Cost-Effective Synthesis of Efficient CoWO4/Ni Nanocomposite Electrode Material for Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ni Nanoparticles
2.3. Preparation of CoWO4/Ni Nanoparticles
2.4. Preparation of CoWO4 and CoWO4/Ni Nanoparticles as Working Electrodes
2.5. Material Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
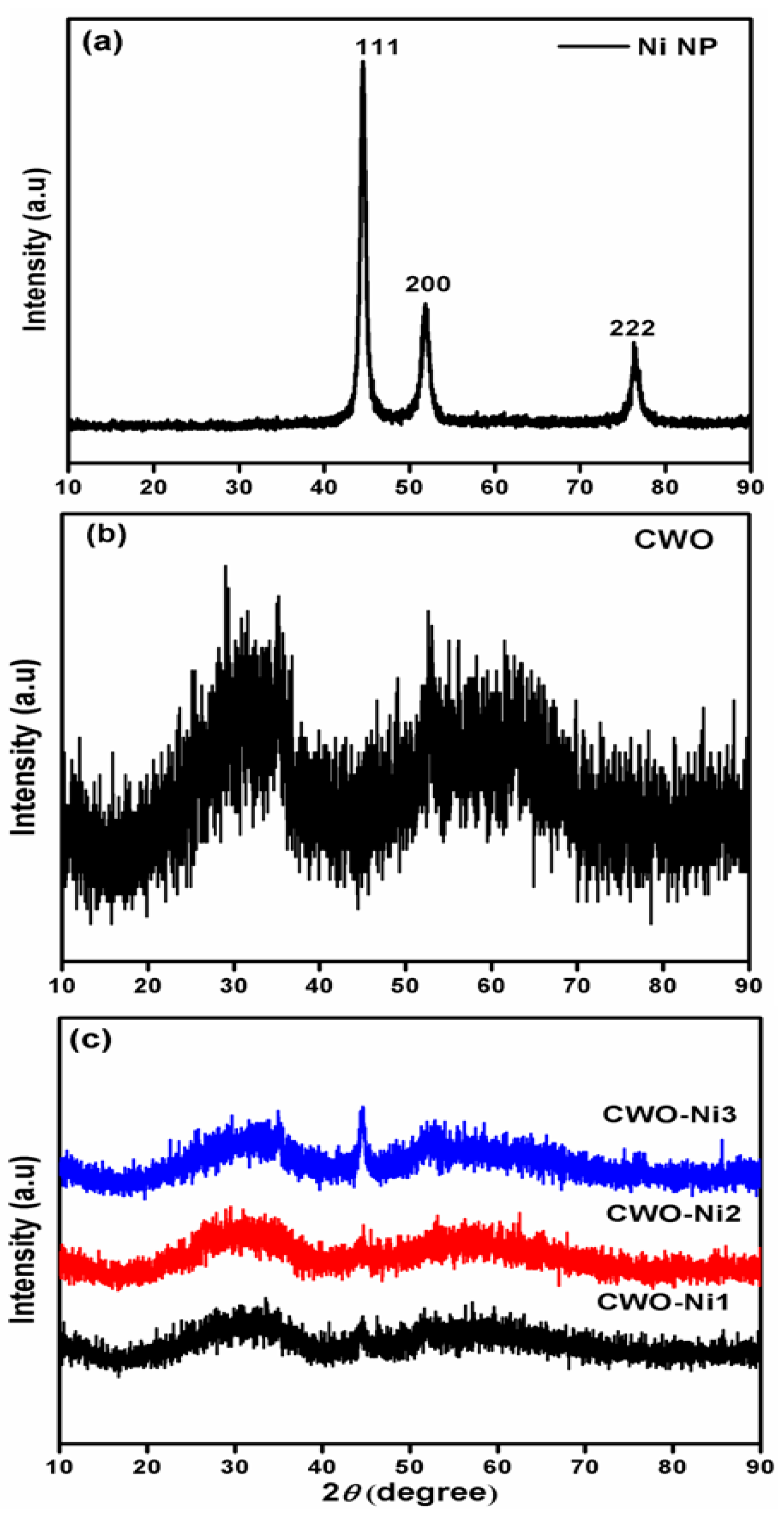
3.1. XRD Analysis
3.2. FT-IR Analysis
3.3. Surface Morphology Analysis
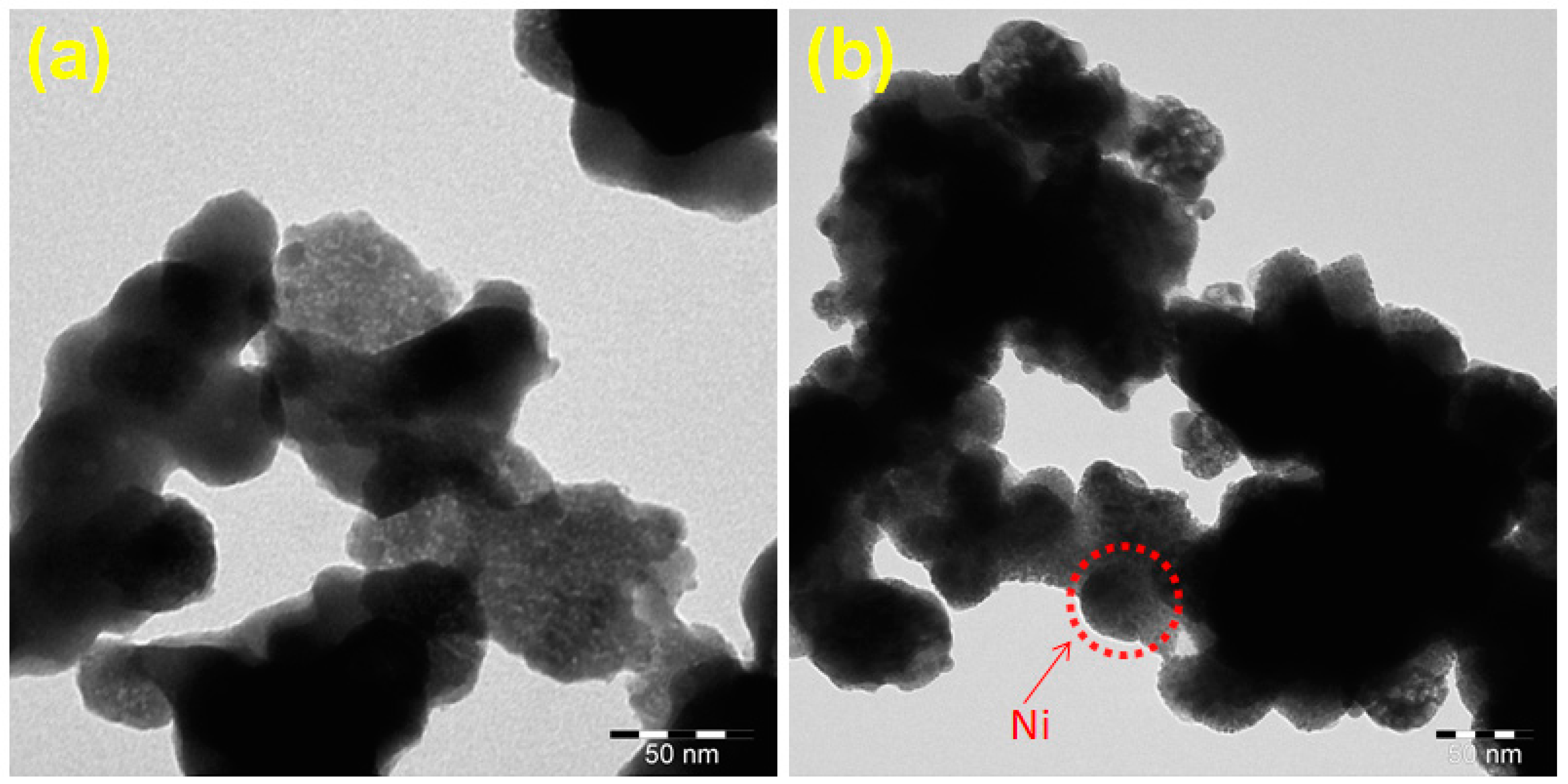
3.4. TEM Analysis
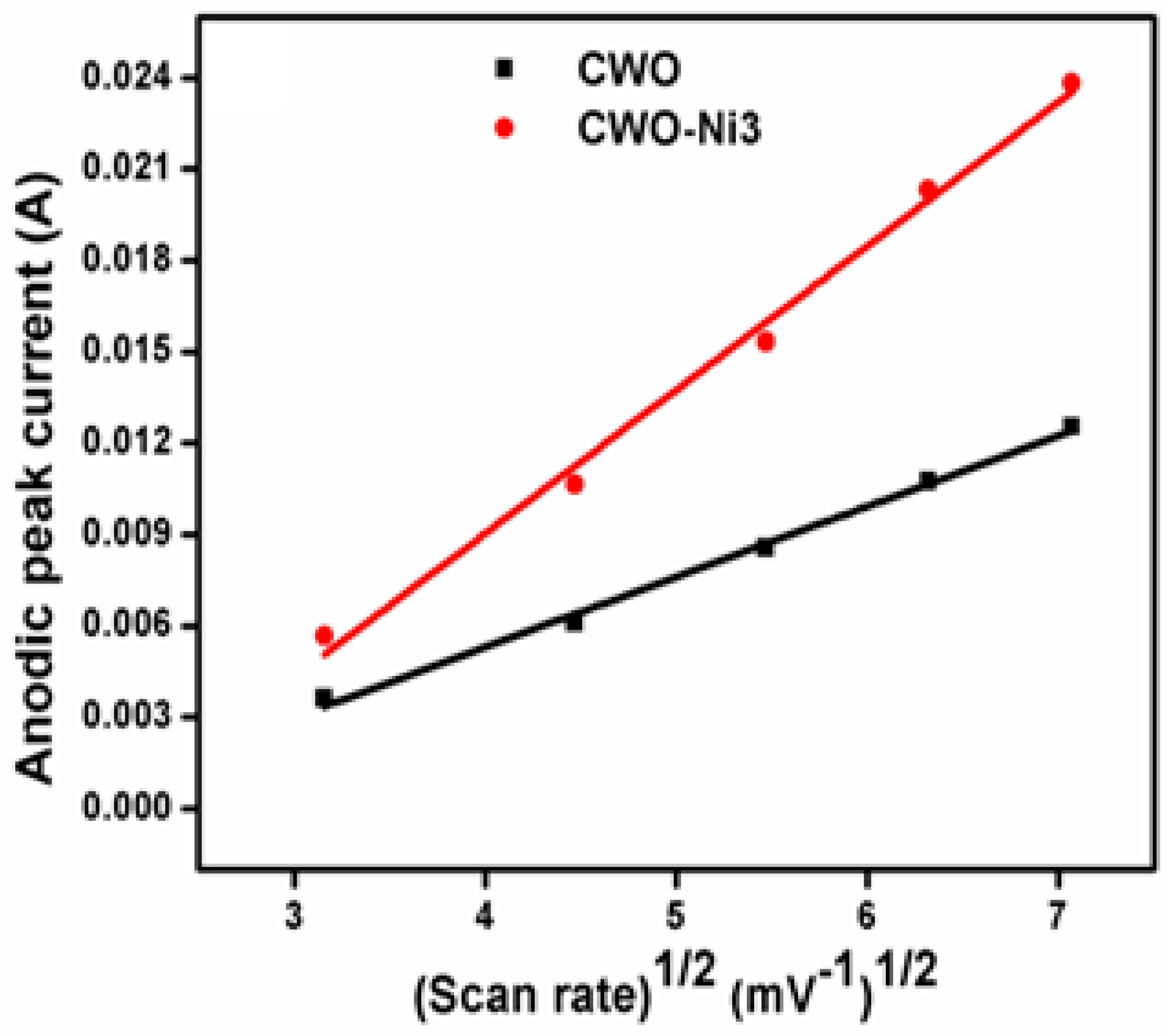
3.5. Analysis of the Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nature Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Qi, D.; Liu, Y.; Liu, Z.; Zhang, L.; Chen, X. Design of architectures and materials in in-plane micro-supercapacitors: Current status and future challenges. Adv. Mater. 2017, 29, 1602802. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, L.; Fan, W.; Zhang, Y.; Huang, Y.; Gao, W.; Liu, T. Bacterial cellulose-based sheet-like carbon aerogels for the in situ growth of nickel sulfide as high performance electrode materials for asymmetric supercapacitors. Nanoscale 2017, 9, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.S. Recent advances in metal chalcogenides (MX; X=S, Se) nanostructures for electrochemical supercapacitor applications: A brief review. Nanomaterials 2018, 8, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theerthagiri, J.; Durai, G.; Karuppasamy, K.; Arunachalam, P.; Elakkiya, V.; Kuppusami, P.; Maiyalagan, T.; Kim, H.S. Recent advances in 2-D nanostructured metal nitrides, carbides, and phosphides electrodes for electrochemical supercapacitors—A brief review. J. Ind. Eng. Chem. 2018, 67, 12–27. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Theerthagiri, J.; Senthil, R.A.; Arunachalam, P.; Madhavan, J.; Ghanem, M.A. Synthesis of Ni3V2O8@ graphene oxide nanocomposite as an efficient electrode material for supercapacitor applications. J. Solid State Electrochem. 2018, 22, 527–536. [Google Scholar] [CrossRef]
