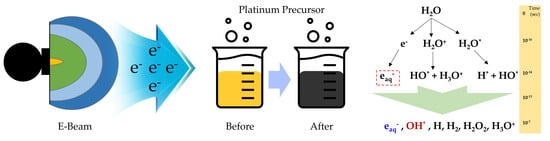
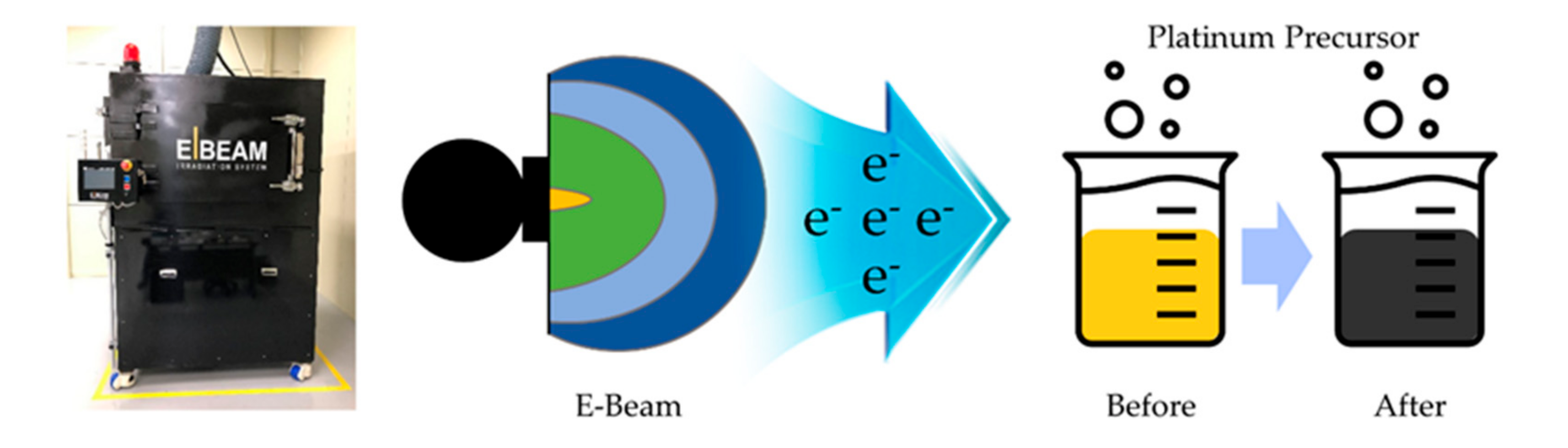
Facile Strategy for Mass Production of Pt Catalysts for Polymer Electrolyte Membrane Fuel Cells Using Low-Energy Electron Beam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pt/C Catalyst
2.3. Characterizations
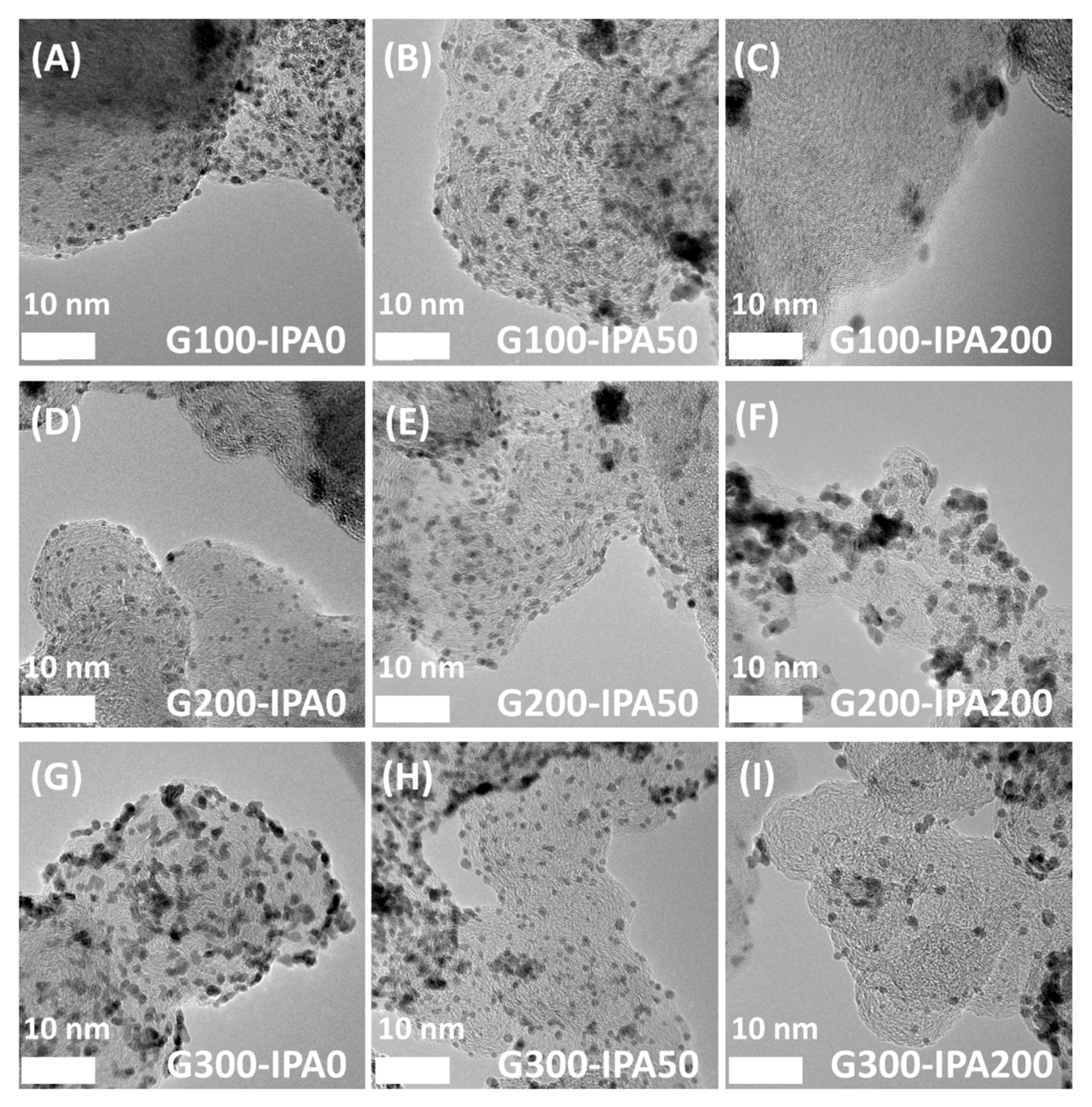
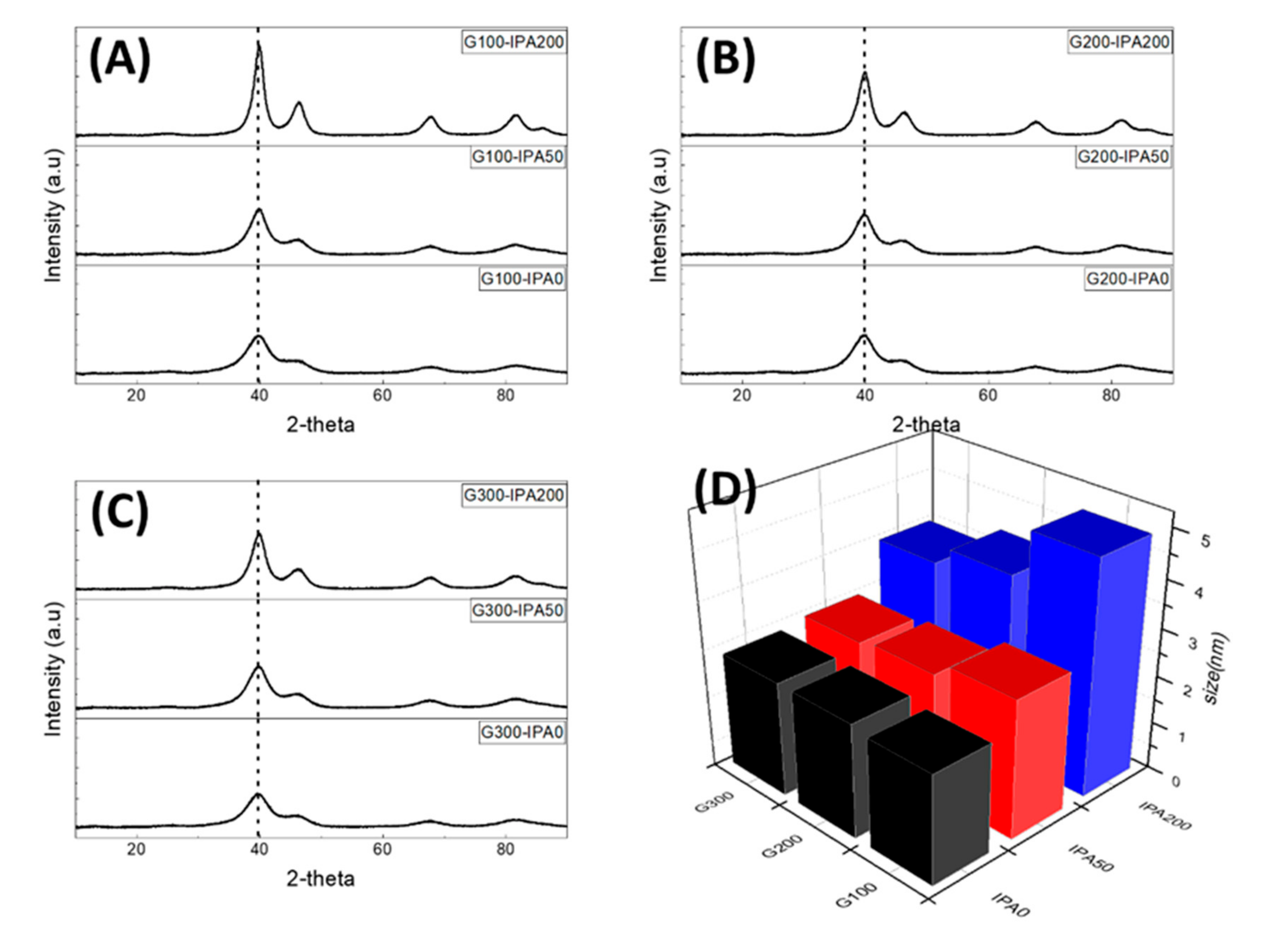
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sharma:, M.; Jang, J.-H.; Shin, D.Y.; Kwon, J.A.; Lim, D.-H.; Choi, D.; Sung, H.; Jang, J.; Lee, S.-Y.; Lee, K.Y. Work function-tailored graphene via transition metal encapsulation as a highly active and durable catalyst for the oxygen reduction reaction. Energy Environ. Sci. 2019, 12, 2200–2211. [Google Scholar] [CrossRef]
- Min, J.; Jeffery, A.A.; Kim, Y.; Jung, N. Electrochemical Analysis for Demonstrating CO Tolerance of Catalysts in Polymer Electrolyte Membrane Fuel Cells. Nanomaterials 2019, 9, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Jeffery, A.A.; Min, J.; Jung, N. Modulating Catalytic Activity and Durability of PtFe Alloy Catalysts for Oxygen Reduction Reaction Through Controlled Carbon Shell Formation. Nanomaterials 2019, 9, 1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Kim, S.-H.; Kim, J.-W.; Lee, M.-H.; Kim, Y.-D. Synthesis of platinum nanoparticles by liquid phase reduction. J. Korean Powder Metall. Inst. 2012, 19, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-N. Synthesis of Electrode Catalyst for Polymer Electrolyte Membrane Fuel Cells Using Colloidal Method. Clean Technol. 