A Review on New 3-D Printed Materials’ Geometries for Catalysis and Adsorption: Paradigms from Reforming Reactions and CO2 Capture
Abstract
:1. Introduction
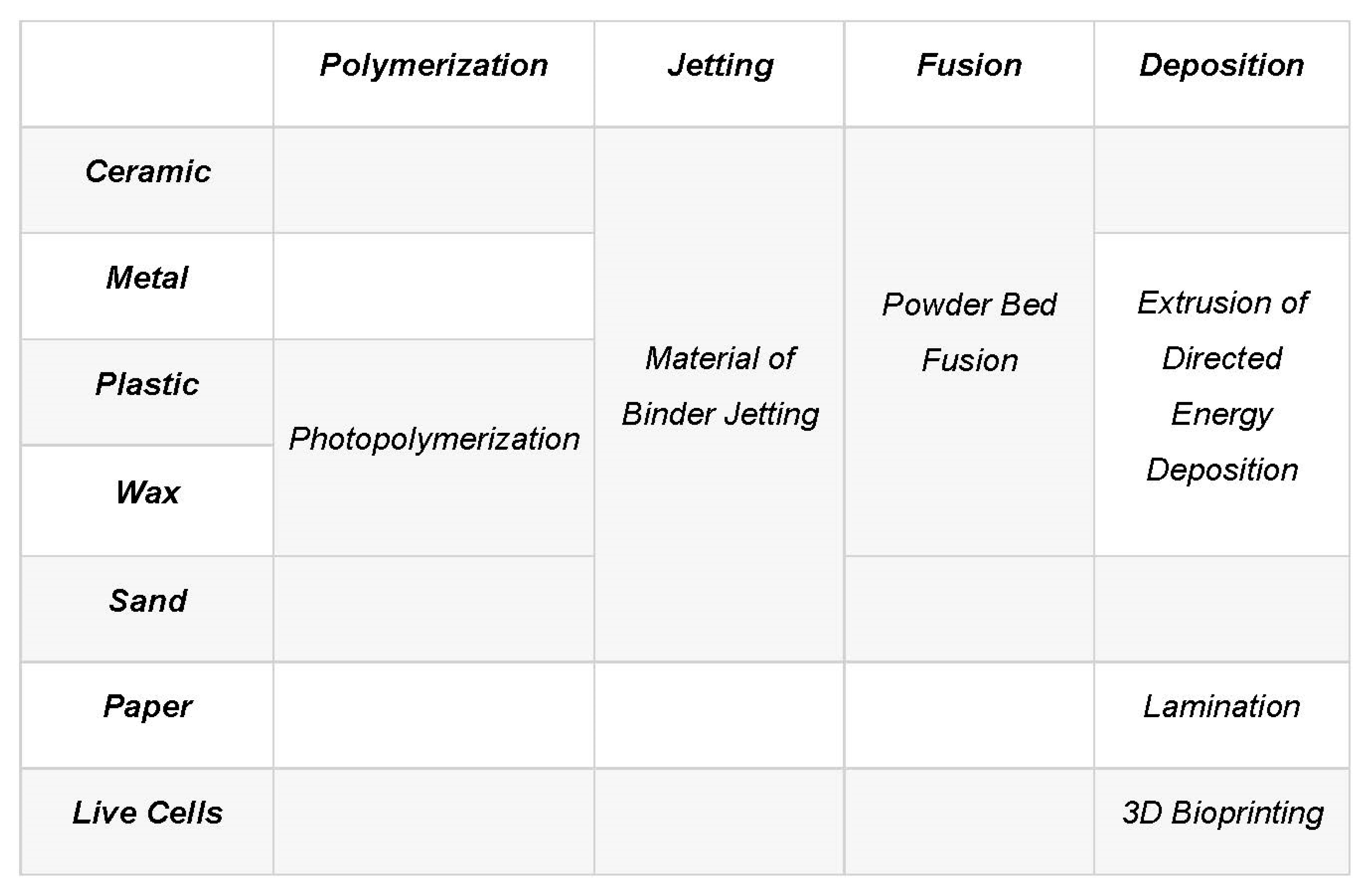
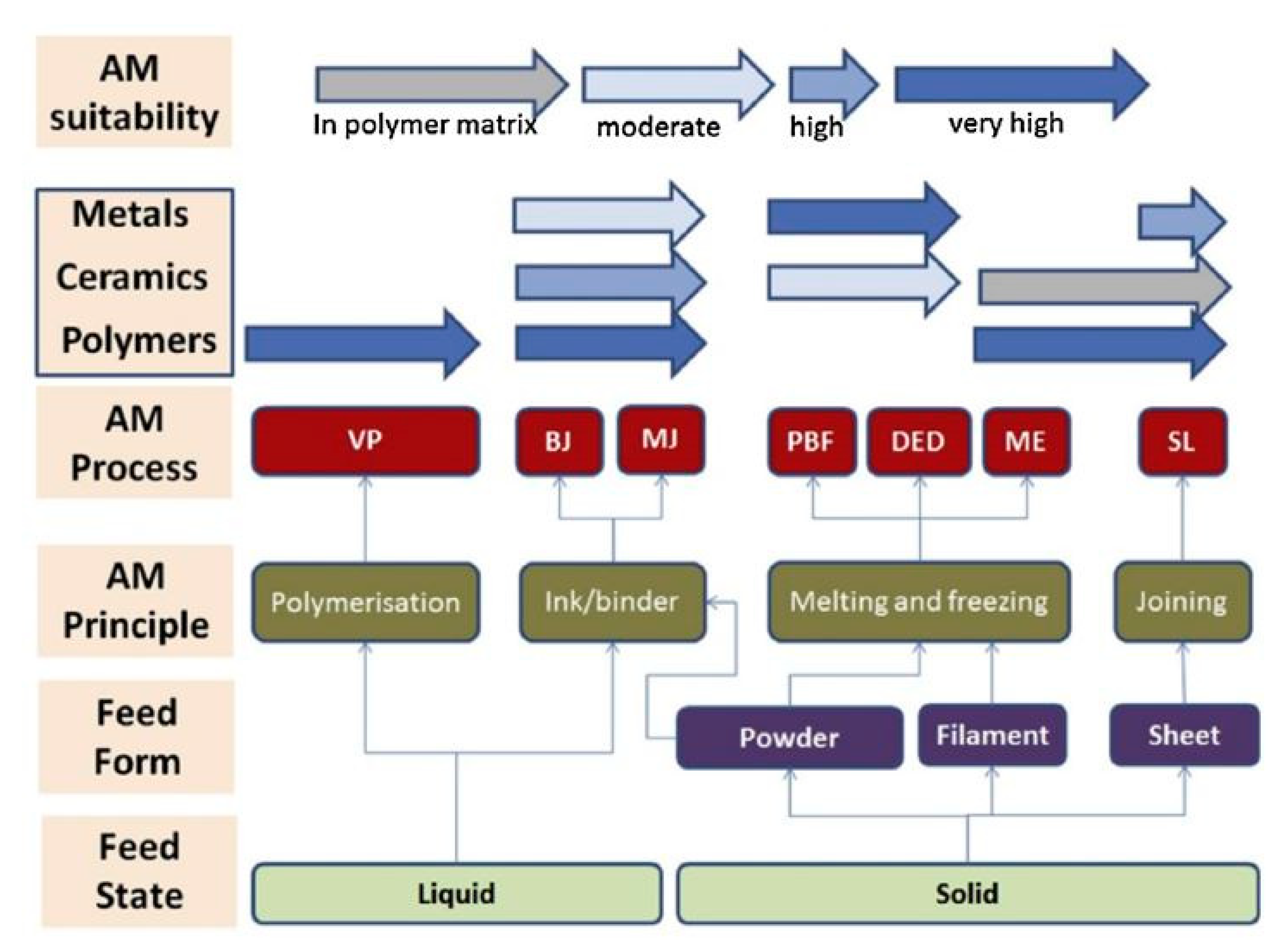
2. Additive Manufacturing Technologies
2.1. Introductory Remarks
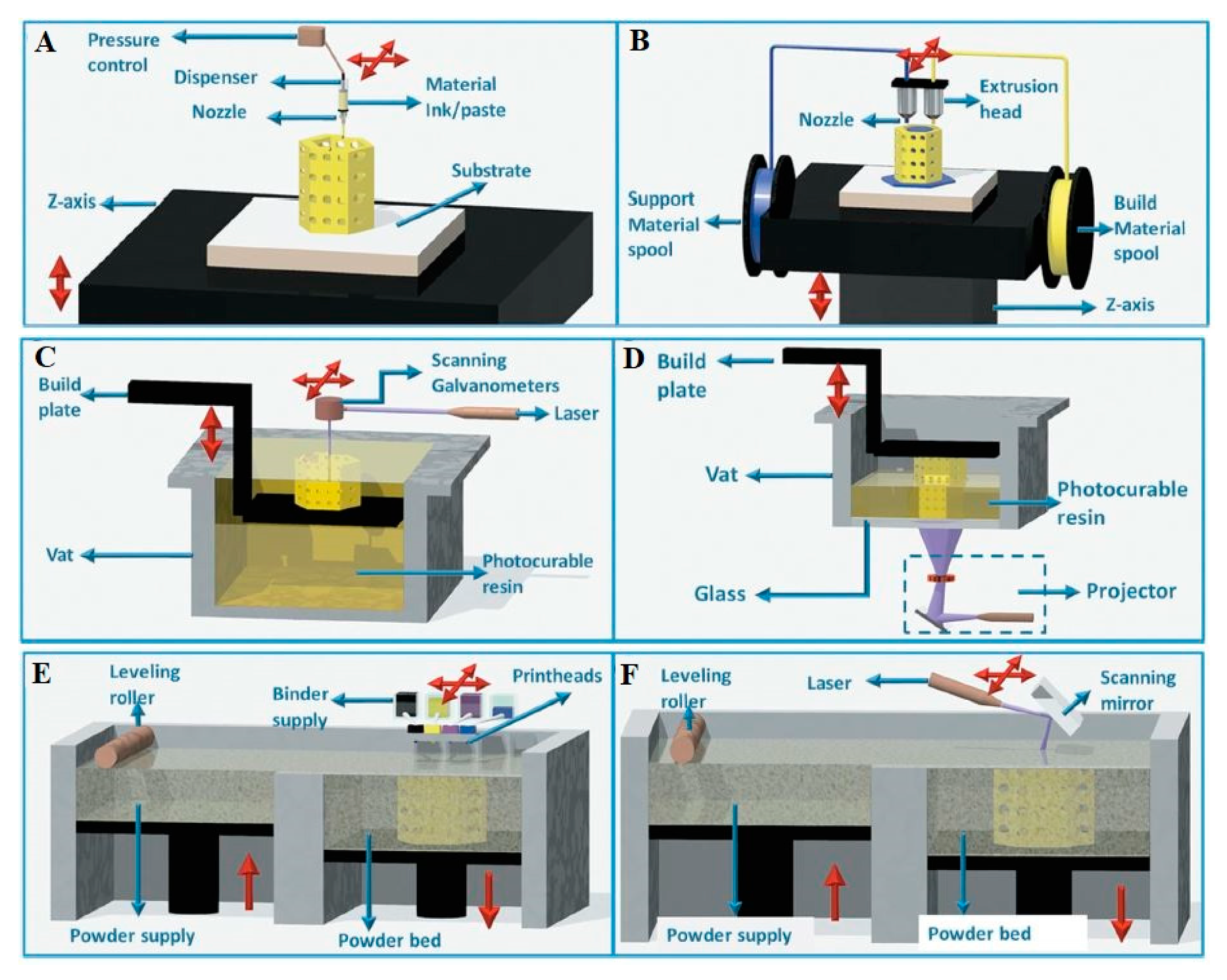
2.2. An Insight into Additive Manufacturing Techniques
2.2.1. Robocasting
2.2.2. Fused Deposition Modeling
2.2.3. Stereolithography
2.2.4. Selective Laser Sintering (SLS)/ Selective Laser Melting (SLM)
2.3. Factors Influencing the Quality of 3D Printed Structures
2.4. Bringing AM to Catalytic and Adsorbent Structures
2.4.1. Types of Catalysts/Adsorbents
2.4.2. Transferring Basic Concepts of Catalysis into 3D-Printing Technology
