au/ZnO nanorod | 86.45 | 32.864 | 56.599 |
| Cu/ZnO nanorod | 71.39 | 29.851 | 31.108 |
| ZnO nanoplate | 36.06 | 195.691 | 172.005 |
| Au/ZnO nanoplate | 77.34 | 26.053 | 24.749 |
| Cu/ZnO nanoplate | 70.51 | 26.172 | 23.743 |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| ZnO nanorod | 11.3021 | 0.040563 | 12.945 |
| ZnO nanoplate | 5.9611 | 0.034951 | 23.453 |
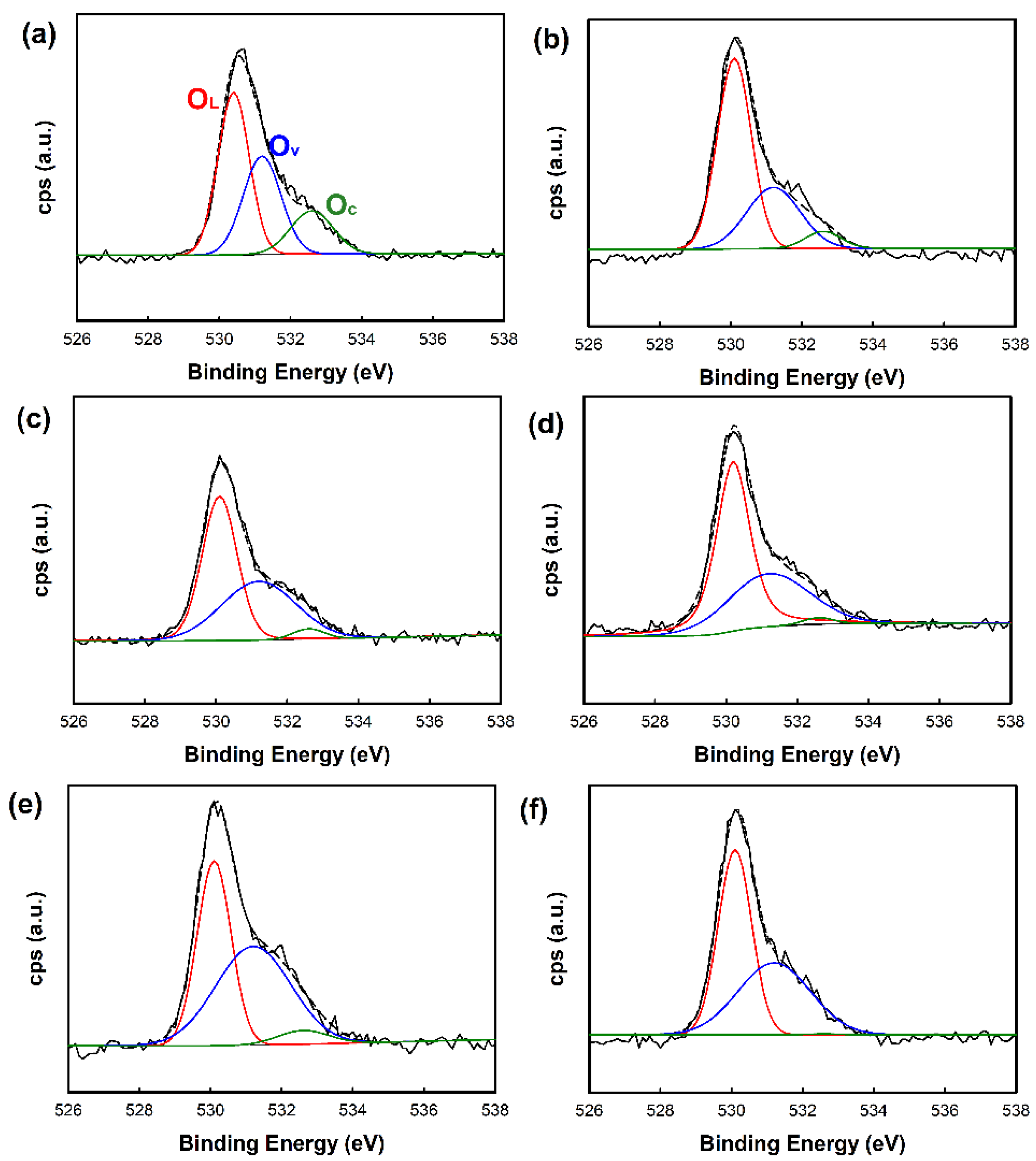
| Sample | OL1(%) | Ov2(%) | Oc3(%) |
|---|---|---|---|
| ZnO nanorod | 39.63 | 46.39 | 13.98 |
| Au/ZnO nanorod | 43.49 | 51.66 | 4.85 |
| Cu/ZnO nanorod | 52.78 | 43.91 | 3.31 |
| ZnO nanoplate | 62.59 | 31.47 | 5.94 |
| Au/ZnO nanoplate | 50.00 | 48.31 | 1.69 |
| Cu/ZnO nanoplate | 54.20 | 45.60 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.-H.; Kim, Y.K.; Hong, S.H.; Lim, S.K. Effect of the Morphology and Electrical Property of Metal-Deposited ZnO Nanostructures on CO Gas Sensitivity. Nanomaterials 2020, 10, 2124. https://doi.org/10.3390/nano10112124
Hwang S-H, Kim YK, Hong SH, Lim SK. Effect of the Morphology and Electrical Property of Metal-Deposited ZnO Nanostructures on CO Gas Sensitivity. Nanomaterials. 2020; 10(11):2124. https://doi.org/10.3390/nano10112124
Chicago/Turabian StyleHwang, Sung-Ho, Young Kwang Kim, Seong Hui Hong, and Sang Kyoo Lim. 2020. "Effect of the Morphology and Electrical Property of Metal-Deposited ZnO Nanostructures on CO Gas Sensitivity" Nanomaterials 10, no. 11: 2124. https://doi.org/10.3390/nano10112124
APA StyleHwang, S.-H., Kim, Y. K., Hong, S. H., & Lim, S. K. (2020). Effect of the Morphology and Electrical Property of Metal-Deposited ZnO Nanostructures on CO Gas Sensitivity. Nanomaterials, 10(11), 2124. https://doi.org/10.3390/nano10112124