2013, 19, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.; Kim, J.W.; Hwang, S.J.; Too, S.J.; Cho, E.; Lim, T.-H.; Kim, S.-K. Fabrication and characterization of high-activity Pt/C electrocatalysts for oxygen reduction. Bull. Korean Chem. Soc. 2010, 31, 1577–1582. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, H.A.; Marković, N.M. Just a dream—Or future reality? Science 2009, 324, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.-J.; Schüth, F. Colloidal metal nanoparticles as a component of designed catalyst. Phys. Chem. Chem. Phys. 2011, 13, 2457–2487. [Google Scholar] [CrossRef]
- Yang, H.; Vogel, W.; Lamy, C.; Alonso-Vante, N. Structure and electrocatalytic activity of carbon-supported Pt−Ni alloy nanoparticles toward the oxygen reduction reaction. J. Phys. Chem. B 2004, 108, 11024–11034. [Google Scholar] [CrossRef]
- Okaya, K.; Yano, H.; Uchida, H.; Watanabe, M. Control of particle size of Pt and Pt alloy electrocatalysts supported on carbon black by the nanocapsule method. ACS Appl. Mater. Interfaces 2010, 2, 888–895. [Google Scholar] [CrossRef]
- Zhang, J.; Lima, F.; Shao, M.; Sasaki, K.; Wang, J.; Hanson, J.; Adzic, R. Platinum monolayer on nonnoble metal-noble metal core-shell nanoparticle electrocatalysts for O2 reduction. J. Phys. Chem. B 2005, 109, 22701–22704. [Google Scholar] [CrossRef]
- Tsuji, M.; Kubokawa, M.; Yano, R.; Miyamae, N.; Tsuji, T.; Jun, M.-S.; Hong, S.; Lim, S.; Yoon, S.-H.; Mochida, I. Fast preparation of PtRu catalysts supported on carbon nanofibers by the microwave-polyol method and their application to fuel cells. Langmuir 2007, 23, 387–390. [Google Scholar] [CrossRef]