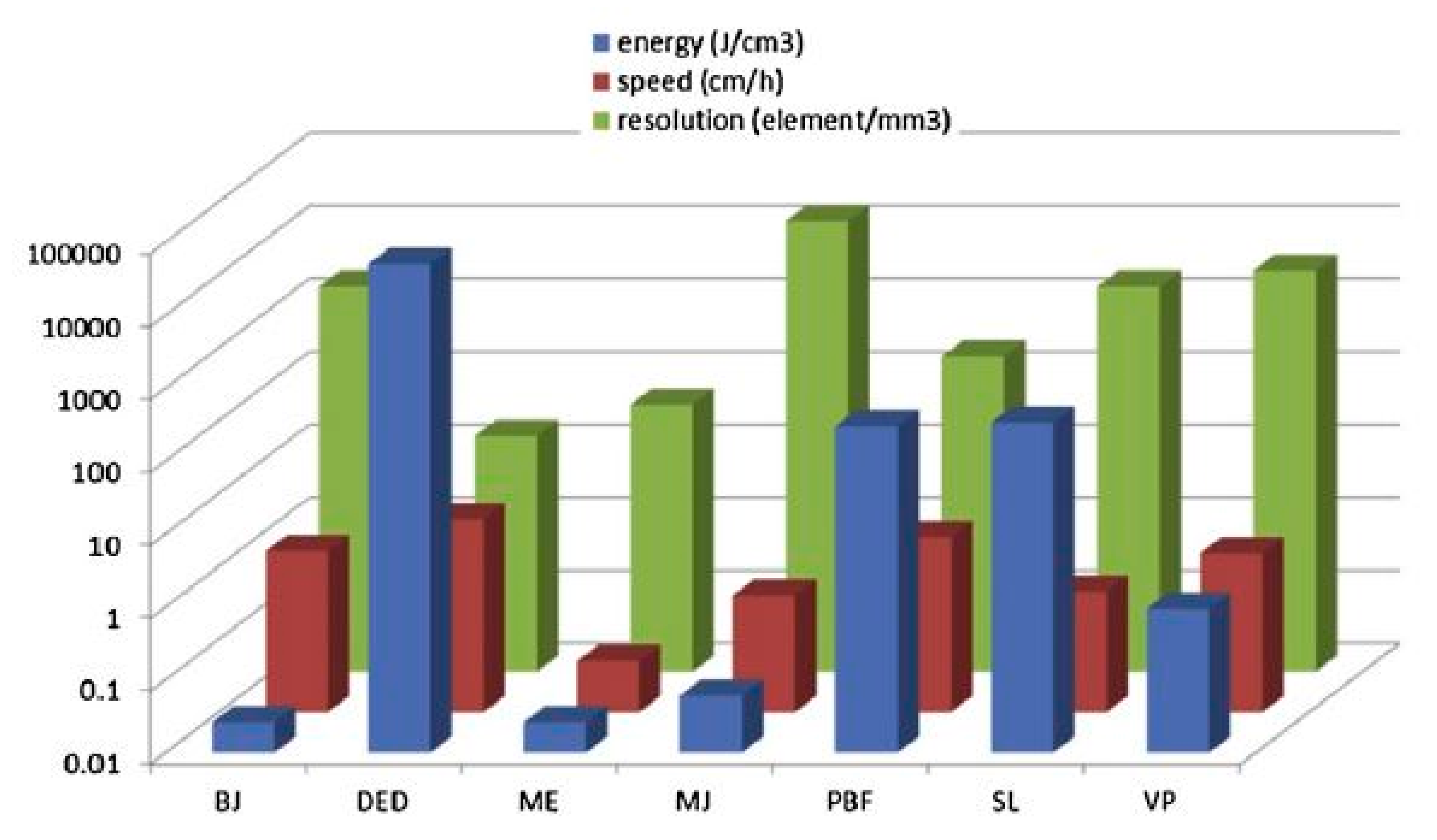
2.5. Metrology
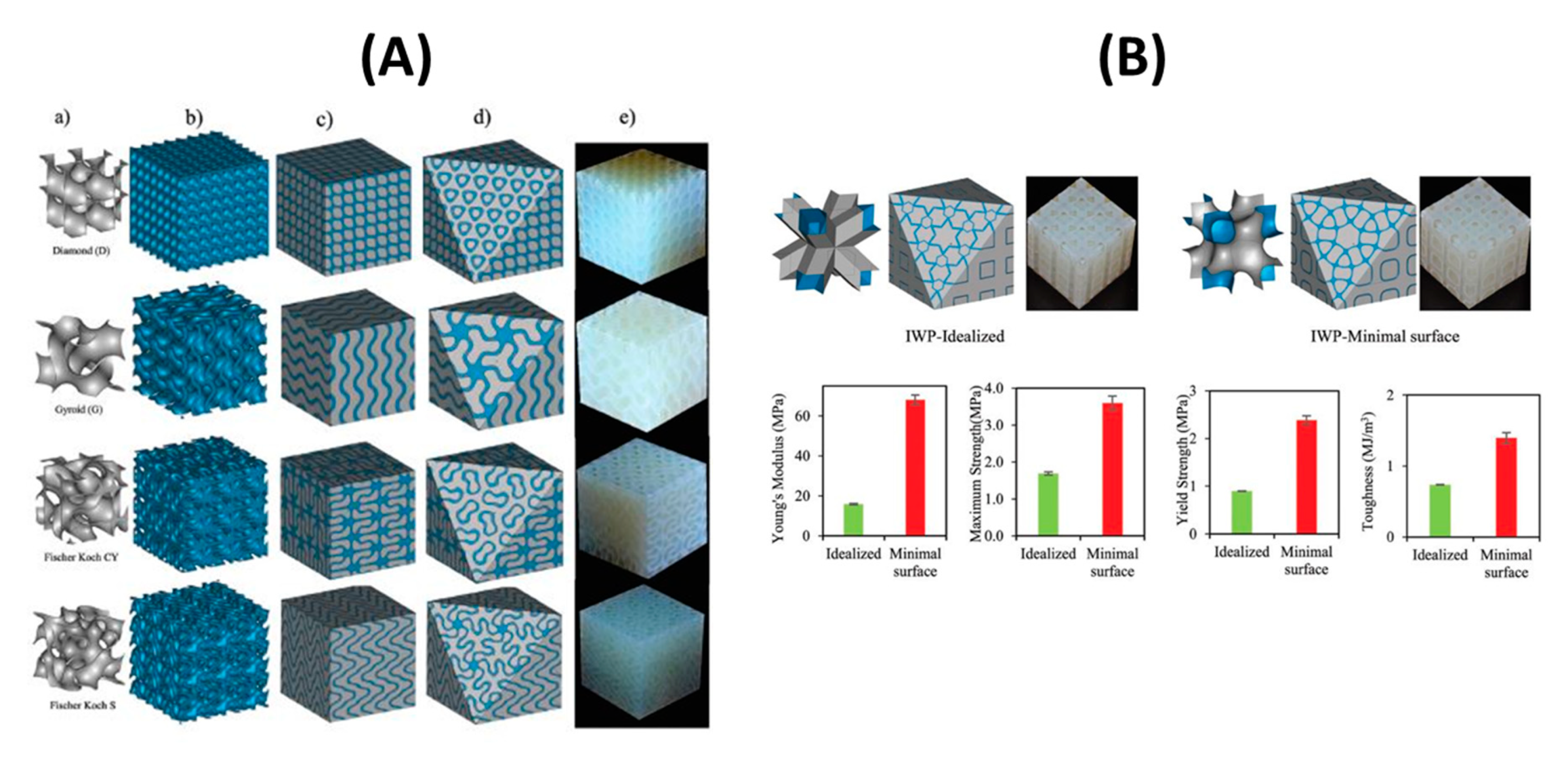
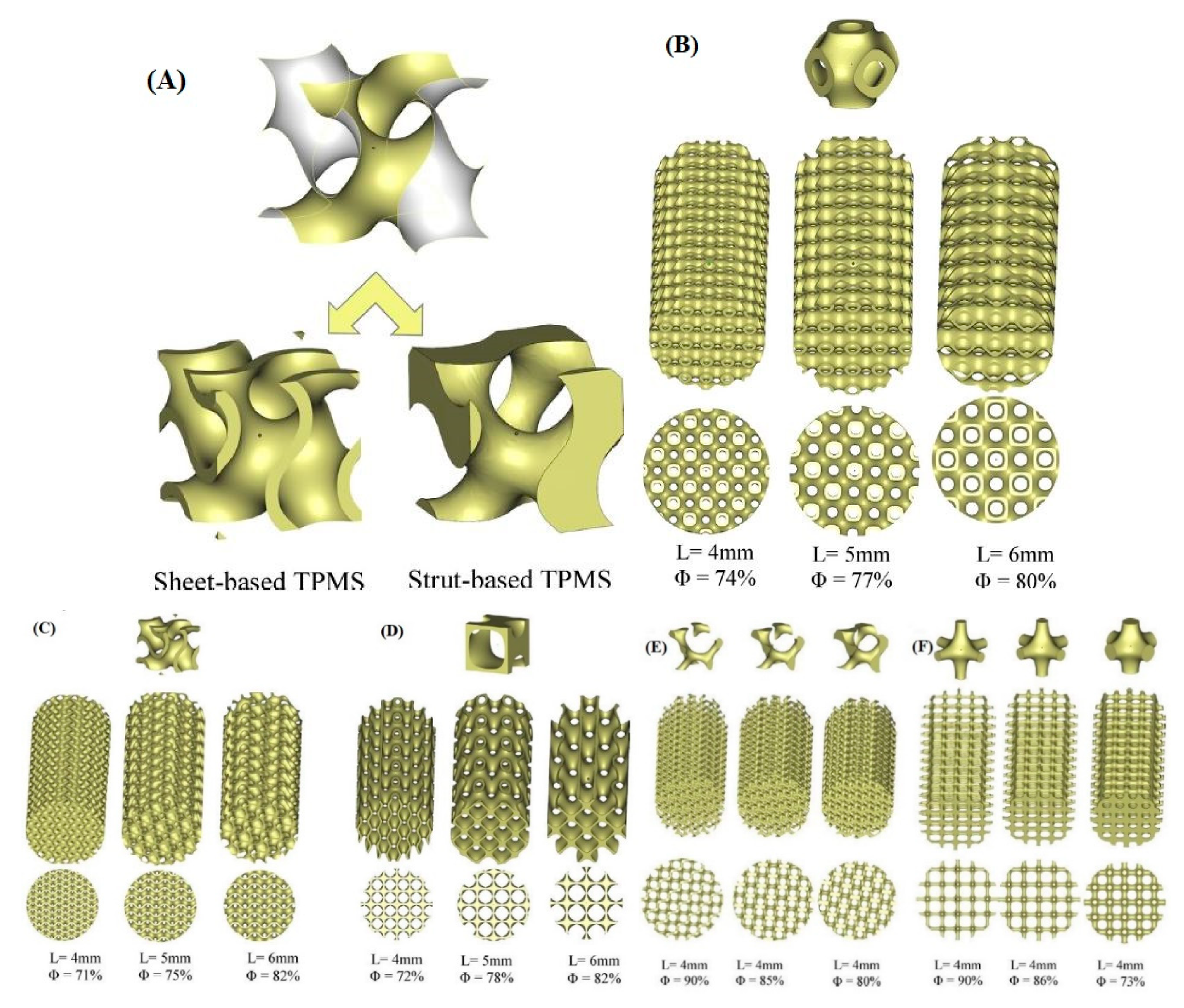
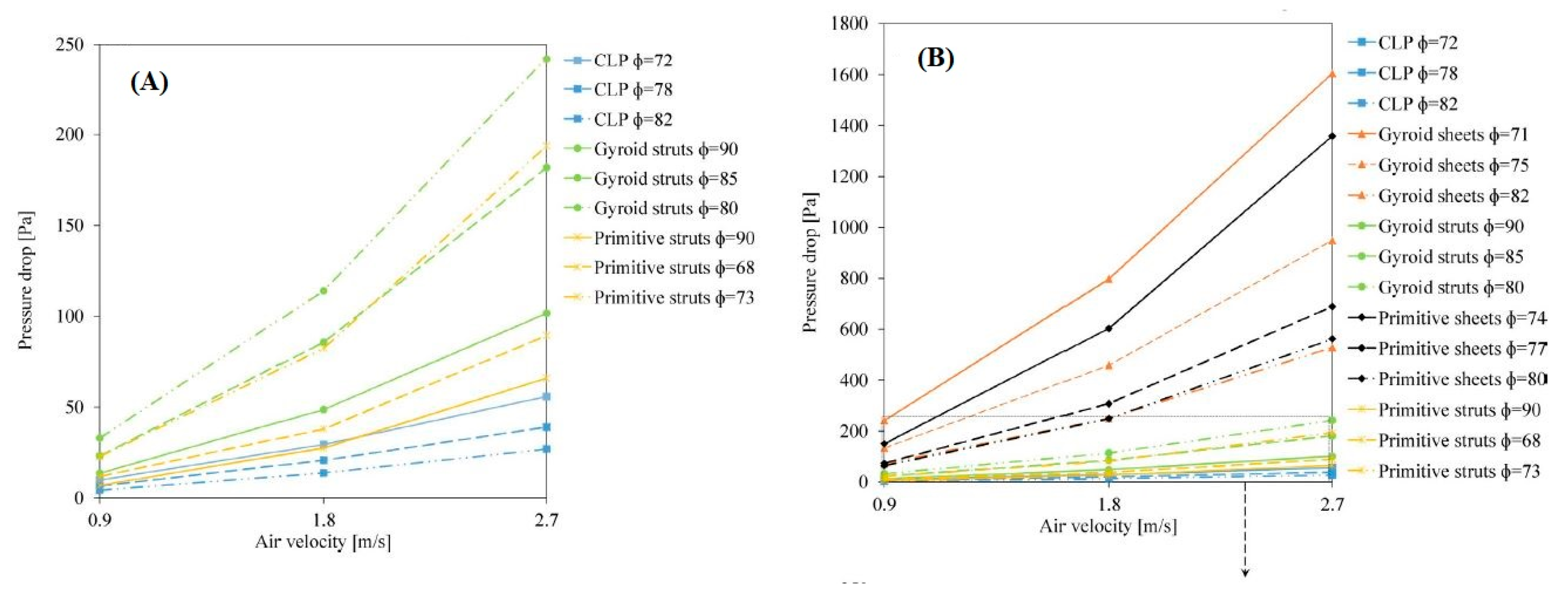
2.6. Nature-Inspired Structures: Triply Periodical Minimal Surfaces (TPMS)
3. Applications of 3D-Printing in Catalysis and Separations
3.1. 3D Printed Adsorbents for CO2 Capturing
3.2. 3D Printed Catalysts for Reforming Reactions
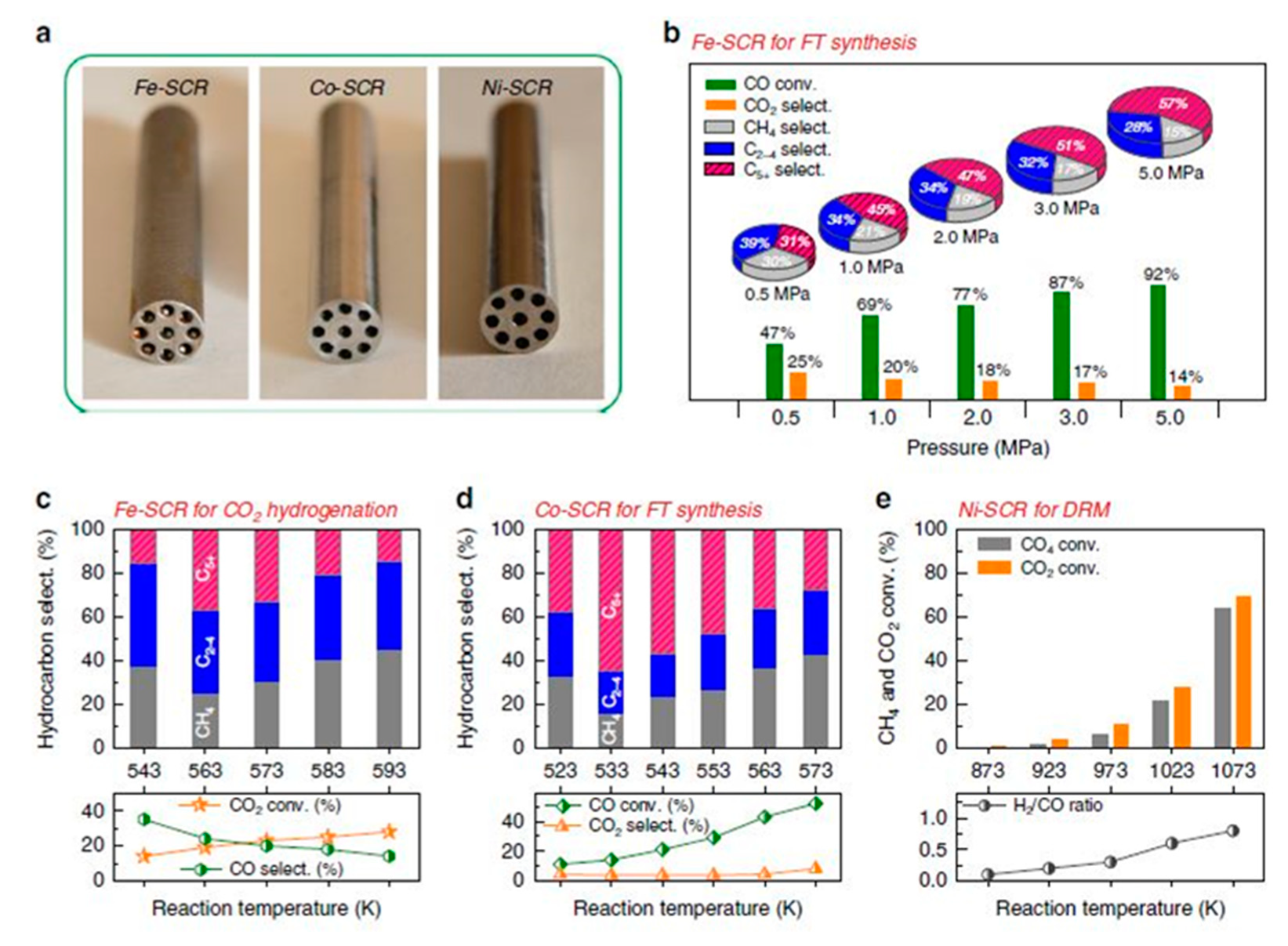
3.2.1. Metal/Ceramic 3-D Printed Catalysts
3.2.2. Case Studies of 3D-Printed Hard Templates Used as Catalyst Matrices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hannah Ritchie, M.R. CO2 and Greenhouse Gas Emissions. 2017. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 2 August 2020).