- Das, A.K.; Sahoo, S.; Arunachalam, P.; Zhang, S.; Shim, J.J. Facile synthesis of Fe3O4 nanorod decorated reduced graphene oxide (RGO) for supercapacitor application. RSC Adv. 2016, 6, 107057–107064. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Thiagarajan, K.; Senthilkumar, B.; Khan, Z.; Senthil, R.A.; Arunachalam, P.; Madhavan, J.; Ashokkumar, M. Synthesis of hierarchical cobalt phosphate nanoflakes and their enhanced electrochemical performances for supercapacitor applications. Chem. Sel. 2017, 2, 201–210. [Google Scholar] [CrossRef]
- Prasad, S.; Durai, G.; Devaraj, D.; AlSalhi, M.S.; Theerthagiri, J.; Arunachalam, P.; Gurulakshmi, M.; Raghavender, M.; Kuppusami, P. 3D nanorhombus nickel nitride as stable and cost-effective counter electrodes for dye-sensitized solar cells and supercapacitor applications. RSC Adv. 2018, 8, 8828–8835. [Google Scholar] [CrossRef] [Green Version]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.; Yang, Y. nanotube arrays and their composites for electrochemical capacitors and lithium-ion batteries. Energy Environ. Sci. 2009, 2, 932–943. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Mastragostino, M.; Arbizzani, C.; Soavi, F. Polymer-based supercapacitors. J. Power Sources 2001, 98, 812–815. [Google Scholar] [CrossRef]
- Bi, R.R.; Wu, X.L.; Cao, F.F.; Jiang, L.Y.; Guo, Y.G.; Wan, L.J. Highly dispersed RuO2 nanoparticles on carbon nanotubes: Facile synthesis and enhanced supercapacitance performance. J. Phys. Chem. C 2010, 114, 2448–2451. [Google Scholar] [CrossRef]
- Meher, S.K.; Justin, P.; Rao, G.R. Microwave-mediated synthesis for improved morphology and pseudocapacitance performance of nickel oxide. ACS Appl. Mater. Interfaces 2011, 3, 2063–2073. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Mai, Y.J.; Wang, X.I.; Gu, C.D.; Zhao, X.B. Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance. J. Mater. Chem. 2011, 21, 9319–9325. [Google Scholar] [CrossRef]