- Cha, I.Y.; Ahn, M.; Yoo, S.J.; Sung, Y.-E. Facile synthesis of carbon supported metal nanoparticles via sputtering onto a liquid substrate and their electrochemical application. RSC Adv. 2014, 4, 38575–38580. [Google Scholar] [CrossRef]
- Brault, P.; Caillard, A.; Thomann, A.; Mathias, J.; Charles, C.; Boswell, R.; Escribano, S.; Durand, J.; Sauvage, T. Plasma sputtering deposition of platinum into porous fuel cell electrodes. J. Phys. D Appl. Phys. 2004, 37, 3419. [Google Scholar] [CrossRef] [Green Version]
- Cavarroc, M.; Ennadjaoui, A.; Mougenot, M.; Brault, P.; Escalier, R.; Tessier, Y.; Durand, J.; Roualdès, S.; Sauvage, T.; Coutanceau, C. Performance of plasma sputtered fuel cell electrodes with ultra-low Pt loadings. Electrochem. Commun. 2009, 11, 859–861. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Luo, N.; Miley, G.H. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. J. Power Sources 2007, 173, 77–85. [Google Scholar] [CrossRef]
- Randhawa, H. Cathodic arc plasma deposition technology. Thin Solid Films 1988, 167, 175–186. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, I.; Bachmatiuk, A.; Bezugly, V.; Kunstmann, J.; Gemming, T.; Liu, Z.; Cuniberti, G.; Rümmeli, M. Electron-beam induced synthesis of nanostructures: A review. Nanoscale 2016, 8, 11340–11362. [Google Scholar] [CrossRef] [Green Version]
- Botman, A.; Mulders, J.; Hagen, C. Creating pure nanostructures from electron-beam-induced deposition using purification techniques: A technology perspective. Nanotechnology 2009, 20, 372001. [Google Scholar] [CrossRef] [Green Version]
