Synthesis, Characterization, and Photocatalytic Evaluation of Manganese (III) Phthalocyanine Sensitized ZnWO4 (ZnWO4MnPc) for Bisphenol A Degradation under UV Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Preparation and Characterization of Photocatalyst Materials
2.3.1. ZnWO4
2.3.2. ZnWO4MnPc
- Synthesis and Characterization of Zwitter-Ionic Water-Soluble Manganese (III) Phthalocyanine (BZ-Mn-PS)
2.4. Determination of pHpzc of ZnWO4MnPc Catalyst Material
2.5. Photocatalytic Experiments
3. Results and Discussion
3.1. Material Characterizations
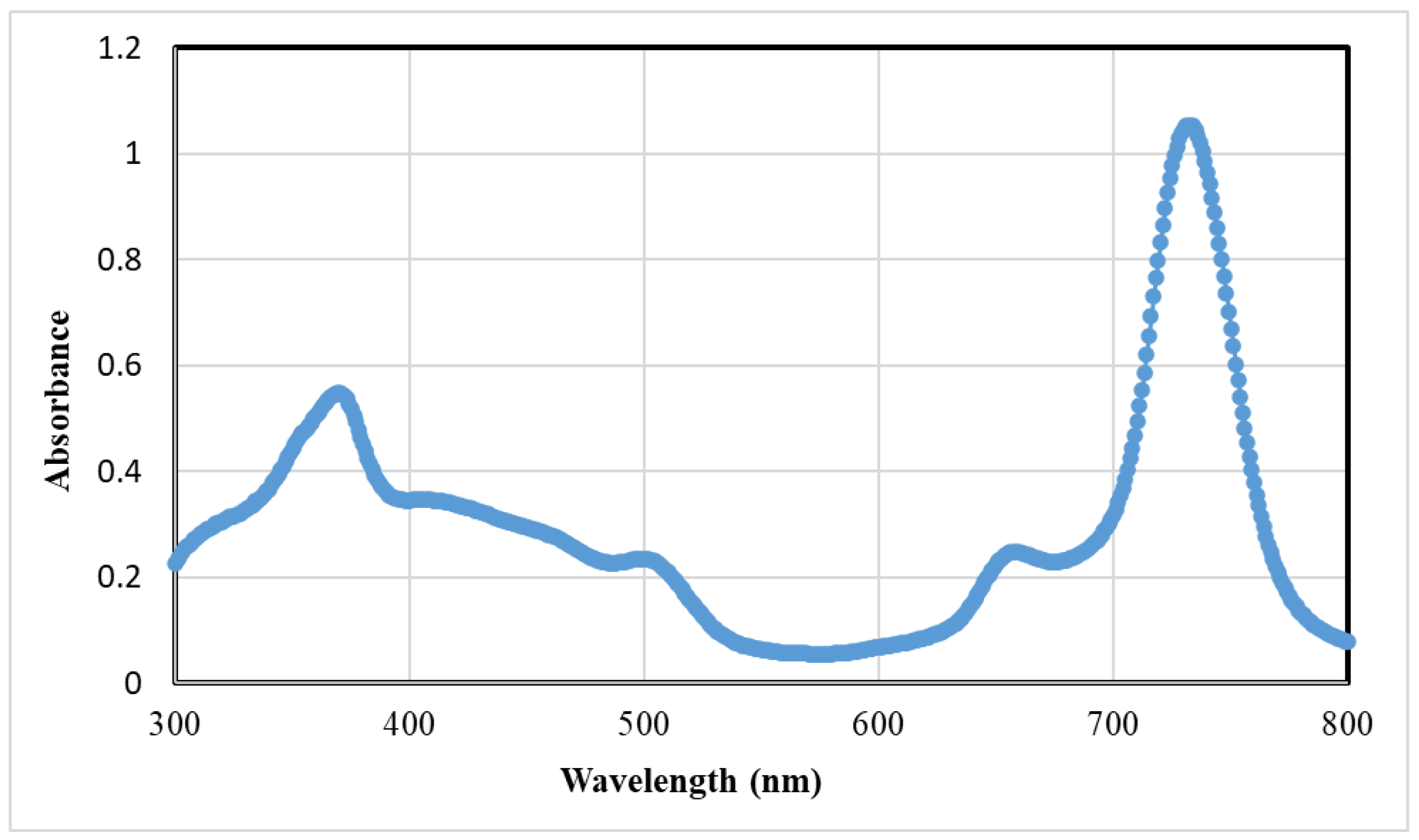
3.1.1. Synthesis and Characterization of BZ-Mn-PS
3.1.2. Characterization of ZnWO4 and ZnWO4MnPc
3.2. Photocatalytic Activity
3.2.1. Effect of pH
- pHpzc Value
3.2.2. Effect of Catalyst Dosage and Initial BPA Concentration
3.2.3. Reactive Oxygen Species (ROS) Probe
3.2.4. Effect of Hydrogen Peroxide (H2O2)
3.2.5. Water Matrix Effects
3.2.6. Evaluation of Visible Light Photocatalytic Activity
3.3. Proposed Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rosenfeld, P.E.; Feng, L.G.H. Emerging Contaminants. Risks Haz. Wastes 2011, 1, 215–222. [Google Scholar]
- Jimenez-Holgado, C.; Chrimatopoulos, C.; Stathopoulos, V.N.; Sakkasv, V. Investigating the Utility of Fabric Phase Sorptive Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples. Separations 2020, 7, 39. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kim, M.-S.; Kim, B.-W. Photodegradation of Bisphenol A with TiO2 Immobilized on the Glass Tubes Including UV Light Lamps. Water Res. 2004, 38, 3605–3613. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Lee, M.K.; Su, T.Y.; Chang, Y.M. Photodegradation of Bisphenol A in a Batch TiO2 Suspension Reactor. J. Hazd. Mat. 2009, 168, 269–275. [Google Scholar] [CrossRef]
