High-Yield Production of Water-Soluble MoS2 Quantum Dots for Fe3+ Detection and Cell Imaging
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Apparatus
2.2. Synthesis of High Water-Solubility Molybdenum Disulfide Quantum Dots (WS-MSQDs)
2.3. Photoluminescence Measurement
2.4. Cell Culture
3. Results and Discussion
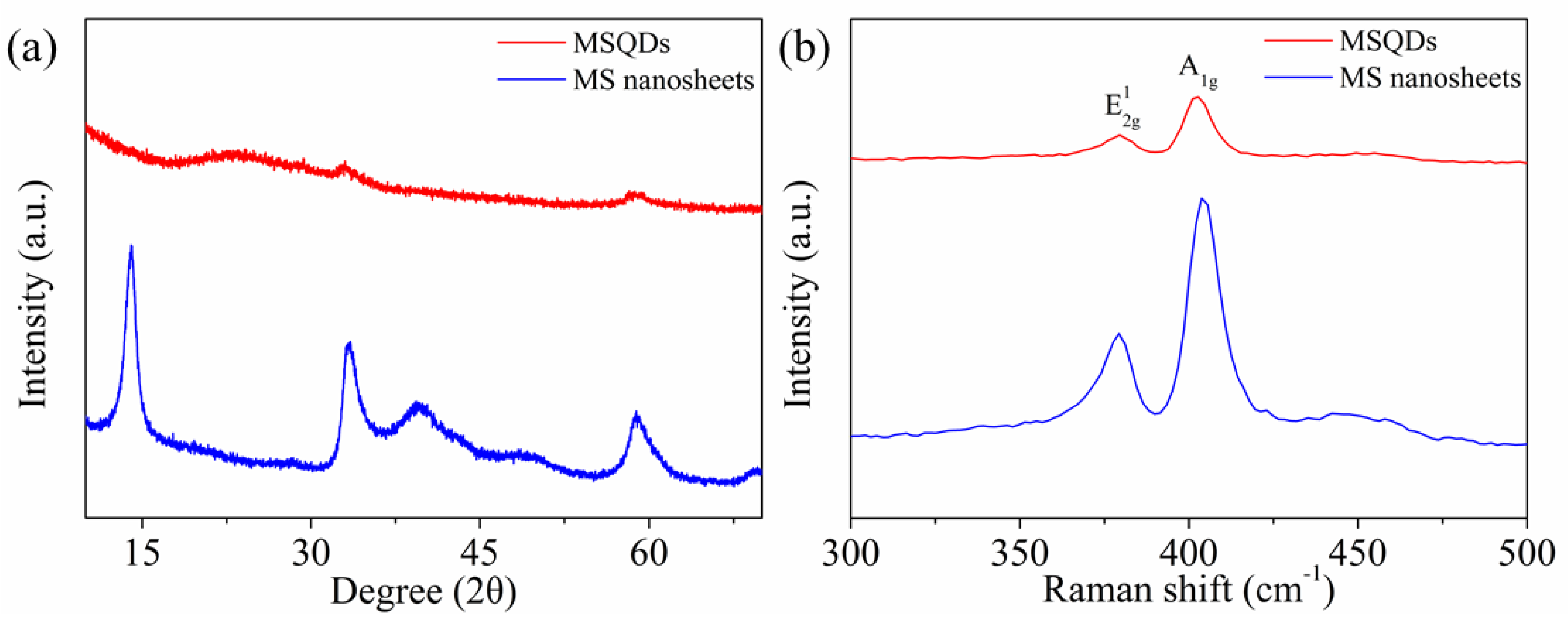
3.1. Characterizations of MSQDs
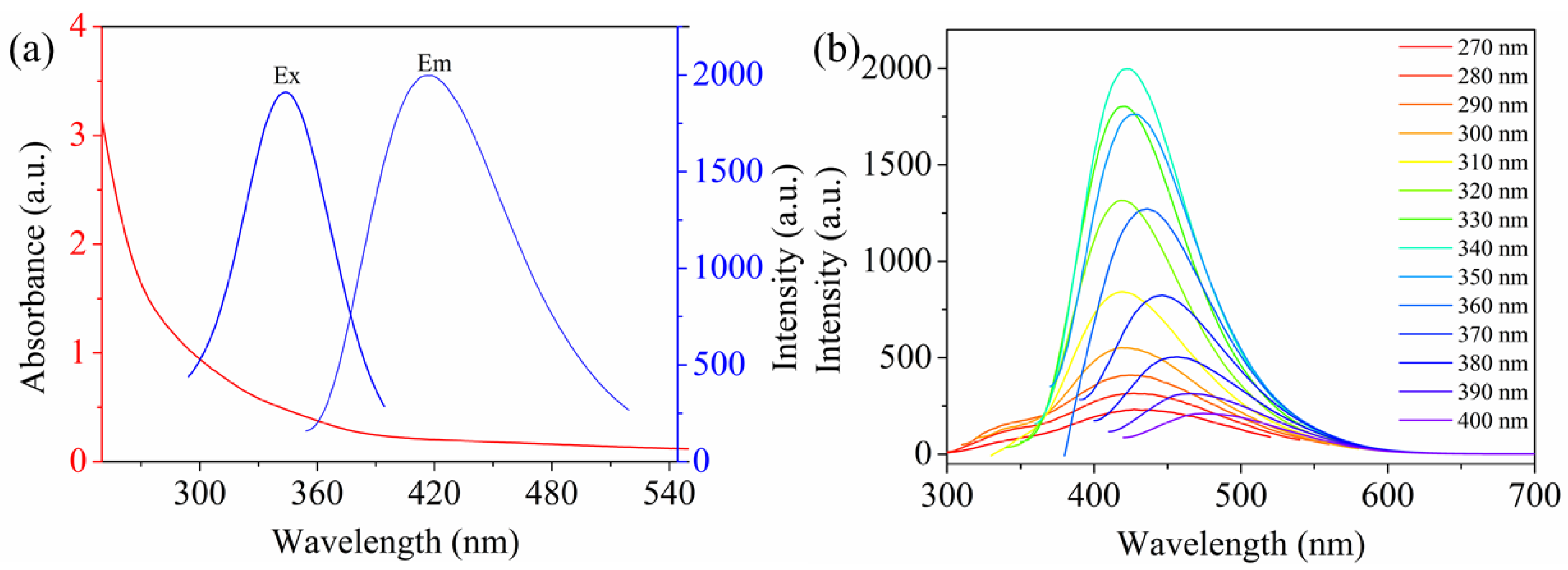
3.2. Optical Properties of MSQDs
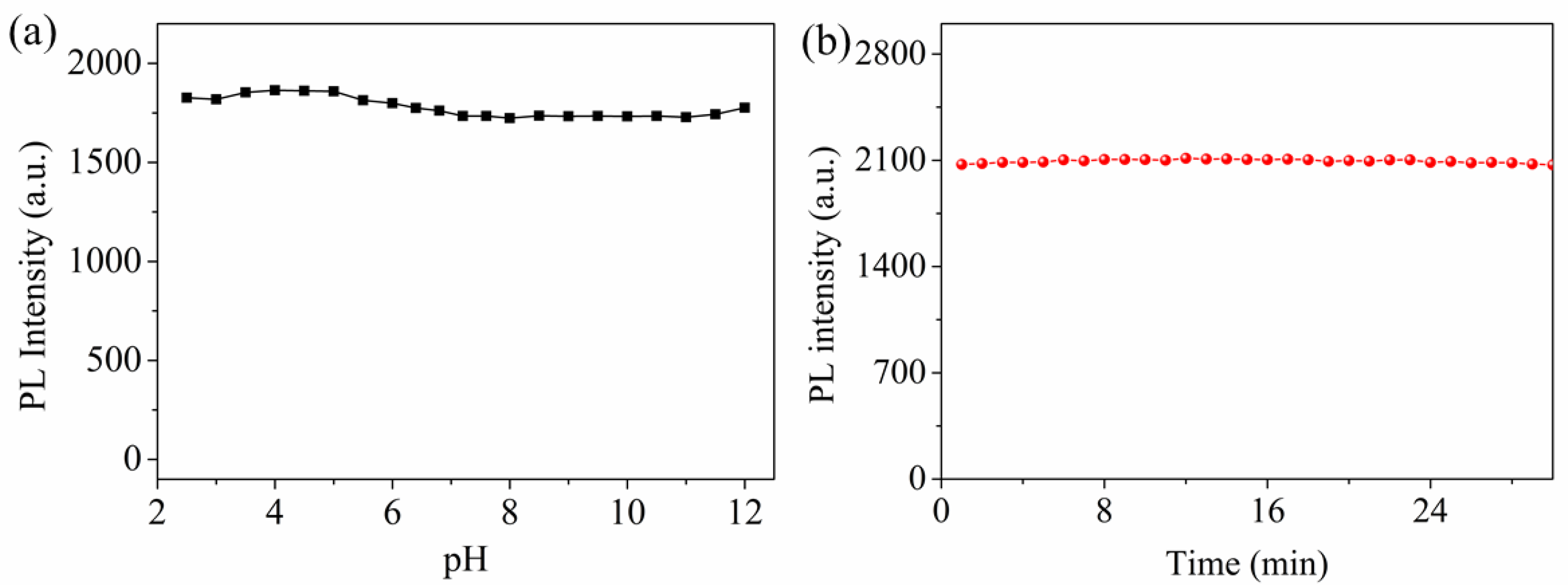
3.3. Effects of pH
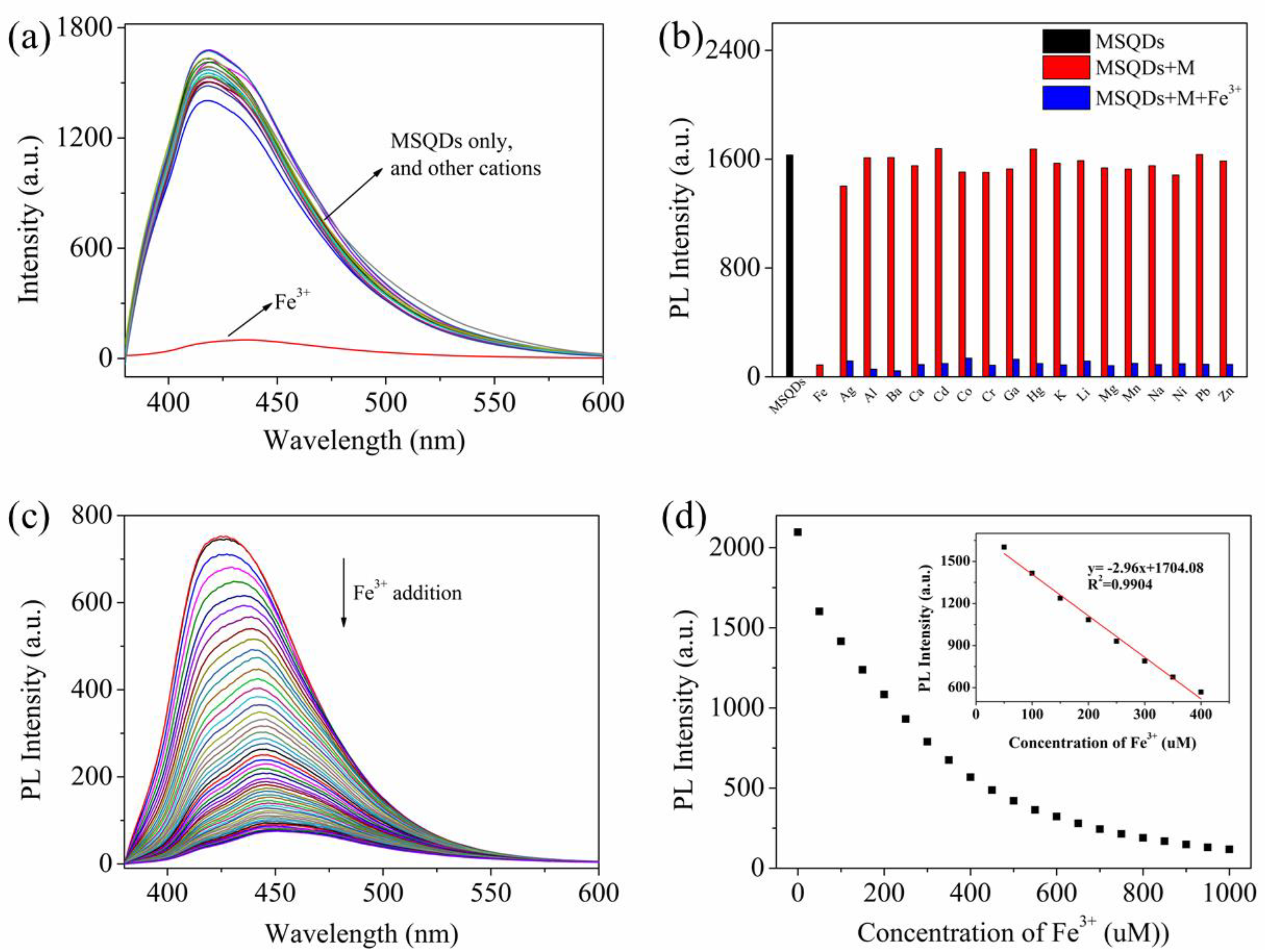
3.4. Detection of Fe3+ Ions and Selectivity Measurements
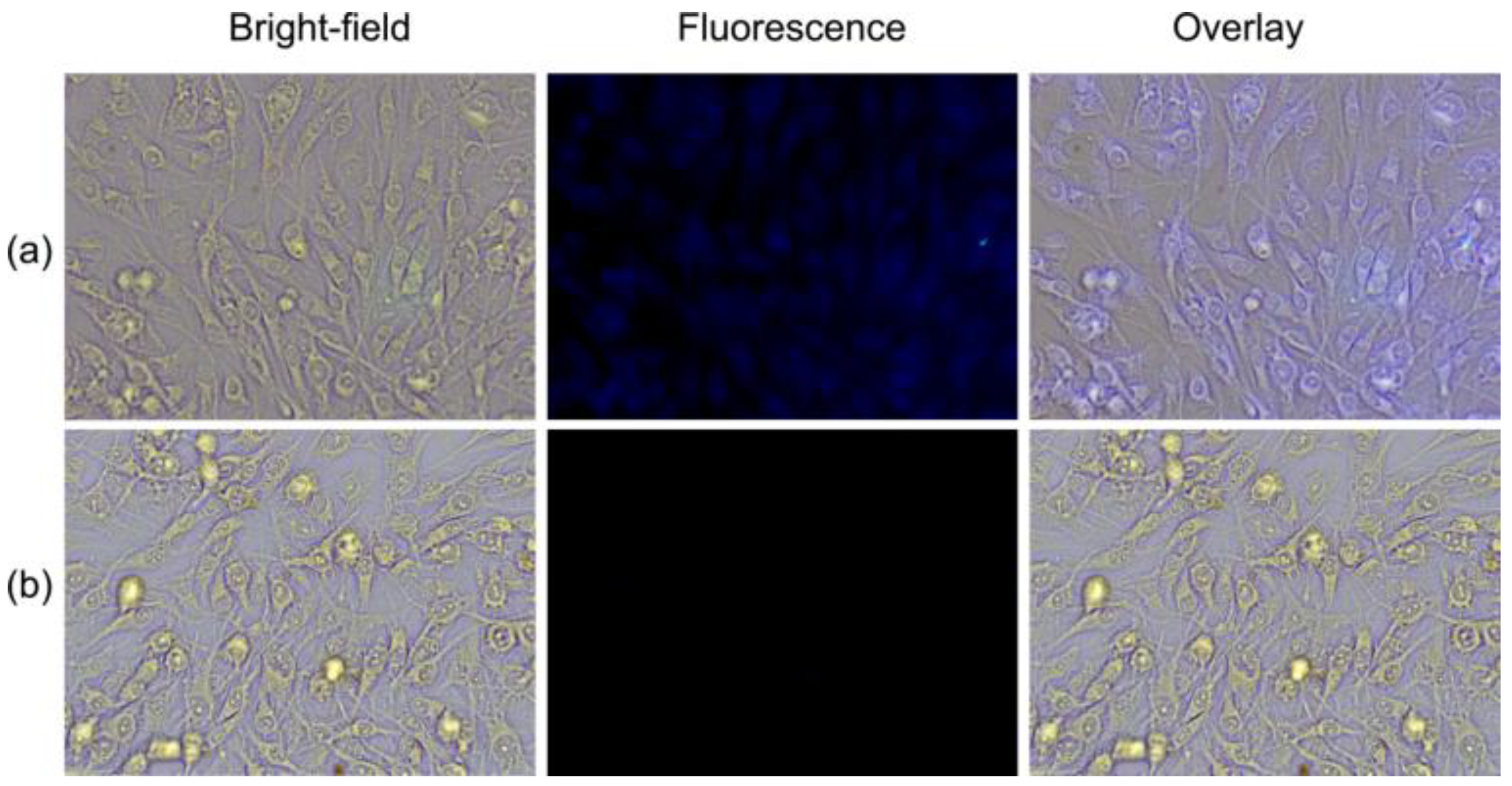
3.5. Fluorescence Imaging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, S.; Chang, J.; Pu, H.; Lu, G.; He, Q.; Zhang, H.; Chen, J. Two-dimensional nanomaterial-based field-effect transistors for chemical and biological sensing. Chem. Soc. Rev. 2017, 46, 6872–6904. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, X.; Wu, X.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Li, N.; Chen, P. Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev. 2016, 45, 2239–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabari Arul, N.; Nithya, V.D. Molybdenum disulfide quantum dots: Synthesis and applications. RSC Adv. 2016, 6, 65670–65682. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J. MoS2 quantum dots: Synthesis, properties and biological applications. Mater. Sci. Eng. C 2020, 109, 110511. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yan, Y.; Zhang, C.; Ding, C.; Xian, Y. One-step synthesis of water-soluble MoS2 quantum dots via a hydrothermal method as a fluorescent probe for hyaluronidase detection. ACS Appl. Mater. Interfaces 2016, 8, 11272–11279. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, S.; Kang, T.W.; Ju, J.; Yim, D.; Kim, H.; Park, J.H.; Kim, J. 2H-WS2 Quantum dots produced by modulating the dimension and phase of 1T-nanosheets for antibody-free optical sensing of neurotransmitters. ACS Appl. Mater. Interfaces 2017, 9, 12316–12323. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, Y. Molybdenum disulfide quantum dots as a photoluminescence sensing platform for 2, 4, 6-trinitrophenol detection. Anal. Chem. 2014, 86, 7463–7470. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Jiang, K.; Wang, C.; Zhang, C. One-step synthesis of water-soluble and highly fluorescent MoS2 quantum dots for detection of hydrogen peroxide and glucose. Sensor. Actuat. B Chem. 2017, 252, 183–190. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Wu, P. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Zhuang, Q.; Ni, Y. Enhancing sensitivity and selectivity in a label-free colorimetric sensor for detection of iron (II) ions with luminescent molybdenum disulfide nanosheet-based peroxidase mimetics. Biosens. Bioelectron. 2016, 80, 111–117. [Google Scholar] [CrossRef]