- Wnuk, J.; Rosenberg, S.; Gorham, J.; Van Dorp, W.; Hagen, C.; Fairbrother, D. Electron beam deposition for nanofabrication: Insights from surface science. Surf. Sci. 2011, 605, 257–266. [Google Scholar] [CrossRef]
- Utke, I.; Hoffmann, P.; Melngailis, J. Gas-assisted focused electron beam and ion beam processing and fabrication. J. Vac. Sci. Technol. B 2008, 26, 1197–1276. [Google Scholar] [CrossRef] [Green Version]
- DeLaRiva, A.T.; Hansen, T.W.; Challa, S.R.; Datye, A.K. In situ Transmission Electron Microscopy of catalyst sintering. J. Catal. 2013, 308, 291–305. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.L.; Niu, K.; Alsem, D.H.; Zheng, H. In situ TEM study of catalytic nanoparticle reactions in atmospheric pressure gas environment. Microsc. Microanal. 2013, 19, 1558–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Chee, S.W.; Sharma, R.; Liu, K.; Xie, X.; Li, Q.; Fan, S.; Jiang, K. In Situ TEM observation of the gasification and growth of carbon nanotubes using iron catalysts. Nano Res. 2011, 4, 767. [Google Scholar] [CrossRef]
- Yasuda, A.; Kawase, N.; Mizutani, W. Carbon-nanotube formation mechanism based on in situ TEM observations. J. Phys. Chem. B 2002, 106, 13294–13298. [Google Scholar] [CrossRef]
- Wang, M.; Park, J.H.; Kabir, S.; Neyerlin, K.C.; Kariuki, N.N.; Lv, H.; Stamenkovic, V.R.; Myers, D.J.; Ulsh, M.; Mauger, S.A. Impact of catalyst ink dispersing methodology on fuel cell performance using in-situ X-ray scattering. ACS Appl. Energy Mater. 2019, 2, 6417–6427. [Google Scholar] [CrossRef]