- Hertwich, E. The Carbon Footprint of Material Production Rises to 23% of Global Greenhouse Gas Emissions. SocArXiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Kontos, A.G.; Likodimos, V.; Veziri, C.M.; Kouvelos, E.; Moustakas, N.; Karanikolos, G.N.; Romanos, G.E.; Falaras, P. CO2 Captured in Zeolitic Imidazolate Frameworks: Raman Spectroscopic Analysis of Uptake and Host-Guest Interactions. ChemSusChem 2014, 7, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Department of the Environment and Heritage. Sulfur Dioxide (SO2), Air Quality Fact Sheet. 2005. Available online: https://www.environment.gov.au/protection/publications/factsheet-sulfur-dioxide-so2 (accessed on 2 August 2020).
- Talapaneni, S.N.; Buyukcakir, O.; Je, S.H.; Srinivasan, S.; Seo, Y.; Polychronopoulou, K.; Coskun, A. Nanoporous Polymers Incorporating Sterically Confined N-Heterocyclic Carbenes for Simultaneous CO2 Capture and Conversion at Ambient Pressure. Chem. Mater. 2015, 27, 6818–6826. [Google Scholar] [CrossRef]
- Kim, D.; Kim, D.W.; Buyukcakir, O.; Kim, M.K.; Polychronopoulou, K.; Coskun, A. Highly Hydrophobic ZIF-8/Carbon Nitride Foam with Hierarchical Porosity for Oil Capture and Chemical Fixation of CO2. Adv. Funct. Mater. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 2 August 2020).
- Charisiou, N.; Siakavelas, G.; Papageridis, K.; Baklavaridis, A.; Tzounis, L.; Polychronopoulou, K.; Goula, M. Hydrogen production via the glycerol steam reforming reaction over nickel supported on alumina and lanthana-alumina catalysts. Int. J. Hydrog. Energy 2017, 42, 13039–13060. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Efstathiou, A.M. NOx Control via H2-Selective Catalytic Reduction (H2-SCR) Technology for Stationary and Mobile Applications. Recent Pat. Mater. Sci. 2012, 5, 87–104. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Kalamaras, C.M.; Efstathiou, A.M. Ceria-Based Materials for Hydrogen Production Via Hydrocarbon Steam Reforming and Water-Gas Shift Reactions. Recent Pat. Mater. Sci. 2011, 4, 122–145. [Google Scholar] [CrossRef]
- Varghese, A.M.; Karanikolos, G.N. CO2 capture adsorbents functionalized by amine–bearing polymers: A review. Int. J. Greenh. Gas Control 2020, 96, 103005. [Google Scholar] [CrossRef]
- Gunawan, T.; Wijiyanti, R.; Widiastuti, N. Adsorption–desorption of CO 2 on zeolite-Y-templated carbon at various temperatures. RSC Adv. 2018, 8, 41594–41602. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO 2 Capture in Metal-Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Kierzkowska, A.M.; Broda, M.; Müller, C.R. Sol-gel Synthesis of MgAl2O4-stabilized CaO for CO2 Capture. Energy Procedia 2017, 114, 220–229. [Google Scholar] [CrossRef]
- Broda, M.; Müller, C.R. Sol–gel-derived, CaO-based, ZrO2-stabilized CO2 sorbents. Fuel 2014, 127, 94–100. [Google Scholar] [CrossRef]
- Varghese, A.M.; Reddy, K.S.K.; Singh, S.; Karanikolos, G.N. Performance enhancement of CO2 capture adsorbents by UV treatment: The case of self-supported graphene oxide foam. Chem. Eng. J. 2020, 386, 124022. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen Production From Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Ampelli, C.; Genovese, C.; Lanzafame, P.; Perathoner, S.; Centi, G. A sustainable production of h2 by water splitting and photo-reforming of organic wastes on Au/TiO2nanotube arrays. Chem. Eng. Trans. 2014, 39, 1627–1632. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Li, R. Bioconversion and Chemical Conversion of Biogas for Fuel Production. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2019; pp. 187–205. [Google Scholar]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming; Springer: Berlin/Heidelberg, Germany, 1984; pp. 1–117. [Google Scholar]
- García, L. Hydrogen production by steam reforming of natural gas and other nonrenewable feedstocks. In Compendium of Hydrogen Energy; Elsevier: Berlin/Heidelberg, Germany, 2015; pp. 83–107. [Google Scholar]
- Melo, F.; Morlanés, N. Naphtha steam reforming for hydrogen production. Catal. Today 2005, 107–108, 458–466. [Google Scholar] [CrossRef]
- Nabgan, W.; Abdullah, T.A.T.; Mat, R.; Nabgan, B.; Triwahyono, S.; Ripin, A. Hydrogen production from catalytic steam reforming of phenol with bimetallic nickel-cobalt catalyst on various supports. Appl. Catal. A Gen. 2016, 527, 161–170. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen production from ethanol reforming: Catalysts and reaction mechanism. Renew. Sustain. Energy Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Costa, C.N.; Efstathiou, A.M. The steam reforming of phenol reaction over supported-Rh catalysts. Appl. Catal. A Gen. 2004, 272, 37–52. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Fierro, J.L.G.; Efstathiou, A.M. The phenol steam reforming reaction over MgO-based supported Rh catalysts. J. Catal. 2004, 228, 417–432. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Bakandritsos, A.; Tzitzios, V.; Fierro, J.; Efstathiou, A. Absorption-enhanced reforming of phenol by steam over supported Fe catalysts. J. Catal. 2006, 241, 132–148. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Steam reforming of hydrocarbons. A historical perspective. Stud. Surf. Sci. Catal. 2004, 147, 121–126. [Google Scholar]
- Boyano, A.; Morosuk, T.; Blanco-Marigorta, A.M.; Tsatsaronis, G. Conventional and advanced exergoenvironmental analysis of a steam methane reforming reactor for hydrogen production. J. Clean. Prod. 2012, 20, 152–160. [Google Scholar] [CrossRef]
- Office of Energy Efficiency & Renewable Energy. Hydrogen Production: Natural Gas Reforming. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming (accessed on 5 May 2020).
- Wang, Y.; Chin, Y.; Rozmiarek, R.; Johnson, B.; Gao, Y.; Watson, J.; Tonkovich, A.; Wiel, D.V. Highly active and stable Rh/MgOAl2O3 catalysts for methane steam reforming. Catal. Today 2004, 98, 575–581. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. A Gen. 2004, 258, 107–114. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Sachs, E.; Cima, M.; Williams, P.; Brancazio, D.; Cornie, J. Three Dimensional Printing: Rapid Tooling and Prototypes Directly from a CAD Model. J. Eng. Ind. 1992, 114, 481–488. [Google Scholar] [CrossRef]
- Duda, T.; Raghavan, L.V. 3D Metal Printing Technology. IFAC-PapersOnLine 2016, 49, 103–110. [Google Scholar] [CrossRef]
- Huang, Y.J.; Schwarz, J.A. The effect of catalyst preparation on catalytic activity. Appl. Catal. 1987, 30, 255–263. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Yu, J.; Wu, T.; Wang, G. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and sol–gel methods. Appl. Catal. A Gen. 2003, 239, 87–94. [Google Scholar] [CrossRef]
- Van Steen, E.; Sewell, G.S.; Makhothe, R.A.; Micklethwaite, C.; Manstein, H.; De Lange, M.; O’Connor, C.T. TPR Study on the Preparation of Impregnated Co/SiO2Catalysts. J. Catal. 1996, 162, 220–229. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Li, G.; Hu, L.; Hill, J.M. Comparison of reducibility and stability of alumina-supported Ni catalysts prepared by impregnation and co-precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- Farhadi, S.; Panahandehjoo, S. Spinel-type zinc aluminate (ZnAl2O4) nanoparticles prepared by the co-precipitation method: A novel, green and recyclable heterogeneous catalyst for the acetylation of amines, alcohols and phenols under solvent-free conditions. Appl. Catal. A Gen. 2010, 382, 293–302. [Google Scholar] [CrossRef]
- Sun, H.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. Preparation of well-adhered γ-Al2O3 washcoat on metallic wire mesh monoliths by electrophoretic deposition. Appl. Surf. Sci. 2007, 253, 3303–3310. [Google Scholar] [CrossRef]
- Nedyalkova, R.; Casanovas, A.; Llorca, J.; Montané, D. Electrophoretic deposition of Co–Me/ZnO (Me=Mn,Fe) ethanol steam reforming catalysts on stainless steel plates. Int. J. Hydrog. Energy 2009, 34, 2591–2599. [Google Scholar] [CrossRef]
- Cundy, C.S. Microwave Techniques in the Synthesis and Modification of Zeolite Catalysts. A Review. Collect. Czechoslov. Chem. Commun. 1998, 63, 1699–1723. [Google Scholar] [CrossRef]
- Montoya, J. Methane reforming with CO2 over Ni/ZrO2–CeO2 catalysts prepared by sol–gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Serp, P.; Kalck, P.; Feurer, R. Chemical Vapor Deposition Methods for the Controlled Preparation of Supported Catalytic Materials. Chem. Rev. 2002, 102, 3085–3128. [Google Scholar] [CrossRef]
- Lu, J.; Elam, J.W.; Stair, P.C. Synthesis and Stabilization of Supported Metal Catalysts by Atomic Layer Deposition. Acc. Chem. Res. 2013, 46, 1806–1815. [Google Scholar] [CrossRef]
- Serp, P.; Castillejos, E. Catalysis in Carbon Nanotubes. ChemCatChem 2010, 2, 41–47. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Hod Lipson, M.K. Fabricated: The New World of 3D Printing; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kodama, H. Automatic method for fabricating a three-dimensional plastic model with photo-hardening polymer. Rev. Sci. Instrum. 1981, 52, 1770–1773. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Soliman, A.; AlQubaisi, A.M.; Al-Rub, R.K.A. Nature-Inspired Lightweight Cellular Co-Continuous Composites with Architected Periodic Gyroidal Structures. Adv. Eng. Mater. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-Based 3D Printing of Polymer and Associated Composite: A Review on Mechanical Properties, Defects and Treatments. Polymers (Basel) 2020, 12, 1529. [Google Scholar] [CrossRef]
- Wohlers Associates. Wohlers Report 2019; Wohlers: Fort Collins, CO, USA, 2019. [Google Scholar]
- Adams, S. Half Million 3d Printers Sold in 2017–on Track for 100 m Sold in 2030. 2018. Available online: https://3dprintingindustry.com/news/half-million-3d-printers-sold-2017-track-100m-sold-2030-131642/ (accessed on 5 May 2020).