- Wu, J.S.; Liu, W.M.; Ge, J.C.; Zhang, H.Y.; Wang, P.F. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, M.; Favereau, L.; Planchat, A.; Akdas-Kilig, H.; Szuwarski, N.; Pellegrin, Y.; Blart, E.; Bozec, H.L.; Boujtita, M.; Odobel, F. Heteroleptic copper(I)-polypyridine complexes as efficient sensitizers for dye sensitized solar cells. J. Mater. Chem. A 2014, 2, 9944–9947. [Google Scholar] [CrossRef]
- Yang, M.Y.; Li, J.; Chen, P.R. Transition metal-mediated bioorthogonal protein chemistry in living cells. Chem. Soc. Rev. 2014, 43, 6511–6526. [Google Scholar] [CrossRef]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.P.; Pan, D.W.; Lin, M.Y.; Han, H.T.; Li, F. Graphene oxide-assisted synthesis of bismuth nanosheets for catalytic stripping voltammetric determination of iron in coastal waters. Microchim. Acta. 2016, 183, 855–861. [Google Scholar] [CrossRef]
- Wolle, M.M.; Fahrenholz, T.; Rahman, G.M.M.; Pamuku, M.; Kingston, H.M.; Browne, D. Method development for the redox speciation analysis of iron by ion chromatography-inductively coupled plasma mass spectrometry and carryover assessment using isotopically labeled analyte analogues. J. Chromatogr. A 2014, 1347, 96–103. [Google Scholar] [CrossRef]
- Picard, M.; Thakur, S.; Misra, M.; Mohanty, A.K. Miscanthus grass-derived carbon dots to selectively detect Fe3+ ions. RSC Adv. 2019, 9, 8628–8637. [Google Scholar] [CrossRef] [Green Version]