- Le Caër, S. Water radiolysis: Influence of oxide surfaces on H2 production under ionizing radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, A.M.; Hasse, U.; Hermes, M.; Scholz, F. Hydroxyl radicals attack metallic gold. Angew. Chem. Int. Ed. 2010, 122, 1079–1081. [Google Scholar] [CrossRef]
- Lee, S.; Jang, W.; Kim, M.; Shin, J.E.; Park, H.B.; Jung, N.; Whang, D. Rational Design of Ultrathin Gas Barrier Layer via Reconstruction of Hexagonal Boron Nitride Nanoflakes to Enhance the Chemical Stability of Proton Exchange Membrane Fuel Cells. Small 2019, 15, 1903705. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Q.; Marcus, K.; Hao, Y.; Deng, J.; Bi, K.; Yang, Y. Photocatalytic glycerol oxidation on Aux Cu–CuS@TiO2 plasmonic heterostructures. J. Mater. Chem. A 2018, 6, 22005–22012. [Google Scholar] [CrossRef]
- D’Errico, G.; Ciccarelli, D.; Ortona, O. Effect of glycerol on micelle formation by ionic and nonionic surfactants at 25 °C. J. Colloid Interface Sci. 2005, 286, 747–754. [Google Scholar] [CrossRef]
- Liu, B.; Gao, F. Navigating Glycerol Conversion Roadmap and Heterogeneous Catalyst Selection Aided by Density Functional Theory: A Review. Catalysts 2018, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Borchert, H.; Shevchenko, E.V.; Robert, A.; Mekis, I.; Kornowski, A.; Grübel, G.; Weller, H. Determination of nanocrystal sizes: A comparison of TEM, SAXS, and XRD studies of highly monodisperse CoPt3 particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Álvarez, A.E.; Cadierno, V. Glycerol: A promising green solvent and reducing agent for metal-catalyzed transfer hydrogenation reactions and nanoparticles formation. Appl. Sci. 2013, 3, 55–69. [Google Scholar] [CrossRef]