- Rengier, F.; Mehndiratta, A.; Von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Leow, W.R.; Chen, X. 3D Printing of Flexible Electronic Devices. Small Methods 2018, 2, 1700259. [Google Scholar] [CrossRef] [Green Version]
- Bassoli, E.; Gatto, A.; Iuliano, L.; Violante, M.G. 3D printing technique applied to rapid casting. Rapid Prototyp. J. 2007, 13, 148–155. [Google Scholar] [CrossRef]
- Llewellyn-Jones, T.M.; Drinkwater, B.W.; Trask, R.S. 3D printed components with ultrasonically arranged microscale structure. Smart Mater. Struct. 2016, 25, 02LT01. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef]
- Mirzaee, M.; Noghanian, S.; Wiest, L.; Chang, I. Developing flexible 3D printed antenna using conductive ABS materials. In Proceedings of the 2015 IEEE International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, Vancouver, BC, Canada, 19–25 July 2015; pp. 1308–1309. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. 3D-Printed Metal–Organic Framework Monoliths for Gas Adsorption Processes. ACS Appl. Mater. Interfaces 2017, 9, 35908–35916. [Google Scholar] [CrossRef]
- AlShowab, F. Mechanical Properties of 3D-Printed Shell-Core Cellular Co-Continuous Composites Based on Triply Periodic Minimal Surfaces; Masdar Institute of Science and Technology: Masdar City, UAE, 2018. [Google Scholar]
- Zhang, D.; Jonhson, W.; Herng, T.S.; Ang, Y.Q.; Yang, L.; Tan, S.C.; Peng, E.; He, H.; Ding, J. A 3D-printing method of fabrication for metals, ceramics, and multi-materials using a universal self-curable technique for robocasting. Mater. Horizons 2020, 7, 1083–1090. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Hajari, A.; Rownaghi, A.A.; Knox, J.C.; Rezaei, F. 3D-Printed Zeolite Monoliths for CO 2 Removal from Enclosed Environments. ACS Appl. Mater. Interfaces 2016, 8, 27753–27761. [Google Scholar] [CrossRef]
- Michorczyk, P.; Hędrzak, E.; Węgrzyniak, A. Preparation of monolithic catalysts using 3D printed templates for oxidative coupling of methane. J. Mater. Chem. A 2016, 4, 18753–18756. [Google Scholar] [CrossRef]
- Low, Z.X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef] [Green Version]
- Stuecker, J.N.; Miller, J.E.; Ferrizz, R.E.; Mudd, J.E.; Cesarano, J. Advanced Support Structures for Enhanced Catalytic Activity. Ind. Eng. Chem. Res. 2004, 43, 51–55. [Google Scholar] [CrossRef]
- Lefevere, J.; Gysen, M.; Mullens, S.; Meynen, V.; van Noyen, J. The benefit of design of support architectures for zeolite coated structured catalysts for methanol-to-olefin conversion. Catal. Today 2013, 216, 18–23. [Google Scholar] [CrossRef]
- van Noyen, J.; de Wilde, A.; Schroeven, M.; Mullens, S.; Luyten, J. Ceramic processing techniques for catalyst design: Formation, properties, and catalytic example of ZSM-5 on 3-dimensional fiber deposition support structures. Int. J. Appl. Ceram. Technol. 2012, 9, 902–910. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Lefevere, J.; Bedel, L.; Guilet, R.; Marty, P. Efficient CO2 methanation over Ni/Al2O3 coated structured catalysts. Catal. Today 2016, 273, 234–243. [Google Scholar] [CrossRef]
- Parra-Cabrera, C.; Achille, C.; Kuhn, S.; Ameloot, R. 3D printing in chemical engineering and catalytic technology: Structured catalysts, mixers and reactors. Chem. Soc. Rev. 2018, 47, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Morales, J.C.; Tarancón, A.; Canales-Vázquez, J.; Méndez-Ramos, J.; Hernández-Afonso, L.; Acosta-Mora, P.; Rueda, J.R.M.; Fernández-González, R. Three dimensional printing of components and functional devices for energy and environmental applications. Energy Environ. Sci. 2017, 10, 846–859. [Google Scholar] [CrossRef] [Green Version]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef] [Green Version]
- Kitson, P.J.; Glatzel, S.; Chen, W.; Lin, C.-G.; Song, Y.-F.; Cronin, L. 3D printing of versatile reactionware for chemical synthesis. Nat. Protoc. 2016, 11, 920–936. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.F.; Stöwe, K.; Sieg, S. Combinatorial and High-Throughput Materials Science. Angew. Chem. Int. Ed. 2007, 46, 6016–6067. [Google Scholar] [CrossRef]
- Smay, J.E.; Cesarano, J.; Lewis, J.A. Colloidal Inks for Directed Assembly of 3-D Periodic Structures. Langmuir 2002, 18, 5429–5437. [Google Scholar] [CrossRef]
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and robocasting of β-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater. 2006, 2, 457–466. [Google Scholar] [CrossRef]
- Eqtesadi, S.; Motealleh, A.; Miranda, P.; Lemos, A.; Rebelo, A.; Ferreira, J.M.F. A simple recipe for direct writing complex 45S5 Bioglass® 3D scaffolds. Mater. Lett. 2013, 93, 68–71. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C. Three-dimensional Printing for Catalytic Applications: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1701134. [Google Scholar] [CrossRef]
- Varotsis, A.B. Introduction to FDM 3D Printing. Available online: https://www.3dhubs.com/knowledge-base/introduction-fdm-3d-printing/ (accessed on 8 June 2020).
- SMG3D. Stratasys Fortus 900mc. Available online: https://www.smg3d.co.uk/automotive/fortus_900mc (accessed on 8 June 2020).
- ANIWAA. Ultimaker 3 Extended. Available online: https://www.aniwaa.com/product/3d-printers/ultimaker-ultimaker-3-extended/ (accessed on 8 June 2020).
- Johnson, R.D. Chemical creativity with 3D printing. Nat. Chem. 2012, 4, 338–339. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Huang, D.; Fuh, J.Y.H.; Hong, G.S. An Overview of 3D Printing Technologies for Food Fabrication. Food Bioprocess Technol. 2015, 8, 1605–1615. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, M.; Liao, C.; Wu, Q.; Liu, M.; Zhang, Y.; Wu, C.; Cheng, L.; Wang, Q. Printable hybrid hydrogel by dual enzymatic polymerization with superactivity. Chem. Sci. 2016, 7, 2748–2752. [Google Scholar] [CrossRef] [Green Version]