- Li, K.B.; Zang, Y.; Wang, H.; Li, J.; Chen, G.R.; James, T.D.; He, X.P.; Tian, H. Hepatoma-selective imaging of heavy metal ions using a ‘clicked’ galactosylrhodamine probe. Chem. Commun. 2014, 50, 11735–11737. [Google Scholar] [CrossRef]
- Chen, X.Q.; Tian, X.Z.; Shin, I.; Yoon, J.Y. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2011, 40, 4783–4804. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Chen, X.Q.; Yoon, J.Y. Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chatti, M.; Adusumalli, V.N.; Mahalingam, V. Highly selective and sensitive detection of Cu2+ ions using Ce(III)/Tb(III)-doped SrF2 nanocrystals as fluorescent probe. ACS Appl. Mater. Interfaces 2015, 7, 25702–25708. [Google Scholar] [CrossRef]
- Ruan, L.; Zhao, Y.; Chen, Z.; Zeng, W.; Wang, S.; Liang, D.; Zhao, J. Ethylenediamine-assisted hydrothermal method to fabricate MoS2 quantum dots in aqueous solution as a fluorescent probe for Fe3+ ion detection. Appl. Surf. Sci. 2020, 528, 146811. [Google Scholar] [CrossRef]
- Ma, J.; Yu, H.; Jiang, X.; Luo, Z.; Zheng, Y. High sensitivity label-free detection of Fe3+ ion in aqueous solution using fluorescent MoS2 quantum dots. Sens. Actuat. B Chem. 2019, 281, 989–997. [Google Scholar] [CrossRef]