- Grace, A.N.; Pandian, K. One pot synthesis of polymer protected Pt, Pd, Ag and Ru nanoparticles and nanoprisms under reflux and microwave mode of heating in glycerol—A comparative study. Mater. Chem. Phys. 2007, 104, 191–198. [Google Scholar] [CrossRef]
- Koo, M.; Bae, J.-S.; Kim, H.-C.; Nam, D.-G.; Ko, C.H.; Yeum, J.H.; Oh, W. Electrochemical oxidation of some basic alcohols on multiwalled carbon nanotube–platinum composites. Bull. Mater. Sci. 2012, 35, 545–550. [Google Scholar] [CrossRef]
- Selvaraj, V.; Vinoba, M.; Alagar, M. Electrocatalytic oxidation of ethylene glycol on Pt and Pt–Ru nanoparticles modified multi-walled carbon nanotubes. J. Colloid Interface Sci. 2008, 322, 537–544. [Google Scholar] [CrossRef]
- Sharma, R.; Wang, Y.; Li, F.; Chamier, J.; Andersen, S.M. Particle Size-Controlled Growth of Carbon-Supported Platinum Nanoparticles (Pt/C) through Water-Assisted Polyol Synthesis. Acs Omega 2019, 4, 15711–15720. [Google Scholar] [CrossRef] [Green Version]
- Min, M.; Park, C.; Kim, H.; Kwak, C.; Serov, A.; Kweon, H.; Lee, S. Nano-fabrication and characterization of new conceptual platinum catalysts for low temperature fuel cells. Electrochimica Acta 2006, 52, 1670–1675. [Google Scholar] [CrossRef]
- Dutta, A.; Mondal, A.; Datta, J. Tuning of platinum nano-particles by Au usage in their binary alloy for direct ethanol fuel cell: Controlled synthesis, electrode kinetics and mechanistic interpretation. J. Power Sources 2015, 283, 104–114. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Liang, C.-C.; Chung, C.-P. Characterization of Platinum Nanoparticles Deposited on Functionalized Graphene Sheets. Materials 2015, 8, 6484–6497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoda, E.; Jinnouchi, R.; Hatanaka, T.; Morimoto, Y.; Mitsuhara, K.; Visikovskiy, A.; Kido, Y. The d-Band Structure of Pt Nanoclusters Correlated with the Catalytic Activity for an Oxygen Reduction Reaction. J. Phys. Chem. C 2011, 115, 21236–21240. [Google Scholar] [CrossRef]
- Sharma, M.; Jung, N.; Yoo, S.J. Toward High-Performance Pt-Based Nanocatalysts for Oxygen Reduction Reaction through Organic–Inorganic Hybrid Concepts. Chem. Mater. 2017, 30, 2–24. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Blizanac, B.B.; Arenz, M.; Stamenkovic, V.R.; Ross, A.P.N.; Markovic, N.M. The Impact of Geometric and Surface Electronic Properties of Pt-Catalysts on the Particle Size Effect in Electrocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef] [PubMed]
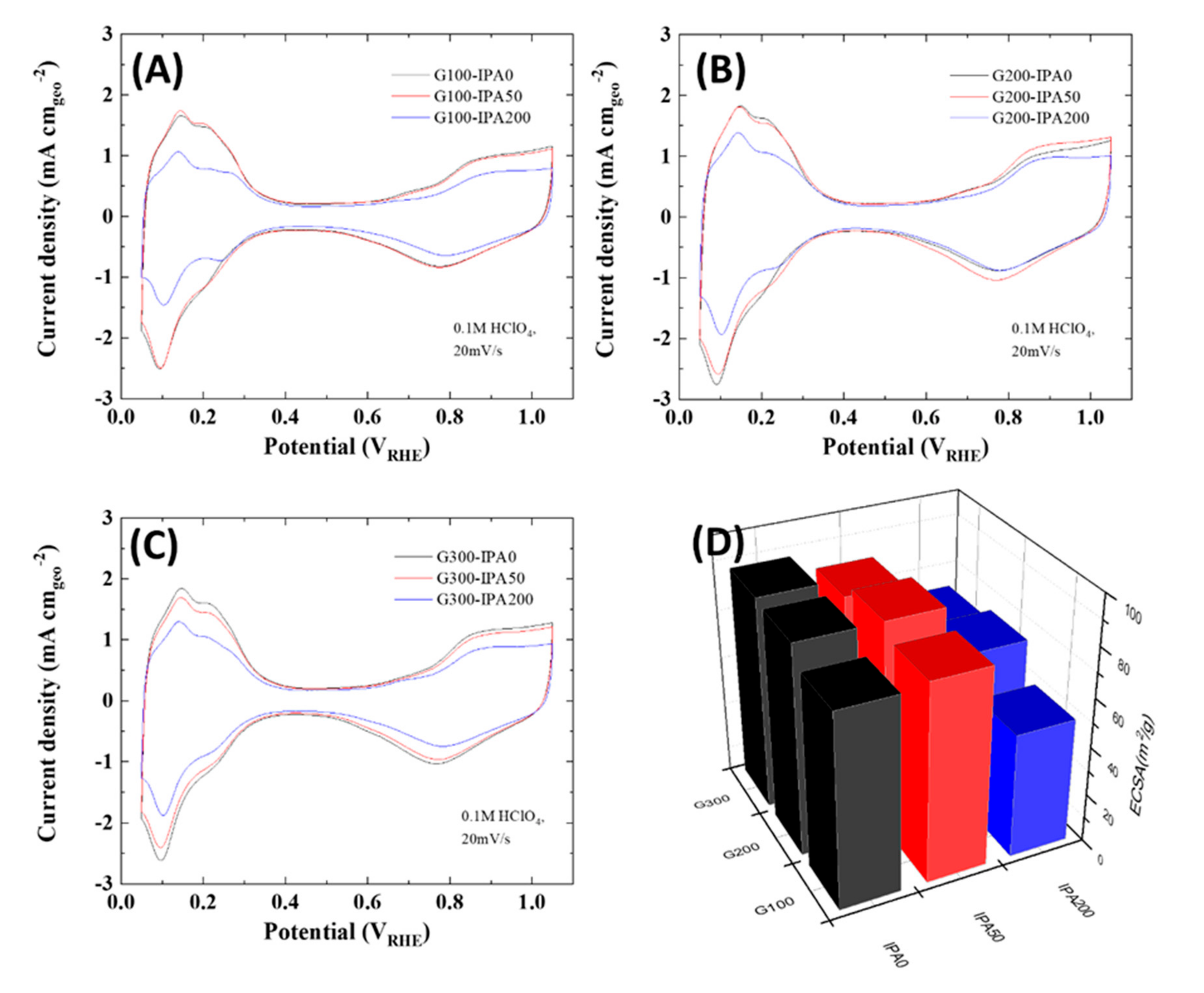
| Samples | Pt Content (wt %) |
|---|---|
| G100-IPA0 | 47.7 |
| G100-IPA50 | 45.3 |
| G100-IPA200 | 46.6 |
| G200-IPA0 | 47.5 |
| G200-IPA50 | 47.5 |
| G200-IPA200 | 49.7 |
| G300-IPA0 | 48.4 |
| G300-IPA50 | 48.3 |
| G300-IPA200 | 49.2 |
| Samples | Particle Size (nm) | Crystallite Size (nm) |
|---|---|---|
| G100-IPA0 | 2.3 | 2.0 |
| G100-IPA50 | 2.9 | 2.3 |
| G100-IPA200 | 4.9 | 3.7 |
| G200-IPA0 | 2.4 | 2.0 |
| G200-IPA50 | 2.6 | 2.3 |
| G200-IPA200 | 3.9 | 3.0 |
| G300-IPA0 | 2.4 | 2.1 |
| G300-IPA50 | 2.5 | 2.2 |
| G300-IPA200 | 3.5 | 2.8 |