- Lu, N.; Lu, Y.; Liu, F.; Zhao, K.; Yuan, X.; Zhao, Y.; Li, Y.; Qin, H.; Zhu, J. H3PW12O40/TiO2 Catalyst Induced Photodegradation of Bisphenol A (BPA): Kinetics, Toxicity and Degradation Pathways. Chemosphere 2013, 91, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Garg, A.; Singhania, T.; Singh, A.; Sharma, S.; Rani, S.; Neogy, A.; Yadav, S.R.; Sangal, V.K.; Garg, N. Photocatalytic Degradation of Bisphenol-A using N, Co Codoped TiO2 Catalyst under Solar Light. Sci. Rep. 2019, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Genco, M.C.; Megrelis, L.; Ruderman, J.V. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl. Acad. Sci. USA 2011, 108, 17732–17737. [Google Scholar] [CrossRef] [Green Version]
- Gong, R.; Jiang, Y.; Cai, W.; Zhang, K.; Yuan, B.; Jiang, J. Enhanced Sorption of Bisphenol A on Alpha- Ketoglutaric Acid -Modified Chitosan Resins by Hydrophobic Sorption Hemimicelles. Desalination 2010, 258, 54–58. [Google Scholar] [CrossRef]
- Huang, R.-P.; Liu, Z.-H.; Yuan, S.-F.; Yin, H.; Dang, Z.; Wu, P. Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000–2016) and its risk analysis. Environ. Pollut. 2017, 230, 143–152. [Google Scholar] [CrossRef]
- Kundakovic, M.; Gudsnuk, K.; Franks, B.; Madrid, J.; Miller, R.L.; Perera, F.P.; Champagne, F.A. Sex-Specific Epigenetic Disruption and Behavioural Changes Following Low Dose in Utero Bisphenol A Exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 9956–9961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetherill, Y.B.; Petre, C.E.; Monk, K.R.; Puga, A.; Knudsen, K.E. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol. Cancer Ther. 2002, 1, 515–524. [Google Scholar]
- NORMAN—Network of Reference Laboratories, Research Centres and Related Organisations for Monitoring of Emerging Environmental Substances 2016. Available online: http://www.norman-network.net/ (accessed on 28 February 2020).