- Wee, G.; Soh, H.Z.; Cheah, Y.L.; Mhaisalkar, S.G.; Srinivasan, M. Synthesis and electrochemical properties of electrospun V2O5 nanofibers as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 6720–6725. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Meng, D.; Qin, X.; Diao, G. Hydrothermal synthesis of hematite nanoparticles and their electrochemical properties. J. Phys. Chem. C 2012, 116, 16276–16285. [Google Scholar] [CrossRef]
- Kokubu, T.; Oaki, Y.; Hosono, E.; Zhou, H.S.; Imai, H. Biomimetic solid-solution precursors of metal carbonate for nanostructured metal oxides: MnO/Co and MnO-CoO nanostructures and their electrochemical properties. Adv. Funct. Mater. 2011, 21, 3673. [Google Scholar] [CrossRef]
- Kumar, D.R.; Karuppuchamy, S. Microwave mediated synthesis of nanostructured Co -WO3 and CoWO4 for supercapacitor applications. J. Alloys Compd. 2016, 674, 384–391. [Google Scholar] [CrossRef]
- Xu, X.; Shen, J.; Li, N.; Ye, M. Facile synthesis of reduced graphene oxide/CoWO4 nanocomposites with enhanced electrochemical performances for supercapacitors. Electrochim. Acta 2014, 150, 23–34. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Wang, M.; Dong, P.; Baines, R.; Shen, J. Straightforward synthesis of hierarchical Co3O4@CoWO4/rGO core-shell arrays on Ni as hybrid electrodes for asymmetric supercapacitors. Ceram. Int. 2016, 42, 10719–10725. [Google Scholar] [CrossRef]
- Yang, Y.H.; Zhu, J.; Shi, W.; Zhou, J.; Gong, D.C.; Guet, S.Z. 3D nanoporous ZnWO4 nanoparticles with excellent electrochemical performances for supercapacitors. Mater. Lett. 2016, 177, 34–38. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahmed, J.; Ahamad, T.; Alhokbany, N.; Arunachalam, P.; Al-Mayouf, A.M.; Ahmad, T. Synthesis, characterization, multifunctional electrochemical (OGR/ORR/SCs) and photodegradable activities of ZnWO4 nanobricks. J. Sol-Gel Sci. Technol. 