- Hurt, C.; Brandt, M.; Priya, S.S.; Bhatelia, T.; Patel, J.; Selvakannan, P.; Bhargava, S. Combining additive manufacturing and catalysis: A review. Catal. Sci. Technol. 2017, 7, 3421–3439. [Google Scholar] [CrossRef]
- Alexandrea, P. The Complete Guide to Selective Laser Sintering (SLS) in 3D Printing. 2019. Available online: https://www.3dnatives.com/en/selective-laser-sintering100420174/ (accessed on 15 April 2020).
- Hussain, G.; Khan, W.A.; Ashraf, H.A.; Ahmad, H.; Ahmed, H.; Imran, A.; Ahmad, I.; Rehman, K.; Abbas, G. Design and development of a lightweight SLS 3D printer with a controlled heating mechanism: Part A. Int. J. Light. Mater. Manuf. 2019, 2, 373–378. [Google Scholar] [CrossRef]
- Mikołajczyk, T.; Kłodowski, A.; Mikołajewska, E.; Walkowiak, P.; Berjano, P.; Villafañe, J.; Aggogeri, F.; Borboni, A.; Fausti, D.; Petrogalli, G. Design and control of system for elbow rehabilitation: Preliminary findings. Adv. Clin. Exp. Med. 2018, 27, 1661–1669. [Google Scholar] [CrossRef]
- Vulcaman. DIY-SLS-3D-Printer. Instructables Workshop. Available online: https://www.instructables.com/id/DIY-SLS-3D-Printer/ (accessed on 8 June 2020).
- Hoy, M.B. 3D Printing: Making Things at the Library. Med. Ref. Serv. Q. 2013, 32, 93–99. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Ioannou, Y.; Doumanidis, C.; Fyrillas, M.M.; Polychronopoulou, K. Analytical model for geometrical characteristics control of laser sintered surfaces. Int. J. Nanomanuf. 2010, 6, 300. [Google Scholar] [CrossRef]
- Koo, J.H.; Ortiz, R.; Ong, B.; Wu, H. Polymer nanocomposites for laser additive manufacturing. In Laser Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2017; pp. 205–235. [Google Scholar]
- Naboychenko, N.A.Y.S. Handbook of Non-Ferrous Metal Powders. In Handbook of Non-Ferrous Metal Powders, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 373–399. [Google Scholar]
- di Mondo, D.; Ashok, D.; Waldie, F.; Schrier, N.; Morrison, M.; Schlaf, M. Stainless Steel As a Catalyst for the Total Deoxygenation of Glycerol and Levulinic Acid in Aqueous Acidic Medium. ACS Catal. 2011, 1, 355–364. [Google Scholar] [CrossRef]
- Sang, L.; Sun, B.; Tan, H.; Du, C.; Wu, Y.; Ma, C. Catalytic reforming of methane with CO2 over metal foam based monolithic catalysts. Int. J. Hydrog. Energy 2012, 37, 13037–13043. [Google Scholar] [CrossRef]
- Elias, Y.; von Rohr, P.R.; Bonrath, W.; Medlock, J.; Buss, A. A porous structured reactor for hydrogenation reactions. Chem. Eng. Process. Process. Intensif. 2015, 95, 175–185. [Google Scholar] [CrossRef]
- Saggiomo, V. 3D Printed Devices for Catalytic Systems. In Catalyst Immobilization; Wiley: Hoboken, NJ, USA, 2020; pp. 369–408. [Google Scholar]
- Marikkannan, S.K.; Ayyasamy, E.P. Synthesis, characterisation and sintering behaviour influencing the mechanical, thermal and physical properties of cordierite-doped TiO2. J. Mater. Res. Technol. 2013, 2, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Ogihara, H.; Randall, C.A.; Trolier-McKinstry, S. High-Energy Density Capacitors Utilizing 0.7 BaTiO 3 -0.3 BiScO 3 Ceramics. J. Am. Ceram. Soc. 2009, 92, 1719–1724. [Google Scholar] [CrossRef]
- Eckel, Z.C.; Zhou, C.; Martin, J.H.; Jacobsen, A.J.; Carter, W.B.; Schaedler, T.A. Additive manufacturing of polymer-derived ceramics. Science (80-.) 2016, 351, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Crew. What are Factors the Quality of 3D Printing Rests upon? Available online: https://www.3dprintwise.com/3d-printing-quality/ (accessed on 5 May 2020).
- Carlota, V. All you Need to Know about ABS for 3D Printing. Available online: https://www.3dnatives.com/en/abs-3d-printing-060620194/ (accessed on 5 May 2020).
- Spoerk, M.; Gonzalez-Gutierrez, J.; Sapkota, J.; Schuschnigg, S.; Holzer, C. Effect of the printing bed temperature on the adhesion of parts produced by fused filament fabrication. Plast. Rubber Compos. 2018, 47, 17–24. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Kim, C.-M.; Jeong, H.-S.; Youn, J.-H. Influence of Bed Temperature on Heat Shrinkage Shape Error in FDM Additive Manufacturing of the ABS-Engineering Plastic. World J. Eng. Technol. 2016, 4, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing–Process parameters, materials, properties, and challenges. Prog. Mater. Sci. 2020, 100707. [Google Scholar] [CrossRef]
- Li, Y.; Chen, K.; Tamura, N. Mechanism of heat affected zone cracking in Ni-based superalloy DZ125L fabricated by laser 3D printing technique. Mater. Des. 2018, 150, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Lederle, F.; Meyer, F.; Brunotte, G.-P.; Kaldun, C.; Hübner, E.G. Improved mechanical properties of 3D-printed parts by fused deposition modeling processed under the exclusion of oxygen. Prog. Addit. Manuf. 2016, 1, 3–7. [Google Scholar] [CrossRef] [Green Version]
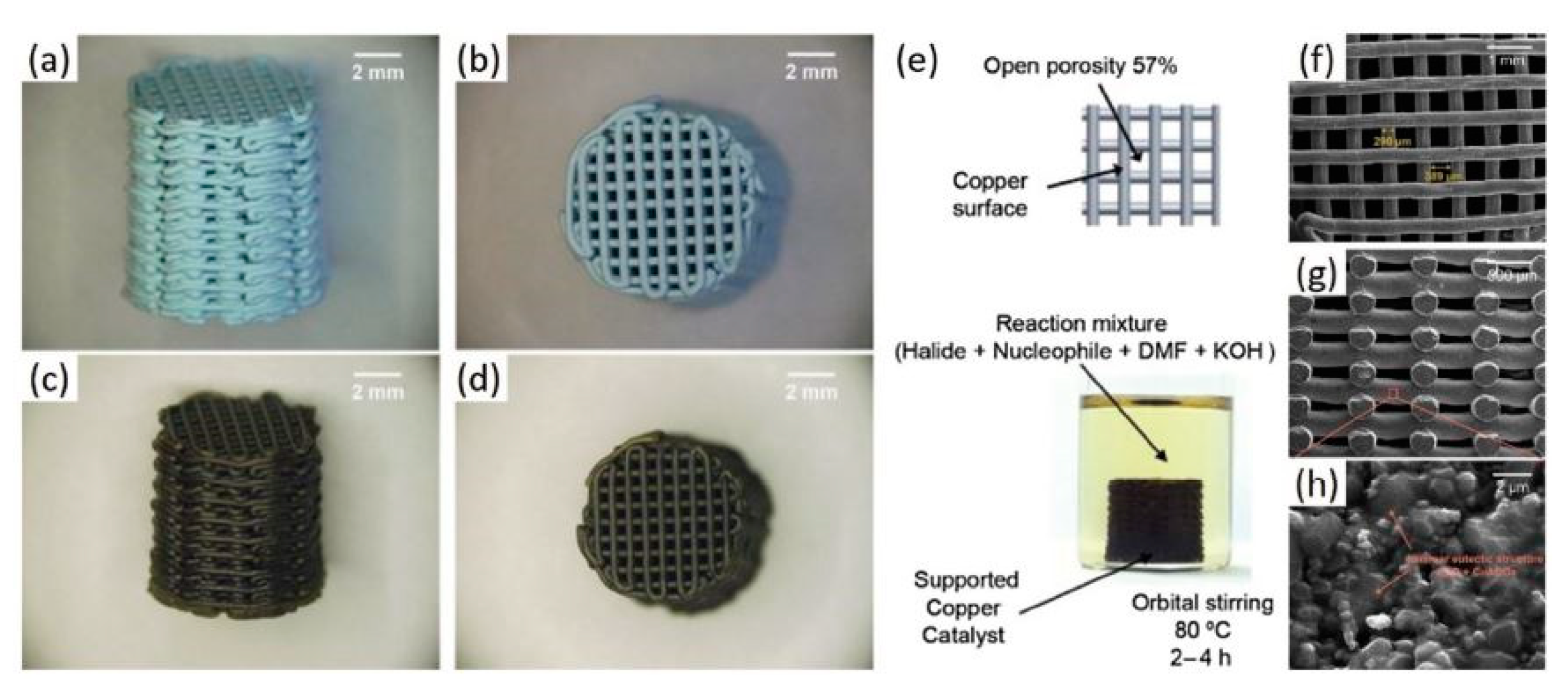
- Tubío, C.R.; Azuaje, J.; Escalante, L.; Coelho, A.; Guitián, F.; Sotelo, E.; Gil, A. 3D printing of a heterogeneous copper-based catalyst. J. Catal. 2016, 334, 110–115. [Google Scholar] [CrossRef]
- Arin, M.; Watté, J.; Pollefeyt, G.; de Buysser, K.; van Driessche, I.; Lommens, P. Low temperature deposition of TiO2 layers from nanoparticle containing suspensions synthesized by microwave hydrothermal treatment. J. Sol-Gel Sci. Technol. 2013, 66, 100–111. [Google Scholar] [CrossRef]