- Wang, H.; Liu, Z.-H.; Zhang, J.; Huang, R.-P.; Yin, H.; Dang, Z.; Wu, P.-X.; Liu, Y. Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci. Total. Environ. 2019, 692, 107–116. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Kowalska, E. On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef] [Green Version]
- Osotsi, M.I.; Macharia, D.K.; Zhu, B.; Wang, Z.; Shen, X.; Liu, Z.; Zhang, L.; Chen, Z. Synthesis of ZnWO4-x nanorods with oxygen vacancy for efficient photocatalytic degradation of tetracycline. Prog. Nat. Sci. Mat. Int. 2018, 28, 408–415. [Google Scholar] [CrossRef]
- Mavrič, T.; Valant, M.; Forster, M.; Cowan, A.J.; Lavrenčič, U.; Emin, S. Design of a highly photocatalytically active ZnO/CuWO4 nanocomposite. J. Colloid Interface Sci. 2016, 483, 93–101. [Google Scholar] [CrossRef]
- Gavade, N.L.; Babar, S.B.; Kadam, A.N.; Gophane, A.D.; Garadkar, K. Fabrication of M@CuxO/ZnO (M = Ag, Au) Heterostructured Nanocomposite with Enhanced Photocatalytic Performance under Sunlight. Ind. Eng. Chem. Res. 2017, 56, 14489–14501. [Google Scholar] [CrossRef]
- Amouzegar, Z.; NaghiZadeh, R.; Rezaie, H.; Ghahari, M.; Aminzare, M. Microwave engineering of ZnWO4 nanostructures: Towards morphologically favorable structures for photocatalytic activity. Ceram. Int. 2015, 41, 8352–8359. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Tong, L.; Chen, X.-B.; Zeng, X.-H. Ethylene glycol-assisted solvothermal fabrication of ZnWO4 nanostructures with tunable size, optical properties, and photocatalytic activities. Appl. Phys. A 2014, 117, 673–679. [Google Scholar] [CrossRef]
- He, D.; Wang, L.; Xu, D.; Zhai, J.; Wang, D.; Xie, T. Investigation of Photocatalytic Activities over Bi2WO6/ZnWO4 Composite under UV Light and Its Photoinduced Charge Transfer Properties. ACS Appl. Mater. Interfaces 2011, 3, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, L.; Zhu, Y. Visible Photocatalytic Activity Enhancement of ZnWO4 by Graphene Hybridization. ACS Catal. 2012, 2, 2769–2778. [Google Scholar] [CrossRef]
- Li, K.; Xue, J.; Zhang, Y.; Wei, H.; Liu, Y.; Dong, C. ZnWO4 nanorods decorated with Ag/AgBr nanoparticles as highly efficient visible-light-responsive photocatalyst for dye AR18 photodegradation. Appl. Surf. Sci. 2014, 320, 1–9. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-Based Hybrid Photocatalyst with Broad Spectrum Photocatalytic Properties under UV, Visible, and Near-Infrared Irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, X.; Cheng, X.; Sun, H.; Li, Y.; Li, P.; Fan, W. Evaluating the C, N, and F Pairwise Codoping Effect on the Enhanced Photoactivity of ZnWO4: The Charge Compensation Mechanism in Donor–Acceptor Pairs. J. Phys. Chem. C 2011, 115, 15516–15524. [Google Scholar] [CrossRef]
- Fu, H.; Lin, J.; Zhang, L.; Li, D. Photocatalytic activities of a novel ZnWO4 catalyst prepared by a hydrothermal process. Appl. Catal. A Gen. 2006, 306, 58–67. [Google Scholar] [CrossRef]
- Keleş, T.; Barut, B.; Bıyıklıoğlu, Z.; Özel, A. A comparative study on DNA/BSA binding, DNA photocleavage and antioxidant activities of water soluble peripherally and non-peripherally tetra-3-pyridin-3-ylpropoxy-substituted Mn(III), Cu(II) phthalocyanines. Dyes Pigm. 2017, 139, 575–586. [Google Scholar] [CrossRef]