2018, 87, 137–146. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Jia, Y.; Zheng, H. Three-dimensional NiCo2O4@NiWO4 core-shell nanowire arrays for high performance supercapacitors. J. Mater. Chem. A 2017, 5, 1028–1034. [Google Scholar] [CrossRef]
- Goubard-Bretesche, N.; Crosnier, O.; Payen, C.; Favier, F.; Brousse, T. Nanocrystalline FeWO4 as a pseudocapacitive electrode material for high volumetric energy density supercapacitors operated in an aqueous electrolyte. Electrochem. Commun. 2015, 57, 61–64. [Google Scholar] [CrossRef]
- Xu, X.; Pei, L.; Yang, Y.; Shen, J.; Ye, M. Facile synthesis of NiWO4/reduced graphene oxide nanocomposite with excellent capacitive performance for supercapacitors. J. Alloy. Compd. 2016, 654, 23–31. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Qiao, L.; Zhou, M.; Yang, Y.; Xiao, P.; Zhang, Y. Ni-Co sulfide nanowires on nickel foam with ultrahigh capacitance for asymmetric supercapacitors. J. Mater. Chem. A. 2014, 2, 6540–6548. [Google Scholar] [CrossRef]
- Yu, C.F.; Lin, L.Y. Effect of the bimetal ratio on the growth of nickel cobalt sulfide on the Ni foam for the battery-like electrode. J. Colloid Interface Sci. 2016, 482, 1–7. [Google Scholar] [CrossRef]
- Sarma, B.; Ray, R.S.; Mohanty, S.K.; Misra, M. Synergistic enhancement in the capacitance of nickel and cobalt based mixed oxide supercapacitor prepared by electrodeposition. Appl. Surf. Sci. 2014, 300, 29–36. [Google Scholar] [CrossRef]
- Lu, X.; Huang, X.; Xie, S.; Zhai, T.; Wang, C.; Zhang, P.; Yu, M.; Li, W.; Liang, C.; Tong, Y. Controllable synthesis of porous nickel-cobalt oxide nanosheets for supercapacitors. J. Mater. Chem. 2012, 22, 13357. [Google Scholar] [CrossRef]
- Lai, F.; Huang, Y.; Miao, Y.-E.; Liu, T. Controllable preparation of multi-dimensional hybrid materials of nickel-cobalt layered double hydroxide nanorods/nanosheets on electrospun carbon nanofibers for high-performance supercapacitors. Electrochim. Acta 2015, 174, 456–463. [Google Scholar] [CrossRef]