- Han, C.; Zhang, Y.; Gao, P.; Chen, S.; Liu, X.; Mi, Y.; Zhang, J.; Ma, Y.; Jiang, W.; Chang, J. High-yield production of MoS2 and WS2 quantum sheets from their bulk materials. Nano Lett. 2017, 17, 7767–7772. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Li, B.; Gullappalli, H.; Pillai, V.K.; Ajayan, P.M.; Shaijumon, M.M. Electrochemical synthesis of luminescent MoS2 quantum dots. Chem. Commun. 2015, 51, 6293–6296. [Google Scholar] [CrossRef]
- Ou, G.; Fan, P.; Ke, X.; Xu, Y.; Huang, K.; Wei, H.; Yu, W.; Zhang, H.; Zhong, M.; Wu, H.; et al. Defective molybdenum sulfide quantum dots as highly active hydrogen evolution electrocatalysts. Nano Res. 2018, 11, 751–761. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Liu, Z.; Tan, C.; Huang, Y.; Li, B.; Zhao, M.; Xie, L.; Huang, W.; Zhang, H. A facile and universal top-down method for preparation of monodisperse transition-metal dichalcogenide nanodots. Angew. Chem. 2015, 54, 5425–5428. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 quantum dot-interspersed exfoliated MoS2 nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Y.; Xia, Z.N.; Wei, W.L. As-prepared MoS2 quantum dot as a facile fluorescent probe for long-term tracing of live cells. Nanotechnology 2016, 27, 275101. [Google Scholar] [CrossRef]
- Huang, H.; Du, C.C.; Shi, H.Y.; Feng, X.; Li, J.; Tan, Y.L.; Song, W.B. Water-Soluble monolayer molybdenum disulfide quantum dots with upconversion fluorescence. Part. Part. Syst. Charact. 2015, 32, 72–79. [Google Scholar] [CrossRef]