- Wang, H.-L.; Li, Y.; Pang, L.; Zhang, W.-Z.; Jiang, W.-F. Preparation and application of thermosensitive poly(NIPAM-co-MAH-β-CD)/(TiO2-MWCNTs) composites for photocatalytic degradation of dinitro butyl phenol (DNBP) under visible light irradiation. Appl. Catal. B Environ. 2013, 130, 132–142. [Google Scholar] [CrossRef]
- Çakır, D.; Çakır, V.; Bıyıklıoğlu, Z.; Durmuş, M.; Kantekin, H. New water-soluble cationic zinc phthalocyanines as potential for photodynamic therapy of cancer. J. Organomet. Chem. 2013, 745, 423–431. [Google Scholar] [CrossRef]
- Bayrak, R.; Akçay, H.T.; Beris, F.S.; Sahin, E.; Bayrak, H.; Demirbaş, Ü. Synthesis, aggregation and spectroscopic studies of novel water soluble metal free, zinc, copper and magnesium phthalocyanines and investigation of their anti-bacterial properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 272–280. [Google Scholar] [CrossRef]
- Akinbulu, A.I.; Nyokong, T. The effects of point of substitution on the electrochemical behavior of new manganese phthalocyanines, tetra-substituted with Diethylaminoethanethiol. Inorganica Chim. Acta 2010, 363, 3229–3237. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Xu, D.; Lin, J.; Li, P.; Lin, S.; Shi, W.; Dongbo, X. Enhanced photocatalytic degradation activity for tetracycline under visible light irradiation of Ag/Bi3.84W0.16O6.24 nanooctahedrons. CrystEngComm 2015, 17, 2421–2428. [Google Scholar] [CrossRef]
- Amouzegar, Z.; NaghiZadeh, R.; Rezaie, H.; Ghahari, M.; Aminzare, M. Cubic ZnWO4 nano-photocatalysts synthesized by the microwave-assisted precipitation technique. Ceram. Int. 2015, 41, 1743–1747. [Google Scholar] [CrossRef]
- You, H.; Zhao, Y. Synthesis, Characterization and Visible Light Photocatalytic Performance of Iron (III) Tetracarboxyphthalocyanine-Sensitized TiO2 Photocatalyst. J. Phys. Chem. Biophys. 2016, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Mancheva, M.; Iordanova, R.; Dimitriev, Y. Mechanochemical synthesis of nanocrystalline ZnWO4 at room temperature. J. Alloys Compd. 2011, 509, 15–20. [Google Scholar] [CrossRef]
- Singh, N.P.; Devi, Y.R.; Singh, N.R.; Singh, N.M. Synthesis of Tb3+ Ion Doped ZnWO4 Phosphors and Investigation of Their Photoluminescence Properties: Concentration Effect. Bull. Mater. Sci. 2019, 42, 96. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yu, T.; Zou, Z.; Ye, J. Degradation in Photocatalytic Activity Induced by Hydrogen Related Defects in Nano-LiNbO3. Appl. Phys. Lett. 2006, 88, 917–923. [Google Scholar] [CrossRef]
- Pan, C.; Xu, J.; Chen, Y.; Zhu, Y. Influence of -OH Related Defects on the Performances of BiPO4 Photocatalyst for the Degradation of Rhodamine B. Appl. Catal. B Environ. 2012, 115–116, 314–315. [Google Scholar] [CrossRef]
- Kanan, S.M.; Lu, Z.; Cox, J.K.; Bernhardt, G.; Tripp, C.P. Identification of Surface Sites on Monoclinic WO3Powders by Infrared Spectroscopy. Langmuir 2002, 18, 1707–1712. [Google Scholar] [CrossRef]