- Wu, S.; Chen, D.H. Synthesis and characterization of nickel nanoparticles by hydrazine reduction in ethylene glycol. J. Colloid. Interface. Sci. 2003, 259, 282–286. [Google Scholar] [CrossRef]
- Senthilkumar, B.; VijayaSankar, K.; KalaiSelvan, R.; Danielle, M.; Manickam, M. Nano α- NiMoO4 as a new electrode for electrochemical supercapacitors. RSC Adv. 2013, 3, 352–357. [Google Scholar] [CrossRef]
- Ghosh, D.; Giri, S.; Das, C. Synthesis, characterization and electrochemical performance of graphene decorated with 1D NiMoO4·nH2O nanorods. Nanoscale 2013, 5, 10428–10437. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.W.; Kong, L.B.; Liu, M.; Luo, Y.C.; Kang, L. Co0.56Ni0.44 oxide nanoflake materials and activated carbon for asymmetric supercapacitor. J. Electrochem. Soc. 2010, 157, A1341. [Google Scholar] [CrossRef]
- Lei, F.; Yanw, B. Molten salt synthesis, characterization, and luminescence properties ofGd2MO6:Eu3+ (M=W, Mo) phosphors. J. Am. Ceram. Soc. 2009, 92, 1262–1267. [Google Scholar] [CrossRef]
- Joy, J.; Jaya, N. Structural, magnetic and optical behavior of pristine and Yb doped CoWO4 nanostructure. J. Mater. Sci. Mater. Electron. 2013, 24, 1788–1795. [Google Scholar]
- Xu, X.Y.; Wu, T.; Xia, F.L.; Li, Y.; Zhang, C.C.; Zhang, L.; Chen, M.X.; Li, X.C.; Zhang, L.; Liu, Y.; et al. Redox reaction between graphene oxide and in powder to prepareIn2O3/reduced graphene oxide hybrids for supercapacitors. J. Power Sources. 2014, 266, 282–290. [Google Scholar] [CrossRef]
- Justin, P.; Meher, S.K.; Raob, G.R. Tuning of capacitance behavior of NiO using anionic, cationic and nonionic surfactants by hydrothermal synthesis. J. Phys. Chem. C 2010, 114, 5203–5210. [Google Scholar] [CrossRef]