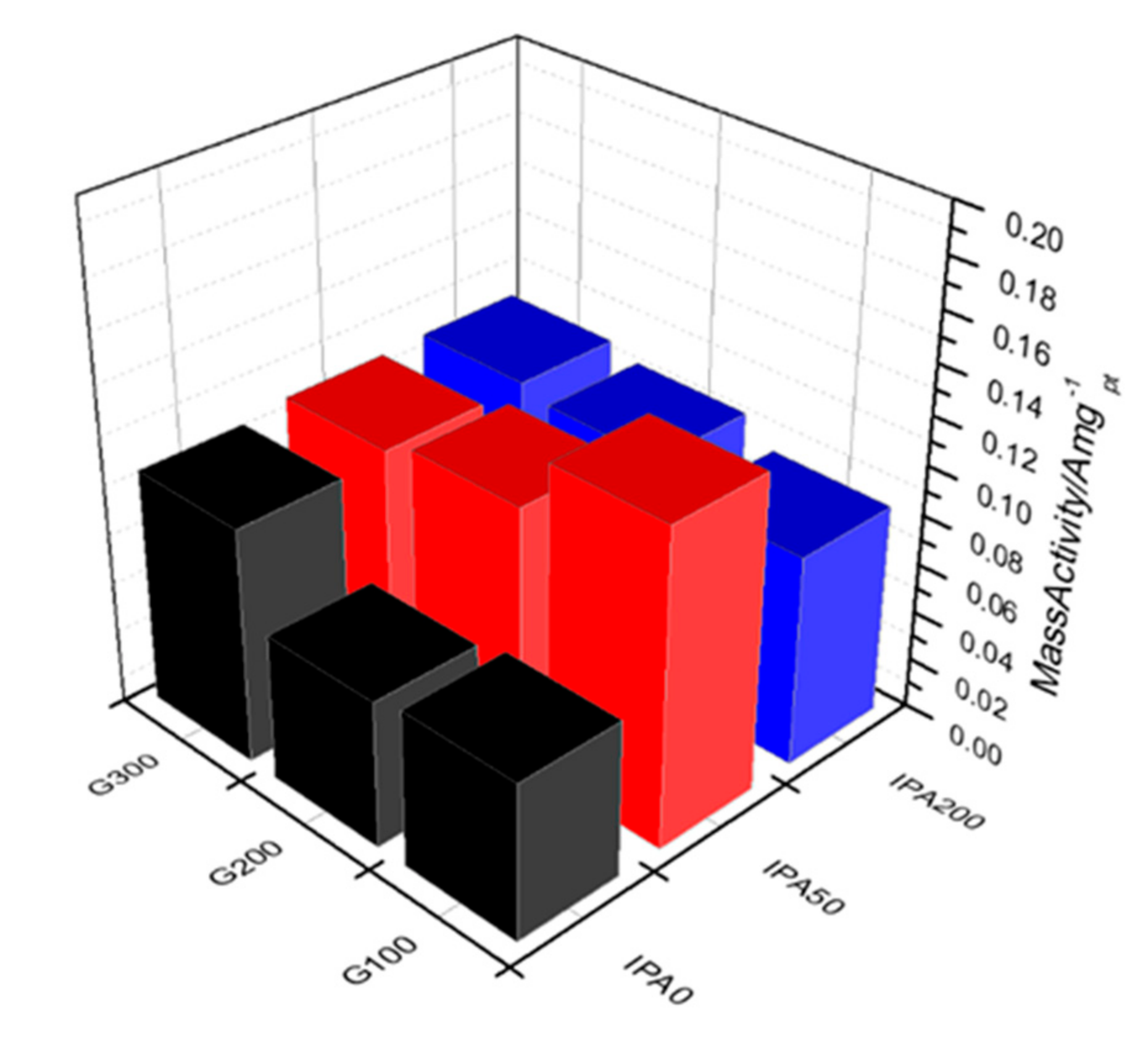
| Samples | Half-Wave Potential (V) | Mass Activity (A/mgPt at 0.9 VRHE) | ECSA (m2/gPt) |
|---|---|---|---|
| G100-IPA0 | 0.906 | 0.063 | 78.7 |
| G100-IPA50 | 0.926 | 0.127 | 80.7 |
| G100-IPA200 | 0.917 | 0.085 | 51.0 |
| G200-IPA0 | 0.901 | 0.059 | 86.1 |
| G200-IPA50 | 0.924 | 0.104 | 86.3 |
| G200-IPA200 | 0.922 | 0.095 | 65.8 |
| G300-IPA0 | 0.922 | 0.095 | 87.4 |
| G300-IPA50 | 0.920 | 0.098 | 80.5 |
| G300-IPA200 | 0.922 | 0.099 | 61.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Min, J.; Kim, Y.; Lee, J.H.; Chai, G.; Jung, N. Facile Strategy for Mass Production of Pt Catalysts for Polymer Electrolyte Membrane Fuel Cells Using Low-Energy Electron Beam. Nanomaterials 2020, 10, 2216. https://doi.org/10.3390/nano10112216
Shin J, Min J, Kim Y, Lee JH, Chai G, Jung N. Facile Strategy for Mass Production of Pt Catalysts for Polymer Electrolyte Membrane Fuel Cells Using Low-Energy Electron Beam. Nanomaterials. 2020; 10(11):2216. https://doi.org/10.3390/nano10112216
Chicago/Turabian StyleShin, Jongmin, Jiho Min, Youngjin Kim, Jin Hee Lee, Geunseok Chai, and Namgee Jung. 2020. "Facile Strategy for Mass Production of Pt Catalysts for Polymer Electrolyte Membrane Fuel Cells Using Low-Energy Electron Beam" Nanomaterials 10, no. 11: 2216. https://doi.org/10.3390/nano10112216
APA StyleShin, J., Min, J., Kim, Y., Lee, J. H., Chai, G., & Jung, N. (2020). Facile Strategy for Mass Production of Pt Catalysts for Polymer Electrolyte Membrane Fuel Cells Using Low-Energy Electron Beam. Nanomaterials, 10(11), 2216. https://doi.org/10.3390/nano10112216