- Kim, S.J.; A’Hearn, M.; Wellnitz, D.; Meier, R.; Lee, Y. The rotational structure of the B–X system of sulfur dimers in the spectra of Comet Hyakutake (C/1996 B2). Icarus 2003, 166, 157–166. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Ao, C.; Lee, S.; Hou, M. Enhanced photocatalytic degradation of VOCs using Ln3+–TiO2 catalysts for indoor air purification. Chemosphere 2005, 59, 787–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ede, S.R.; Ramadoss, A.; Nithiyanantham, U.; Anantharaj, S.; Kundu, S. Bio-molecule Assisted Aggregation of ZnWO4 Nanoparticles (NPs) into Chain-like Assemblies: Material for High Performance Supercapacitor and as Catalyst for Benzyl Alcohol Oxidation. Inorg. Chem. 2015, 54, 3851–3863. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.; Dasari, K.; Palai, R.; Feng, P. The shift of optical band gap in W-doped ZnO with oxygen pressure and doping level. Mater. Res. Bull. 2014, 54, 73–77. [Google Scholar] [CrossRef]
- Chen, S.; Sun, S.; Sun, H.; Fan, W.; Zhao, X.; Sun, X. Experimental and Theoretical Studies on the Enhanced Photocatalytic Activity of ZnWO4 Nanorods by Fluorine Doping. J. Phys. Chem. C 2010, 114, 7680–7688. [Google Scholar] [CrossRef]
- Zhai, B.-G.; Yang, L.; Zhou, F.-F.; Shi, J.-S.; Huang, Y.M. Strong Photo-Oxidative Capability of ZnWO4 Nanoplates with Highly Exposed {0 1 1} Facets. Catalysts 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.J.H.; Bhat, D.K. Novel ZnWO4/RGO Nanocomposite as High Performance Photocatalyst. AIMS Mat. Sci. 2017, 4, 158–171. [Google Scholar]
- Colusso, A.V.; McDonagh, A.; Cortie, M.B. X-ray-induced reduction of a surfactant/polyoxotungstate hybrid compound. Surf. Interface Anal. 2018, 50, 1384–1388. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.P.; Raval, P. Effect of transition metal ion (Cr3+, Mn2+ and Cu2+) doping on the photocatalytic properties of ZnWO4 nanoparticles. J. Photochem. Photobiol. A Chem. 2018, 357, 193–200. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Lacomba-Perales, R.; Ruiz-Fuertes, J.; Errandonea, D.; Martínez-García, D.; Segura, A. Optical absorption of divalent metal tungstates: Correlation between the band-gap energy and the cation ionic radius. EPL (Europhys. Lett.) 2008, 83, 37002–37018. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Zhu, B.; Guan, K.; Gao, S.; Lv, L.; Du, C.; Peng, L.; Hou, L.; Wang, X. Particle Size and Structural Control of ZnWO4 Nanocrystals via Sn2+ Doping for Tunable Optical and Visible Photocatalytic Properties. J. Phys. Chem. C 2012, 116, 18508–18517. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, D.; Chen, X.; Lin, Y. Adsorption of bisphenol A from water by surfactant-modified zeolite. J. Colloid Interface Sci. 2010, 348, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Bechambi, O.; Jlaiel, L.; Najjar, W.; Sayadi, S. Photocatalytic Degradation of Bisphenol A in the Presence of Ce-ZnO: Evolution of Kinetics, Toxicity and Photodegradation Mechanism. Mater. Chem. Phys. 2016, 173, 95–105. [Google Scholar] [CrossRef]
- Anju, S.; Yesodharan, S.; Yesodharan, E. Zinc oxide mediated sonophotocatalytic degradation of phenol in water. Chem. Eng. J. 2012, 189–190, 84–93. [Google Scholar] [CrossRef]