- Gu, W.; Yan, Y.; Cao, X.; Zhang, C.; Ding, C.; Xian, Y. A facile and one-step ethanol-thermal synthesis of MoS2 quantum dots for two-photon fluorescence imaging. J. Mater. Chem. B 2016, 4, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chao, Y.; Gao, M.; Liang, C.; Chen, Q.; Song, G.S.; Cheng, L.; Liu, Z. Ultra-small MoS2 nanodots with rapid body clearance for photothermal cancer therapy. Nano Res. 2016, 9, 3003–3017. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, B.; Chen, G.; Gao, C.; Gao, M. Large-scale synthesis of nitrogen doped MoS2 quantum dots for efficient hydrogen evolution reaction. Electrochim. Acta 2018, 270, 256–263. [Google Scholar] [CrossRef]
- Cai, L.; He, J.; Liu, Q.; Yao, T.; Chen, L.; Yan, W.; Hu, F.; Jiang, Y.; Zhao, Y.; Hu, T. Vacancy-induced ferromagnetism of MoS2 nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, X.; Shi, W.; Wu, J.; Jiang, D.; Tan, P. Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material. Chem. Soc. Rev. 2015, 44, 2757–2785. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Tang, S.; Hao, Y.; Yu, H.; Dai, W.; Zhao, G.; Cao, Y.; Lu, H.; Zhang, X.; Ju, H. Fluorescent MoS2 quantum dots: Ultrasonic preparation, up-conversion and down-conversion bioimaging, and photodynamic therapy. ACS Appl. Mater. Interfaces 2016, 8, 3107–3114. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.