- Arin, M.; Lommens, P.; Hopkins, S.C.; Pollefeyt, G.; Van Der Eycken, J.; Ricart, S.; Granados, X.; A Glowacki, B.; Van Driessche, I. Deposition of photocatalytically active TiO2 films by inkjet printing of TiO2 nanoparticle suspensions obtained from microwave-assisted hydrothermal synthesis. Nanotechnology 2012, 23, 165603. [Google Scholar] [CrossRef]
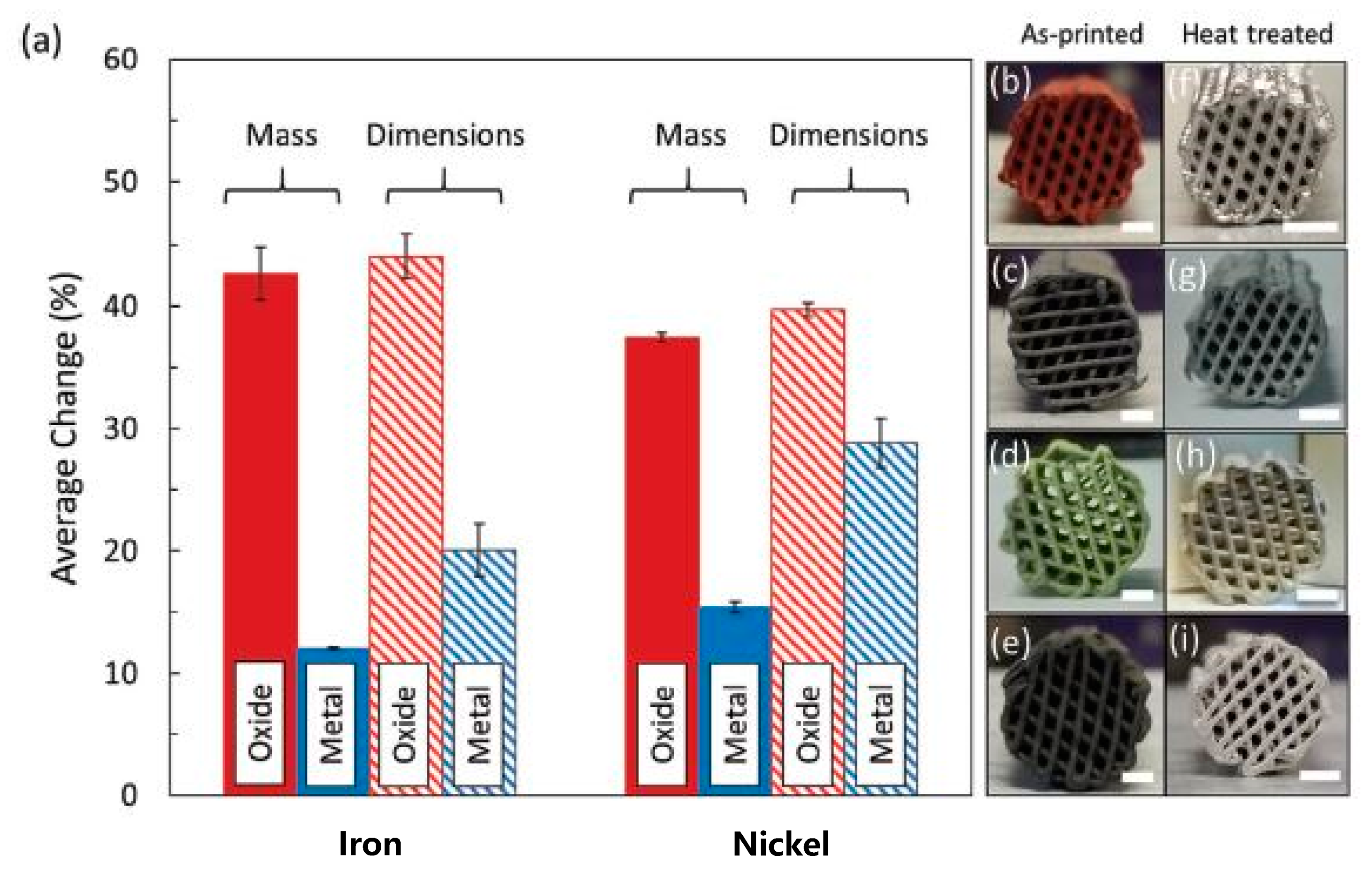
- Taylor, S.L.; Jakus, A.E.; Shah, R.N.; Dunand, D.C. Iron and Nickel Cellular Structures by Sintering of 3D-Printed Oxide or Metallic Particle Inks. Adv. Eng. Mater. 2017, 19, 1600365. [Google Scholar] [CrossRef]
- Couck, S.; Lefevere, J.; Mullens, S.; Protasova, L.; Meynen, V.; Desmet, G.; Baron, G.V.; Denayer, J.F. CO2, CH4 and N2 separation with a 3DFD-printed ZSM-5 monolith. Chem. Eng. J. 2017, 308, 719–726. [Google Scholar] [CrossRef]
- Kramer, M.; McKelvie, M.; Watson, M. Additive Manufacturing of Catalyst Substrates for Steam–Methane Reforming. J. Mater. Eng. Perform. 2018, 27, 21–31. [Google Scholar] [CrossRef]
- Siakavelas, G.; Charisiou, N.; Alkhoori, S.; Alkhoori, A.; Sebastian, V.; Hinder, S.; Baker, M.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly selective and stable nickel catalysts supported on ceria promoted with Sm2O3, Pr2O3 and MgO for the CO2 methanation. Appl. Catal. B Environ. 2020, 119562. [Google Scholar] [CrossRef]
- Basina, G.; Polychronopoulou, K.; Zedan, A.F.; Dimos, K.; Katsiotis, M.S.; Fotopoulos, A.P.; Ismail, I.; Tzitzios, V. Ultrasmall Metal-Doped CeO 2 Nanoparticles for Low-Temperature CO Oxidation. ACS Appl. Nano Mater. 2020, acsanm.0c02090. [Google Scholar] [CrossRef]
- Zhu, C.; Qi, Z.; Beck, V.A.; Luneau, M.; Lattimer, J.; Chen, W.; Worsley, M.A.; Ye, J.; Duoss, E.B.; Spadaccini, C.M.; et al. Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing. Sci. Adv. 2018, 4, eaas9459. [Google Scholar] [CrossRef] [Green Version]
- Elkoro, A.; Soler, L.; Llorca, J.; Casanova, I. 3D printed microstructured Au/TiO2 catalyst for hydrogen photoproduction. Appl. Mater. Today 2019, 16, 265–272. [Google Scholar] [CrossRef]
- Kitson, P.J.; Symes, M.D.; Dragone, V.; Cronin, L. Combining 3D printing and liquid handling to produce user-friendly reactionware for chemical synthesis and purification. Chem. Sci. 2013, 4, 3099–3103. [Google Scholar] [CrossRef] [Green Version]
- Skorski, M.R.; Esenther, J.M.; Ahmed, Z.; Miller, A.E.; Hartings, M.R. The chemical, mechanical, and physical properties of 3D printed materials composed of TiO 2 -ABS nanocomposites. Sci. Technol. Adv. Mater. 2016, 17, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Yan, Y.; Zhang, L.; Ma, G.; Liu, Y.; Yu, Y.; An, Q.; Tao, S. Direct 3D Printing of Reactive Agitating Impellers for the Convenient Treatment of Various Pollutants in Water. Adv. Mater. Interfaces 2018, 5, 1701626. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Li, M.; Sun, K.; Liu, C. Three-dimensional Printed Acrylonitrile Butadiene Styrene Framework Coated with Cu-BTC Metal-organic Frameworks for the Removal of Methylene Blue. Sci. Rep. 2015, 4, 5939. [Google Scholar] [CrossRef]
- Díaz-Marta, A.S.; Tubío, C.R.; Carbajales, C.; Fernández, C.; Escalante, L.; Sotelo, E.; Guitián, F.; Barrio, V.L.; Gil, A.; Coelho, A. Three-Dimensional Printing in Catalysis: Combining 3D Heterogeneous Copper and Palladium Catalysts for Multicatalytic Multicomponent Reactions. ACS Catal. 2018, 8, 392–404. [Google Scholar] [CrossRef]
- Yee, D.W.; Schulz, M.D.; Grubbs, R.H.; Greer, J.R. Functionalized 3D Architected Materials via Thiol-Michael Addition and Two-Photon Lithography. Adv. Mater. 2017, 29, 1605293. [Google Scholar] [CrossRef]
- Wei, Q.; Li, H.; Liu, G.; He, Y.; Wang, Y.; Tan, Y.E.; Wang, D.; Peng, X.; Yang, G.; Tsubaki, N. Metal 3D printing technology for functional integration of catalytic system. Nat. Commun. 2020, 11, 4098. [Google Scholar] [CrossRef] [PubMed]
- Fratalocchi, L.; Groppi, G.; Visconti, C.G.; Lietti, L.; Tronconi, E. Adoption of 3D printed highly conductive periodic open cellular structures as an effective solution to enhance the heat transfer performances of compact Fischer-Tropsch fixed-bed reactors. Chem. Eng. J. 2020, 386, 123988. [Google Scholar] [CrossRef]
- Kim, H.; Lin, Y.; Tseng, T.-L.B. A review on quality control in additive manufacturing. Rapid Prototyp. J. 2018, 24, 645–669. [Google Scholar] [CrossRef] [Green Version]
- Townsend, A.; Senin, N.; Blunt, L.; Leach, R.K.; Taylor, J.S. Surface texture metrology for metal additive manufacturing: A review. Precis. Eng. 2016, 46, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Slotwinski, J.A.; Garboczi, E.J. Metrology Needs for Metal Additive Manufacturing Powders. JOM 2015, 67, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Clayton, J. Characterising Powders to Optimise Additive Manufacturing. Available online: http://www.tctmagazine (accessed on 5 May 2020).
- Lyckfeldt, O. Powder Rheology of Steel Powders for Additive Manufacturing. Swerea IVF AB 2013, 2, 225–230. [Google Scholar]
- Starr, C.M.S.; H, T.L.; Rafi, K.; Stucker, B. Controlling phase composition in selective laser melted stainless steels. Powder (W) 2012, 195, 195. [Google Scholar]
- Murr, L.E.; Martinez, E.; Hernandez, J.; Collins, S.; Amato, K.N.; Gaytan, S.M.; Shindo, P.W. Microstructures and Properties of 17-4 PH Stainless Steel Fabricated by Selective Laser Melting. J. Mater. Res. Technol. 2012, 1, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.; O’Conner, A.; Ramulu, M. Electron Beam Additive Manufacturing of Titanium Components: Properties and Performance. J. Manuf. Sci. Eng. 2013, 135, 061016. [Google Scholar] [CrossRef]
- Galantucci, L.M.; Lavecchia, F.; Percoco, G. Experimental study aiming to enhance the surface finish of fused deposition modeled parts. CIRP Ann. 2009, 58, 189–192. [Google Scholar] [CrossRef]