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Onundi, Y.; Drake, B.A.; Malecky, R.T.; DeNardo, M.A.; Mills, M.R.; Kundu, S.; Ryabov, A.D.; Beach, E.S.; Horwitz, C.P.; Simonich, M.T.; et al. A multidisciplinary investigation of the technical and environmental performances of TAML/peroxide elimination of Bisphenol A compounds from water. Green Chem. 2017, 19, 4234–4262. [Google Scholar] [CrossRef] [Green Version]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. J. Photochem. Photobiol. A Chem. 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Goto, H.; Hanada, Y.; Ohno, T.; Matsumura, M. Quantitative analysis of superoxide ion and hydrogen peroxide produced from molecular oxygen on photoirradiated TiO2 particles. J. Catal. 2004, 225, 223–229. [Google Scholar] [CrossRef]
- Tseng, D.-H.; Juang, L.-C.; Huang, H.-H. Effect of Oxygen and Hydrogen Peroxide on the Photocatalytic Degradation of Monochlorobenzene in Aqueous Suspension. Int. J. Photoenergy 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of Pharmaceuticals and Personal Care Products in Water Using Carbonacious -TiO2 Composites: A Critical Review of Recent Literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Sojic-Merkulov, D.; Lazarevic, M.; Despotović, V.N.; Banić, N.D.; Fincur, N.; Maletic, S.; Abramović, B.F. The effect of inorganic anions and organic matter on mesotrione (Callisto®) removal from environmental waters. J. Serbian Chem. Soc. 2017, 82, 343–355. [Google Scholar] [CrossRef]
- Kudlek, E.; Dudziak, M.; Bohdziewicz, J. Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix. Water 2016, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Lee, H.; Choi, Y.; Kim, S.; Lee, S.; Lee, S.; Choi, W.; Lee, J. Heterogeneous photocatalytic treatment of pharmaceutical micropollutants: Effects of wastewater effluent matrix and catalyst modifications. Appl. Catal. B Environ. 2014, 147, 8–16. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Skoutelis, C.; Daikopoulos, C.; Deligiannakis, Y.; Konstantinou, I. Probing the photolytic–photocatalytic degradation mechanism of DEET in the presence of natural or synthetic humic macromolecules using molecular-scavenging techniques and EPR spectroscopy. J. Environ. Chem. Eng. 2015, 3, 3005–3014. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kim, K.-H.; Kavitha, B.; Kumar, V.; Raza, N.; Kalagara, S. Photocatalytic degradation of bisphenol A in aqueous media: A review. J. Environ. Manag. 2018, 213, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, Y. Sensitization of TiO2 with Aluminum Phthalocyanine: Factors Influencing the Efficiency for Chlorophenol Degradation in Water under Visible Light. J. Phys. Chem. C 2009, 113, 12387–12394. [Google Scholar] [CrossRef]