-T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Ou, J.; Chrimes, A.F.; Wang, Y.; Tang, S.; Strano, M.S.; Kalantar-Zadeh, K. Ion-driven Photoluminescence Modulation of Quasi-two-dimensional MoS2 Nanoflakes for Applications in Biological Systems. Nano Lett. 2014, 14, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ou, J.; Balendhran, S.; Chrimes, A.; Mortazavi, M.; Yao, D.; Field, M.; Latham, K.; Bansal, V.; Friend, J.; et al. Electrochemical Control of Photoluminescence in Two-dimensional MoS2 Nanoflakes. ACS Nano 2013, 7, 10083–10093. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Mishra, A.K. Green and cost-effective fluorescent carbon nanoparticles for the selective and sensitive detection of iron (III) ions in aqueous solution: Mechanistic insights and cell line imaging studies. Sens. Actuat. B Chem. 2016, 227, 467–474. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Zhang, Z.; Zhang, P.; Wang, L.; Yuan, R.; Ju, Z.; Liu, W. High-Yield Production of Water-Soluble MoS2 Quantum Dots for Fe3+ Detection and Cell Imaging. Nanomaterials 2020, 10, 2155. https://doi.org/10.3390/nano10112155
Xu B, Zhang Z, Zhang P, Wang L, Yuan R, Ju Z, Liu W. High-Yield Production of Water-Soluble MoS2 Quantum Dots for Fe3+ Detection and Cell Imaging. Nanomaterials. 2020; 10(11):2155. https://doi.org/10.3390/nano10112155
Chicago/Turabian StyleXu, Benhua, Zhiqi Zhang, Peng Zhang, Li Wang, Rui Yuan, Zhenghua Ju, and Weisheng Liu. 2020. "High-Yield Production of Water-Soluble MoS2 Quantum Dots for Fe3+ Detection and Cell Imaging" Nanomaterials 10, no. 11: 2155. https://doi.org/10.3390/nano10112155