- Beard, M.A.; Ghita, O.R.; Evans, K.E. Using Raman spectroscopy to monitor surface finish and roughness of components manufactured by selective laser sintering. J. Raman Spectrosc. 2011, 42, 744–748. [Google Scholar] [CrossRef]
- Calignano, F.; Manfredi, D.; Ambrosio, E.P.; Iuliano, L.; Fino, P. Influence of process parameters on surface roughness of aluminum parts produced by DMLS. Int. J. Adv. Manuf. Technol. 2013, 67, 2743–2751. [Google Scholar] [CrossRef] [Green Version]
- Köpplmayr, T.; Häusler, L.; Bergmair, I.; Mühlberger, M. Nanoimprint Lithography on curved surfaces prepared by fused deposition modelling. Surf. Topogr. Metrol. Prop. 2015, 3, 024003. [Google Scholar] [CrossRef]
- Safdar, A.; He, H.Z.; Wei, L.; Snis, A.; de Paz, L.E.C. Effect of process parameters settings and thickness on surface roughness of EBM produced Ti-6Al-4V. Rapid Prototyp. J. 2012, 18, 401–408. [Google Scholar] [CrossRef]
- Grimm, T.; Wiora, G.; Witt, G. Characterization of typical surface effects in additive manufacturing with confocal microscopy. Surf. Topogr. Metrol. Prop. 2015, 3, 014001. [Google Scholar] [CrossRef]
- Triantaphyllou, A.; Giusca, C.L.; Macaulay, G.D.; Roerig, F.; Hoebel, M.; Leach, R.K.; Tomita, B.; A Milne, K. Surface texture measurement for additive manufacturing. Surf. Topogr. Metrol. Prop. 2015, 3, 024002. [Google Scholar] [CrossRef]
- Temmler, A.; Willenborg, E.; Wissenbach, K. Laser Polishing. Proc. SPIE 2012, 82430W. [Google Scholar] [CrossRef]
- Jamshidinia, M.; Kovacevic, R. The influence of heat accumulation on the surface roughness in powder-bed additive manufacturing. Surf. Topogr. Metrol. Prop. 2015, 3, 014003. [Google Scholar] [CrossRef]
- Johnson, M.K.; Cole, F.; Raj, A.; Adelson, E.H. Microgeometry capture using an elastomeric sensor. In ACM SIGGRAPH 2011 Papers on—SIGGRAPH ’11; ACM: New York, NY, USA, 2011; p. 1. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.K.; Adelson, E.H. Retrographic sensing for the measurement of surface texture and shape. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; pp. 1070–1077. [Google Scholar] [CrossRef]
- Mumtaz, K.; Hopkinson, N. Top surface and side roughness of Inconel 625 parts processed using selective laser melting. Rapid Prototyp. J. 2009, 15, 96–103. [Google Scholar] [CrossRef]
- Kerckhofs, G.; Pyka, G.; Moesen, M.; van Bael, S.; Schrooten, J.; Wevers, M. High-Resolution Microfocus X-Ray Computed Tomography for 3D Surface Roughness Measurements of Additive Manufactured Porous Materials. Adv. Eng. Mater. 2013, 15, 153–158. [Google Scholar] [CrossRef]
- Pyka, G.; Kerckhofs, G.; Papantoniou, I.; Speirs, M.; Schrooten, J.; Wevers, M. Surface Roughness and Morphology Customization of Additive Manufactured Open Porous Ti6Al4V Structures. Materials (Basel) 2013, 6, 4737–4757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive Manufacturing of Ceramics: Issues, Potentialities, and Opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- Santoliquido, O.; Bianchi, G.; Eggenschwiler, P.D.; Ortona, A. Additive manufacturing of periodic ceramic substrates for automotive catalyst supports. Int. J. Appl. Ceram. Technol. 2017, 14, 1164–1173. [Google Scholar] [CrossRef]
- Jung, Y.; Torquato, S. Fluid permeabilities of triply periodic minimal surfaces. Phys. Rev. E 2005, 72, 056319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreedhar, N.; Thomas, N.; Al-Ketan, O.; Rowshan, R.; Hernandez, H.H.; Abu Al-Rub, R.K.; Arafat, H.A. Mass transfer analysis of ultrafiltration using spacers based on triply periodic minimal surfaces: Effects of spacer design, directionality and voidage. J. Memb. Sci. 2018, 561, 89–98. [Google Scholar] [CrossRef]
- Yuzo Hitachim, H.S. Metal-Made Honeycomb Carrier Body. Patent 5,374,402, 20 December 1994. [Google Scholar]
- Torquato, S.; Donev, A. Minimal surfaces and multifunctionality. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 460, 1849–1856. [Google Scholar] [CrossRef]
- Abueidda, D.W.; Dalaq, A.S.; Al-Rub, R.K.A.; Younes, H.A. Finite element predictions of effective multifunctional properties of interpenetrating phase composites with novel triply periodic solid shell architectured reinforcements. Int. J. Mech. Sci. 2015, 92, 80–89. [Google Scholar] [CrossRef]
- Aldrich, D.E.; Fan, Z.; Mummery, P. Processing, microstructure, and physical properties of interpenetrating Al 2 O 3 /Ni composites. Mater. Sci. Technol. 2000, 16, 747–752. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, A.; Fu, H.; Zhu, Z.; Zhang, Z.; Hu, Z.; Wang, L.; Cheng, H. Preparation, microstructure and deformation behavior of Zr-based metallic glass/porous SiC interpenetrating phase composites. Mater. Sci. Eng. A 2011, 530, 15–20. [Google Scholar] [CrossRef]
- Marchi, C.S.; Kouzeli, M.; Rao, R.; Lewis, J.A.; Dunand, D.C. Alumina–aluminum interpenetrating-phase composites with three-dimensional periodic architecture. Scr. Mater. 2003, 49, 861–866. [Google Scholar] [CrossRef]
- del Rio, E.; Nash, J.M.; Williams, J.C.; Breslin, M.C.; Daehn, G.S. Co-continuous composites for high-temperature applications. Mater. Sci. Eng. A 2007, 463, 115–121. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Al-Rub, R.K.A.; Rowshan, R. Mechanical Properties of a New Type of Architected Interpenetrating Phase Composite Materials. Adv. Mater. Technol. 2017, 2, 1600235. [Google Scholar] [CrossRef]
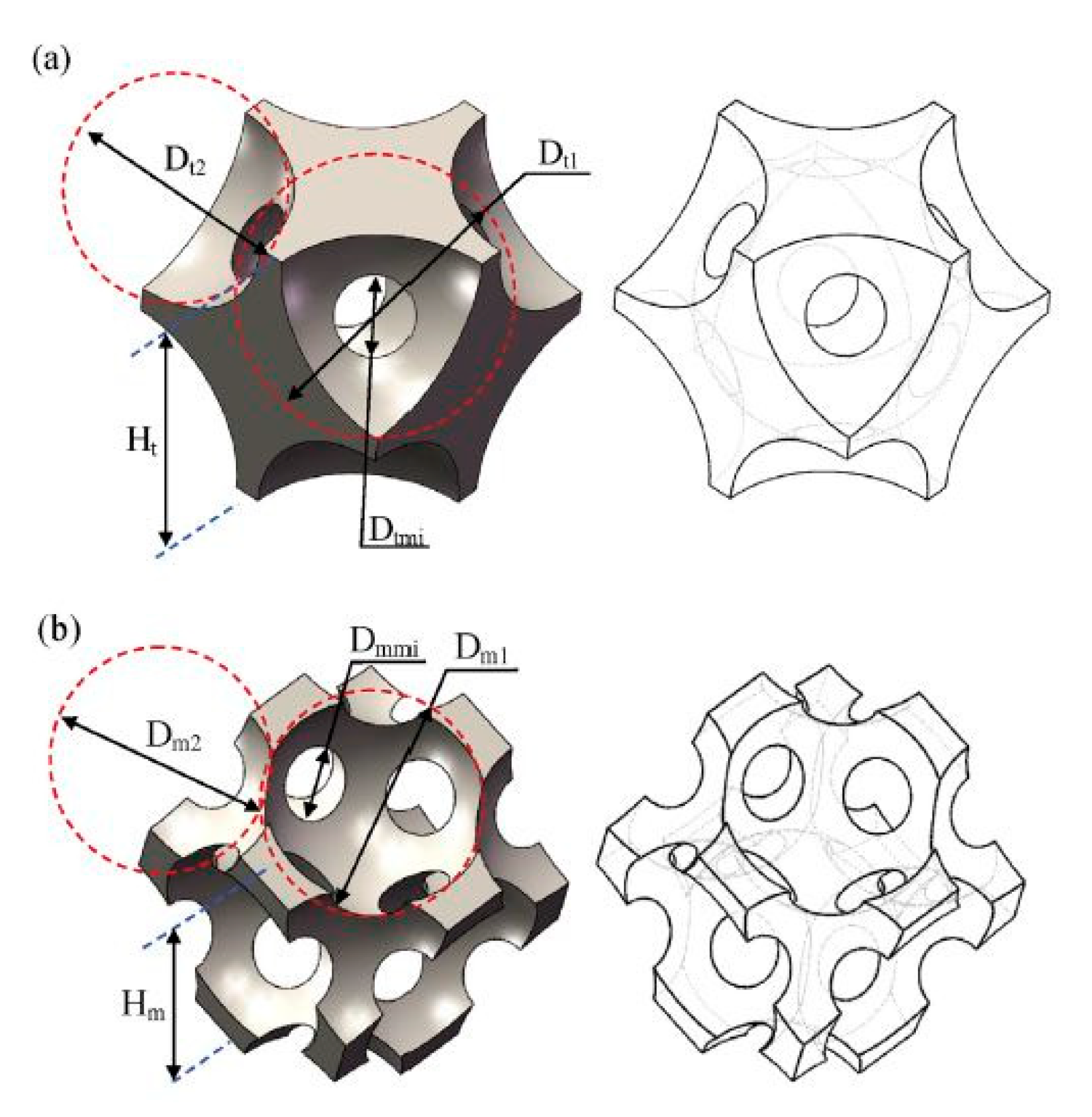
- Al-Ketan, O.; Pelanconi, M.; Ortona, A.; Al-Rub, R.K.A. Additive manufacturing of architected catalytic ceramic substrates based on triply periodic minimal surfaces. J. Am. Ceram. Soc. 2019, 102, 6176–6193. [Google Scholar] [CrossRef]