- Souza, J.D.; Pinheiro, M.V.B.; Krambrock, K.; Alves, W.A. Dye Degradation Mechanisms Using Nitrogen Doped and Copper(II) Phthalocyanine Tetracarboxylate Sensitized Titanate and TiO2 Nanotubes. J. Phys. Chem. C 2016, 120, 11561–11571. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; De La Cruz, A.A.; Dunlop, P.S.; Byrne, J.A.; Dionysiou, D.D. Use of selected scavengers for the determination of NF-TiO2 reactive oxygen species during the degradation of microcystin-LR under visible light irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Bian, J.; Sun, N.; Sun, J.; Chen, L.; Li, Z.; Jing, L. Controlled synthesis of novel Z-scheme iron phthalocyanine/porous WO3 nanocomposites as efficient photocatalysts for CO2 reduction. Appl. Catal. B Environ. 2020, 270, 118849. [Google Scholar] [CrossRef]
- Wang, S.L.; Gu, Y.; Cao, T.T.; Wang, P.; Zhang, A.; Huang, Y.P.; Ma, W. Photodegradation of Toxic Organic Pollutants Catalyzed by MnPcTC Immobilized on WO3. Fresen. Environ. Bull. 2011, 20, 2071–2077. [Google Scholar]

















| Materials | dXRD (nm) | dTEM (nm) | SBET (m2/g) | Vp (cm3/g) | dp (nm) | Eg (eV) |
|---|---|---|---|---|---|---|
| ZnWO4 | 13.1 | 13.4 | 15.1 | 0.040 | 14.0 | 4.0 |
| ZnWO4MnPc | 15.1 | 20.3 | 11.4 | 0.035 | 19.0 | 4.1 |
| Test | Degradation Rate Constant K (h−1) | R2 |
|---|---|---|
| Scavenger effects on ZnWO4MnPc # | ||
| No Scavenger | 0.2661 | 0.9842 |
| Benzoquinone (BQ) | 0.1942 | 0.9125 |
| Isopropyl alcohol (IPA) | 0.182 | 0.9516 |
| Etheylenediamine tetracetic acid (EDTA) | 0.1417 | 0.9245 |
| H2O2 effects on ZnWO4MnPc # | ||
| 0 mM H2O2 | 0.2661 | 0.9842 |
| 5 mM H2O2 | 0.5442 | 0.8607 |
| Ion effects on ZnWO4MnPc: Anions # | ||
| No ion | 0.2661 | 0.9842 |
| 20 mg/L NO3− | 0.1735 | 0.9815 |
| 100 mg/L CO32− | 0.1823 | 0.8227 |
| 200 mg/L Cl− | 0.2270 | 0.9938 |
| Ion effects on ZnWO4MnPc: Cations # | ||
| No ion | 0.2661 | 0.9842 |
| 20 mg/L Al3+ | 0.2336 | 0.9674 |
| 50 mg/L Ca2+ | 0.1795 | 0.9006 |
| 50 mg/L K+ | 0.1795 | 0.9006 |
| Humic acid (HA) effects on ZnWO4MnPc # | ||
| 0 mg/L HA | 0.2661 | 0.9842 |
| 10 mg/L HA | 0.1473 | 0.7826 |
| 15 mg/L HA | 0.0974 | 0.9219 |
| Photolysis effect/450 nm Visible range test | ||
| ZnWO4 | 0.05830 | 0.8362 |
| ZnWO4MnPc | 0.2411 | 0.9048 |
| ZnWO4MnPc + 5 mM H2O2 | 11.899 | 1 |
| UV-365 nm # | 0.1595 | 0.8309 |
| 450 nm | 0.0586 | 0.8451 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anucha, C.B.; Altin, I.; Biyiklioglu, Z.; Bacaksiz, E.; Polat, I.; Stathopoulos, V.N. Synthesis, Characterization, and Photocatalytic Evaluation of Manganese (III) Phthalocyanine Sensitized ZnWO4 (ZnWO4MnPc) for Bisphenol A Degradation under UV Irradiation. Nanomaterials 2020, 10, 2139. https://doi.org/10.3390/nano10112139
Anucha CB, Altin I, Biyiklioglu Z, Bacaksiz E, Polat I, Stathopoulos VN. Synthesis, Characterization, and Photocatalytic Evaluation of Manganese (III) Phthalocyanine Sensitized ZnWO4 (ZnWO4MnPc) for Bisphenol A Degradation under UV Irradiation. Nanomaterials. 2020; 10(11):2139. https://doi.org/10.3390/nano10112139
Chicago/Turabian StyleAnucha, Chukwuka Bethel, Ilknur Altin, Zekeriya Biyiklioglu, Emin Bacaksiz, Ismail Polat, and Vassilis N. Stathopoulos. 2020. "Synthesis, Characterization, and Photocatalytic Evaluation of Manganese (III) Phthalocyanine Sensitized ZnWO4 (ZnWO4MnPc) for Bisphenol A Degradation under UV Irradiation" Nanomaterials 10, no. 11: 2139. https://doi.org/10.3390/nano10112139