- Xia, X.; Tu, J.; Zhang, Y.; Wang, X.; Gu, C.; Zhao, X.B.; Fan, H.J. High-quality metal oxidecore/shell nanowire arrays on conductive substrates for electrochemical energy storage. ACS Nano 2012, 6, 5531–5538. [Google Scholar] [CrossRef]
- Bi, Y.; Nautiyal, A.; Zhang, H.; Luo, J.; Zhang, X. One-pot microwave synthesis of NiO/MnO2 composite as a high-performance electrode material for supercapacitors. Electrochim. Acta 2018, 260, 952–958. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Theerthagiri, J.; Senthil, R.A.; Madhavan, J. Simple and low cost electrode material based on Ca2V2O7/PANI nanoplatelets for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17354–17362. [Google Scholar] [CrossRef]
- Yan, J.A.; Khoo, E.; Sumboja, A.; Lee, P.S. Facile coating of manganese oxide on tin oxide nanowires with high-performance capacitive behavior. ACS Nano 2010, 4, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Liu, M.; Li, F.; Cheng, H.M. Frequency response characteristic of single-walled carbon nanotubes as supercapacitor electrode material. Appl. Phys. Lett. 2008, 92, 143108. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiagarajan, K.; Balaji, D.; Madhavan, J.; Theerthagiri, J.; Lee, S.J.; Kwon, K.-Y.; Choi, M.Y. Cost-Effective Synthesis of Efficient CoWO4/Ni Nanocomposite Electrode Material for Supercapacitor Applications. Nanomaterials 2020, 10, 2195. https://doi.org/10.3390/nano10112195
Thiagarajan K, Balaji D, Madhavan J, Theerthagiri J, Lee SJ, Kwon K-Y, Choi MY. Cost-Effective Synthesis of Efficient CoWO4/Ni Nanocomposite Electrode Material for Supercapacitor Applications. Nanomaterials. 2020; 10(11):2195. https://doi.org/10.3390/nano10112195
Chicago/Turabian StyleThiagarajan, Kannadasan, Dhandapani Balaji, Jagannathan Madhavan, Jayaraman Theerthagiri, Seung Jun Lee, Ki-Young Kwon, and Myong Yong Choi. 2020. "Cost-Effective Synthesis of Efficient CoWO4/Ni Nanocomposite Electrode Material for Supercapacitor Applications" Nanomaterials 10, no. 11: 2195. https://doi.org/10.3390/nano10112195