- Niya, A.A. Novel Adsorbents for Natural Gas Separation and Purification. Ph.D. Thesis, The University of Queensland, St Lucia QLD, Australia, 2017. [Google Scholar]
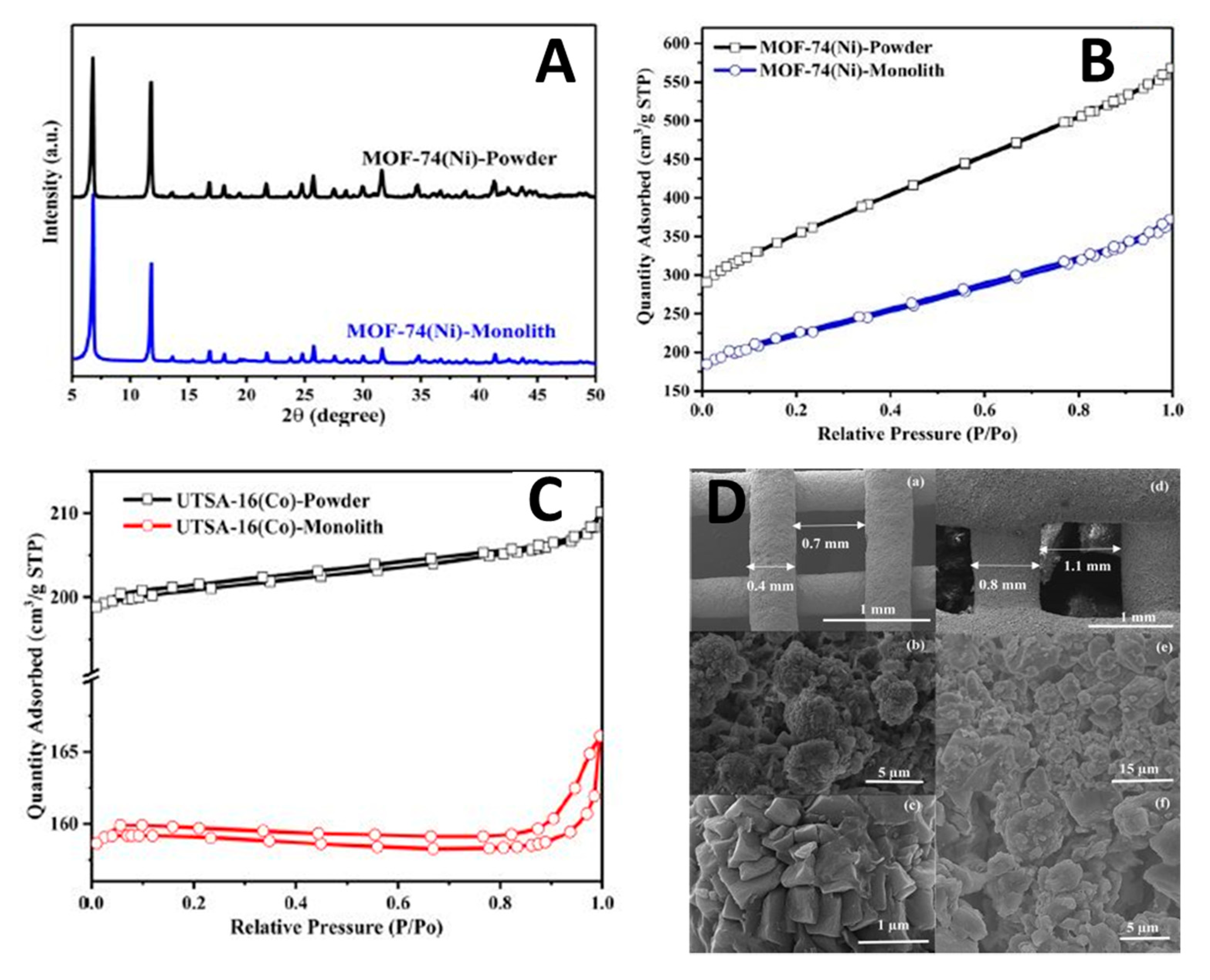
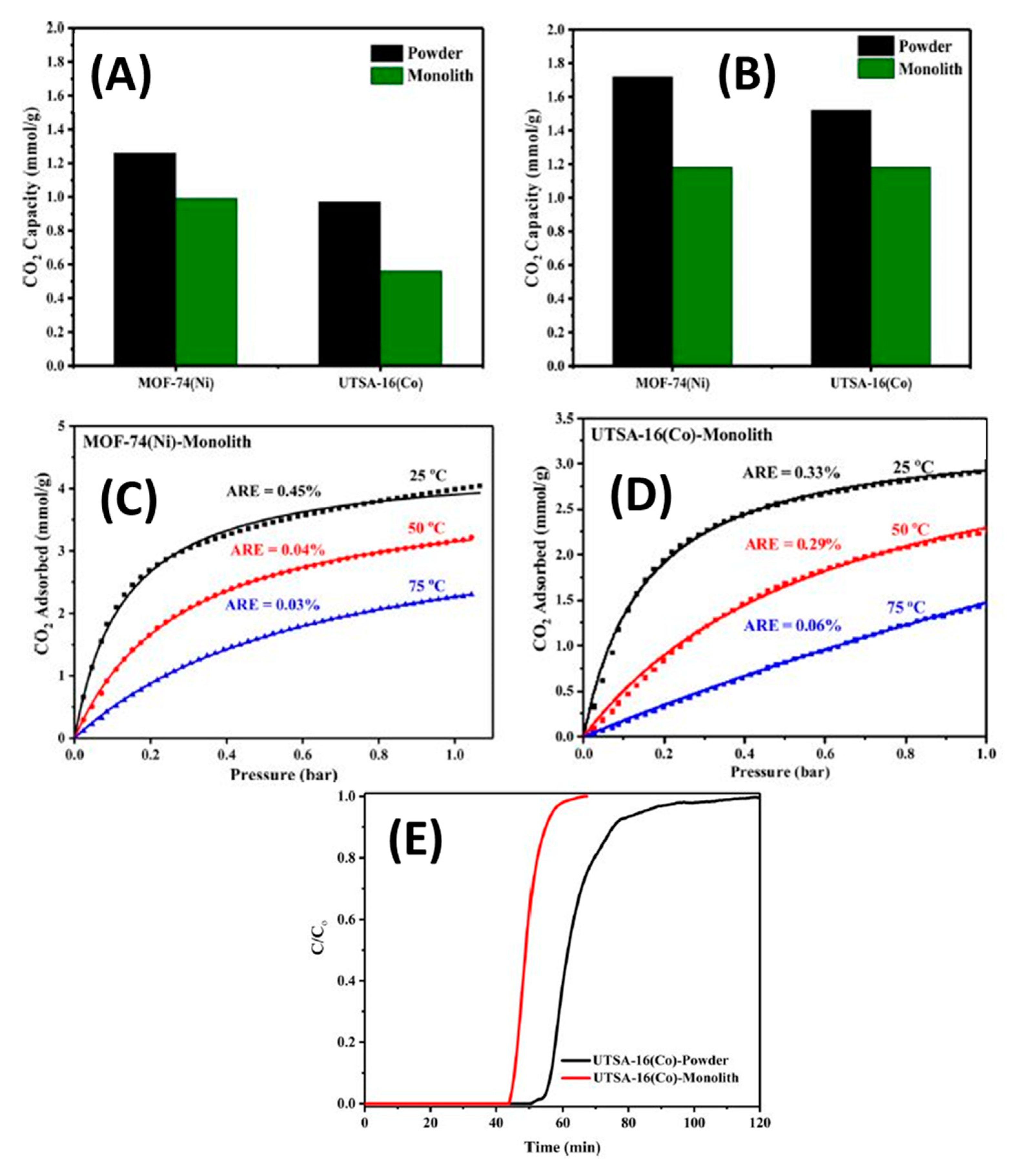
- Lawson, S.; Snarzyk, M.; Hanify, D.; Rownaghi, A.A.; Rezaei, F. Development of 3D-Printed Polymer-MOF Monoliths for CO 2 Adsorption. Ind. Eng. Chem. Res. 2020, 59, 7151–7160. [Google Scholar] [CrossRef]
- Wang, S.; Bai, P.; Sun, M.; Liu, W.; Li, D.; Wu, W.; Yan, W.; Shang, J.; Yu, J. Fabricating Mechanically Robust Binder-Free Structured Zeolites by 3D Printing Coupled with Zeolite Soldering: A Superior Configuration for CO 2 Capture. Adv. Sci. 2019, 6, 1901317. [Google Scholar] [CrossRef] [Green Version]
- Dunne, J.A.; Rao, M.; Sircar, S.; Gorte, R.J.; Myers, A.L. Calorimetric Heats of Adsorption and Adsorption Isotherms. 2. O 2, N 2, Ar, CO 2, CH 4, C 2 H 6, and SF 6 on NaX, H-ZSM-5, and Na-ZSM-5 Zeolites. Langmuir 1996, 12, 5896–5904. [Google Scholar] [CrossRef]
- Khelifa, A.; Benchehida, L.; Derriche, Z. Adsorption of carbon dioxide by X zeolites exchanged with Ni2+ and Cr3+: Isotherms and isosteric heat. J. Colloid Interface Sci. 2004, 278, 9–17. [Google Scholar] [CrossRef]
- Middelkoop, V.; Coenen, K.; Schalck, J.; Annaland, M.V.; Gallucci, F. 3D printed versus spherical adsorbents for gas sweetening. Chem. Eng. J. 2019, 357, 309–319. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Ribeiro, A.M.; Loureiro, J.M.; Evaggelos, O.; Nikolakis, V.; Rodrigues, A.E. Adsorption of H 2, CO 2, CH 4, CO, N 2 and H 2 O in Activated Carbon and Zeolite for Hydrogen Production. Sep. Sci. Technol. 2009, 44, 1045–1073. [Google Scholar] [CrossRef]
- Garshasbi, V.; Jahangiri, M.; Anbia, M. Equilibrium CO2 adsorption on zeolite 13X prepared from natural clays. Appl. Surf. Sci. 2017, 393, 225–233. [Google Scholar] [CrossRef]
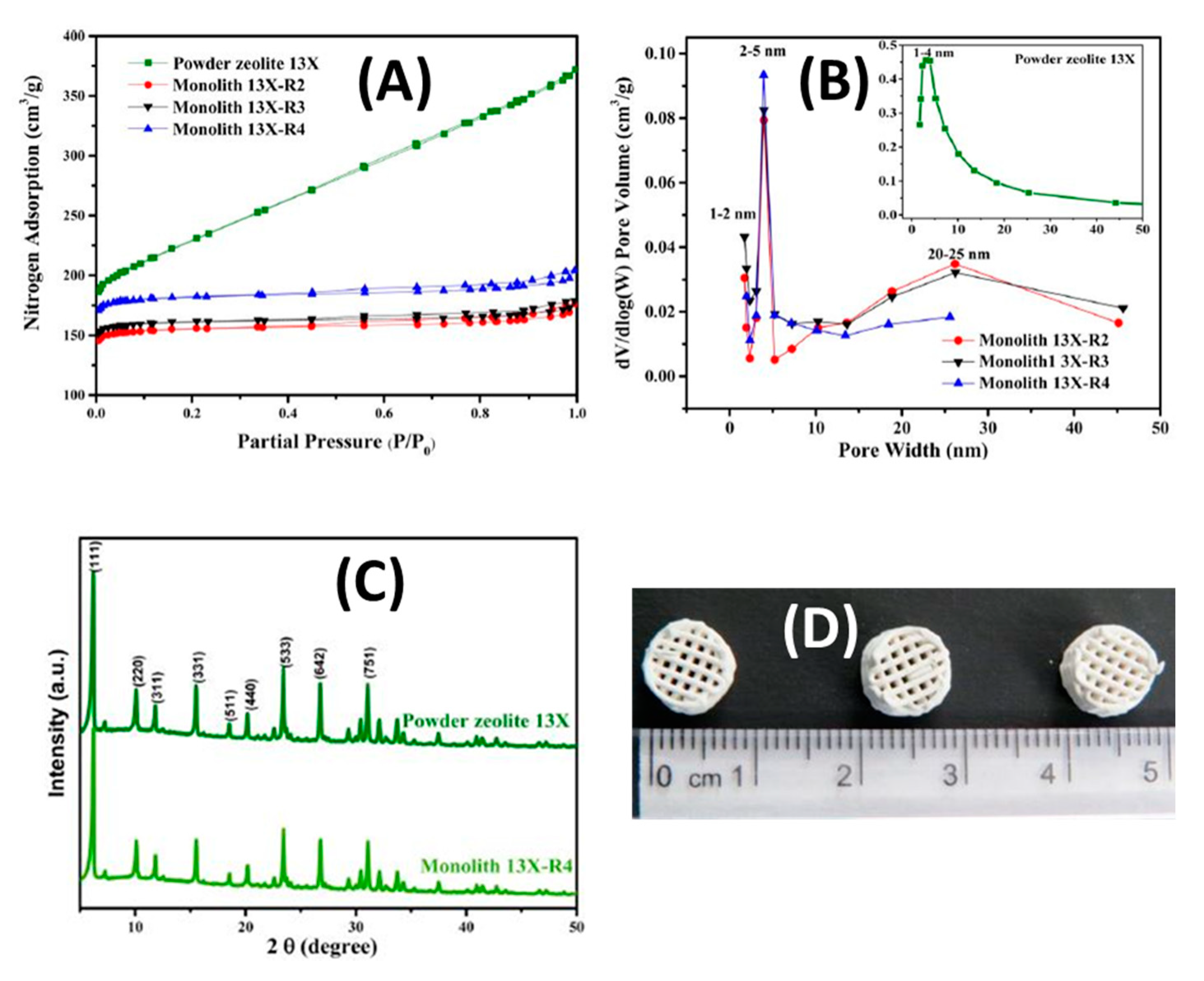
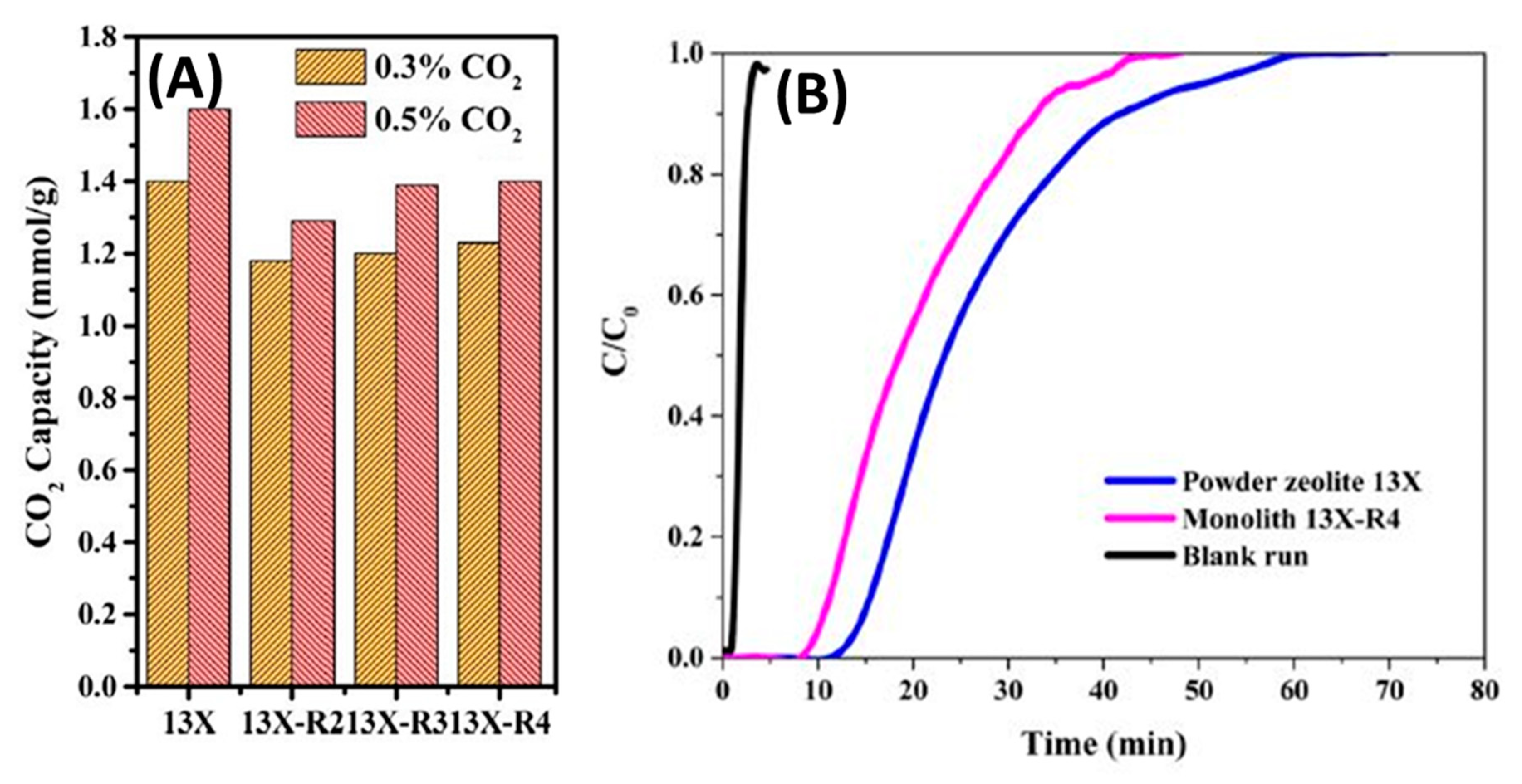
- Lawson, S.; Adebayo, B.; Robinson, C.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. The effects of cell density and intrinsic porosity on structural properties and adsorption kinetics in 3D-printed zeolite monoliths. Chem. Eng. Sci. 2020, 218, 115564. [Google Scholar] [CrossRef]
- Hu, X.; Mangano, E.; Friedrich, D.; Ahn, H.; Brandani, S. Diffusion mechanism of CO2 in 13X zeolite beads. Adsorption 2014, 20, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrog. Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Speight, J. The Refinery of the Future; Gulf Professional Publishing: Houston, TX, USA, 2020. [Google Scholar]
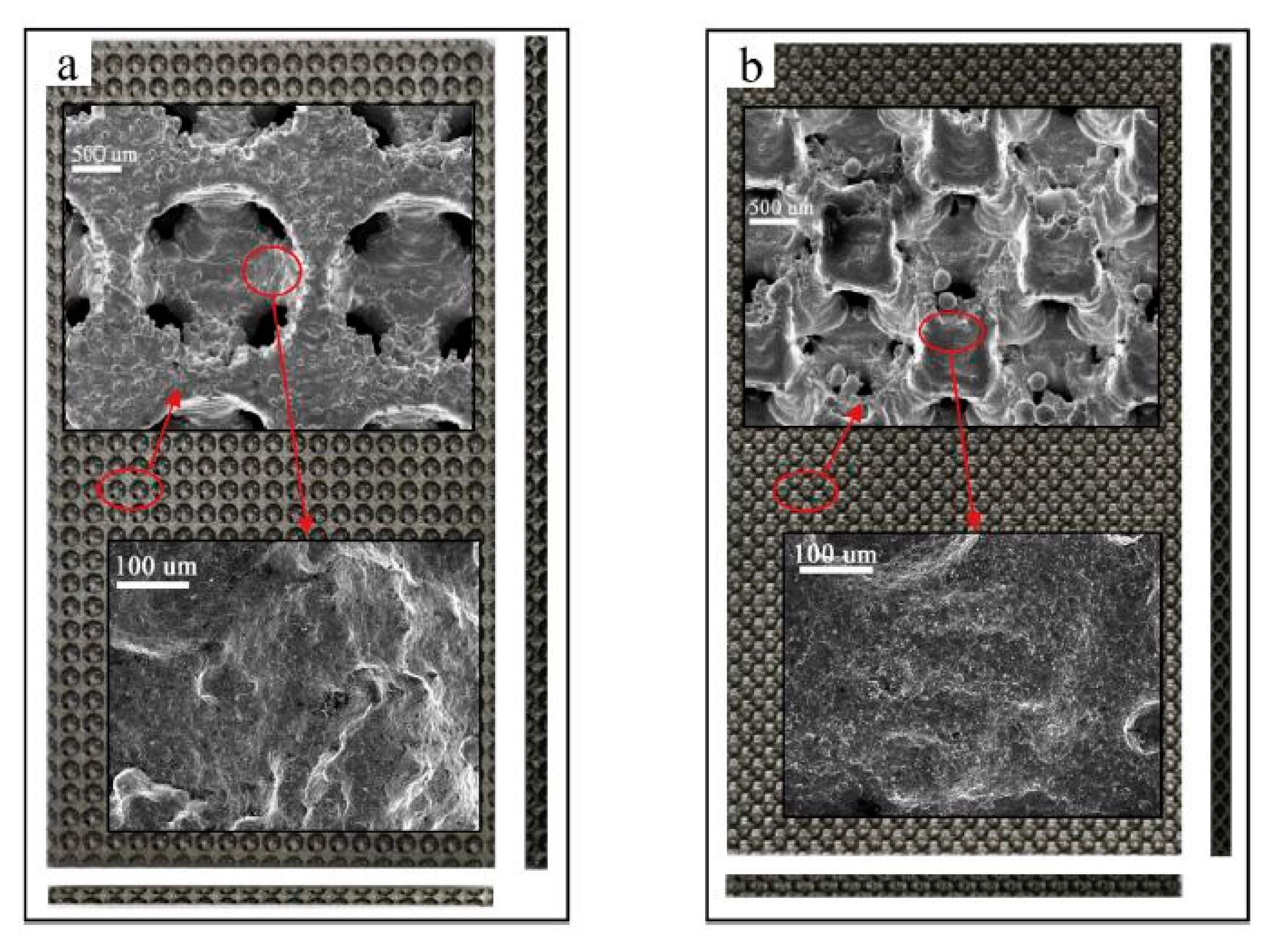
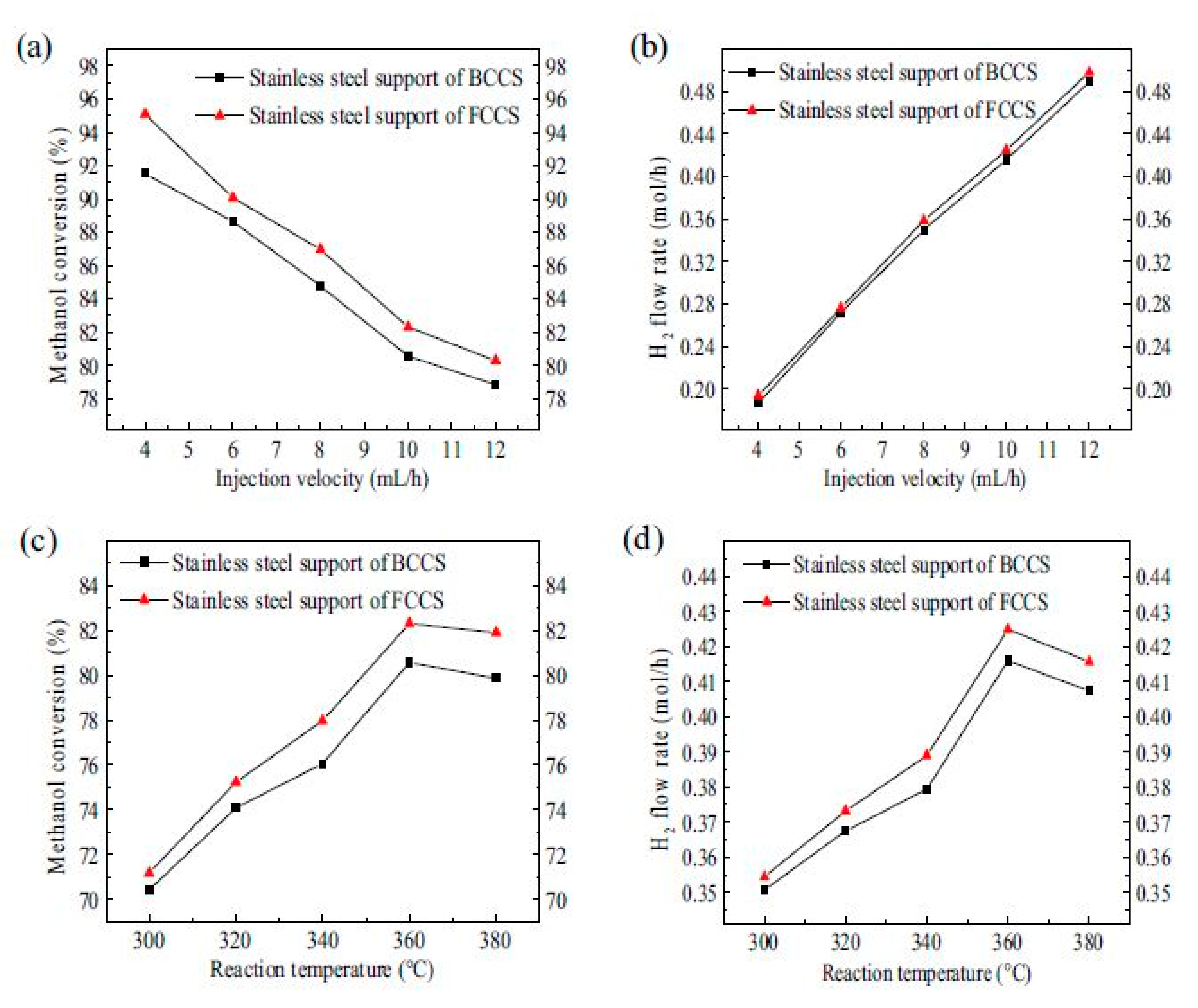
- Zheng, T.; Zhou, W.; Geng, D.; Li, Y.; Liu, Y.; Zhang, C. Methanol steam reforming microreactor with novel 3D-Printed porous stainless steel support as catalyst support. Int. J. Hydrog. Energy 2020, 45, 14006–14016. [Google Scholar] [CrossRef]
- Azuaje, J.; Tubío, C.R.; Escalante, L.; Gómez, M.; Guitián, F.; Coelho, A.; Caamaño, O.; Gil, A.; Sotelo, E. An efficient and recyclable 3D printed α-Al 2 O 3 catalyst for the multicomponent assembly of bioactive heterocycles. Appl. Catal. A Gen. 2017, 530, 203–210. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Middelkoop, V.; Ray, N.; Jouve, M.; Bengaouer, A.; Marty, P. Scaling up of 3D printed and Ni/Al2O3 coated reactors for CO2 methanation. React. Chem. Eng. 2019, 4, 1318–1330. [Google Scholar] [CrossRef]
- Luyten, J.; Mullens, S.; Thijs, I. Designing with Pores-Synthesis and Applications. KONA Powder Part. J. 2010, 28, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Zamaniyan, A.; Mortazavi, Y.; Khodadadi, A.A.; Manafi, H. Tube fitted bulk monolithic catalyst as novel structured reactor for gas–solid reactions. Appl. Catal. A Gen. 2010, 385, 214–223. [Google Scholar] [CrossRef]

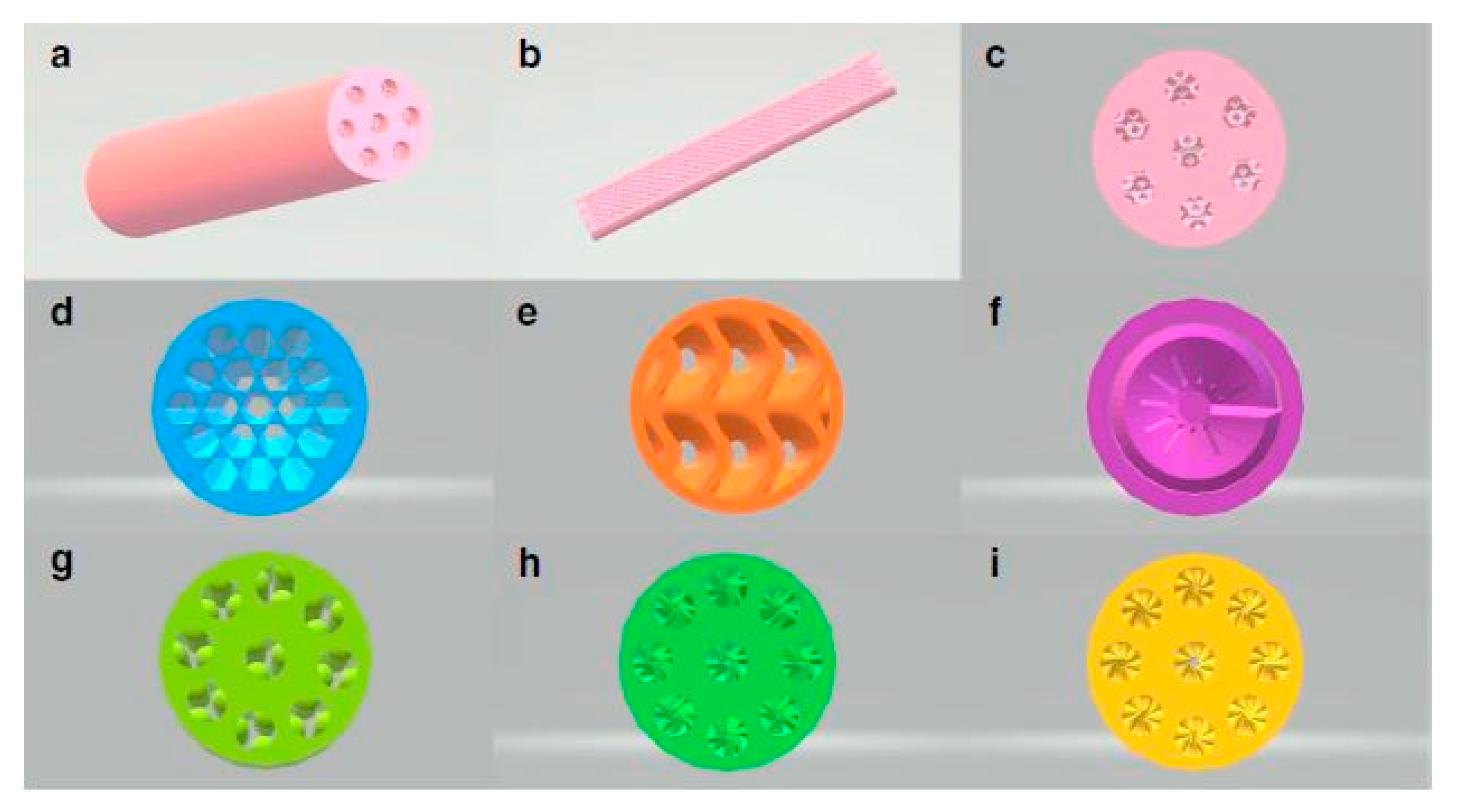

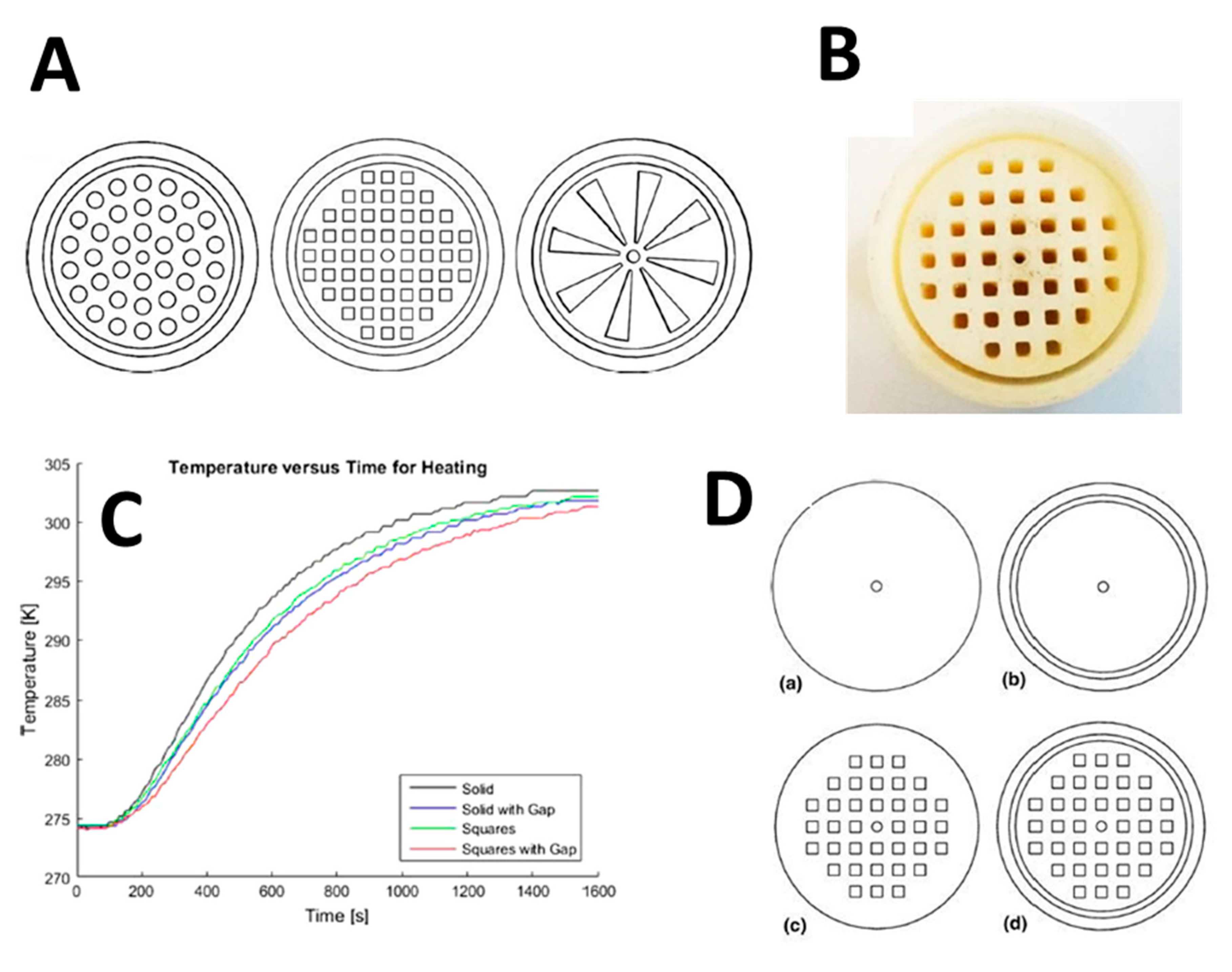
| Unit Cell Representation | TPMS Structure | Level Set Equation |
|---|---|---|
 | Schwarz Primitive | |
 | Schoen IWP | |
 | Schoen Gyroid | |
 | Schwarz Diamond | |
 | Fischer Koch S |
| Sample | t5% (min) | t50% (min) | t95% (min) | Breakthrough Width (min) |
|---|---|---|---|---|
| 13X zeolite powder | 13 | 23 | 53 | 40 |
| 13X-R4 monolith | 9 | 19 | 48 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, A.; AlAmoodi, N.; Karanikolos, G.N.; Doumanidis, C.C.; Polychronopoulou, K. A Review on New 3-D Printed Materials’ Geometries for Catalysis and Adsorption: Paradigms from Reforming Reactions and CO2 Capture. Nanomaterials 2020, 10, 2198. https://doi.org/10.3390/nano10112198
Soliman A, AlAmoodi N, Karanikolos GN, Doumanidis CC, Polychronopoulou K. A Review on New 3-D Printed Materials’ Geometries for Catalysis and Adsorption: Paradigms from Reforming Reactions and CO2 Capture. Nanomaterials. 2020; 10(11):2198. https://doi.org/10.3390/nano10112198
Chicago/Turabian StyleSoliman, Ahmad, Nahla AlAmoodi, Georgios N. Karanikolos, Charalabos C. Doumanidis, and Kyriaki Polychronopoulou. 2020. "A Review on New 3-D Printed Materials’ Geometries for Catalysis and Adsorption: Paradigms from Reforming Reactions and CO2 Capture" Nanomaterials 10, no. 11: 2198. https://doi.org/10.3390/nano10112198
APA StyleSoliman, A., AlAmoodi, N., Karanikolos, G. N., Doumanidis, C. C., & Polychronopoulou, K. (2020). A Review on New 3-D Printed Materials’ Geometries for Catalysis and Adsorption: Paradigms from Reforming Reactions and CO2 Capture. Nanomaterials, 10(11), 2198. https://doi.org/10.3390/nano10112198








