Graphene Oxide Membranes for Trace Hydrocarbon Contaminant Removal from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Methodology
2.3. Lab Setup of Membrane Module
2.4. Characterization
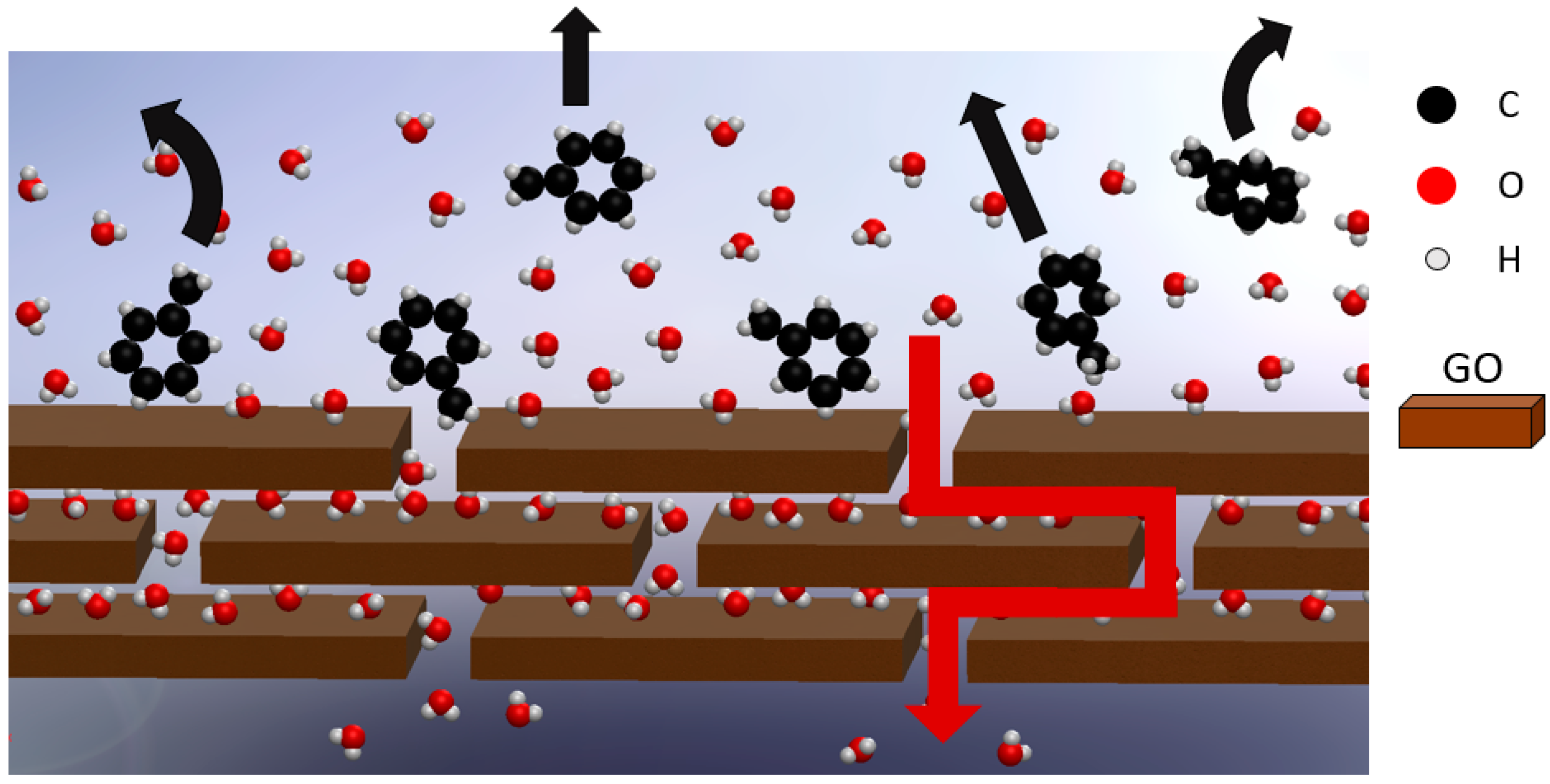
3. Results and Discussion
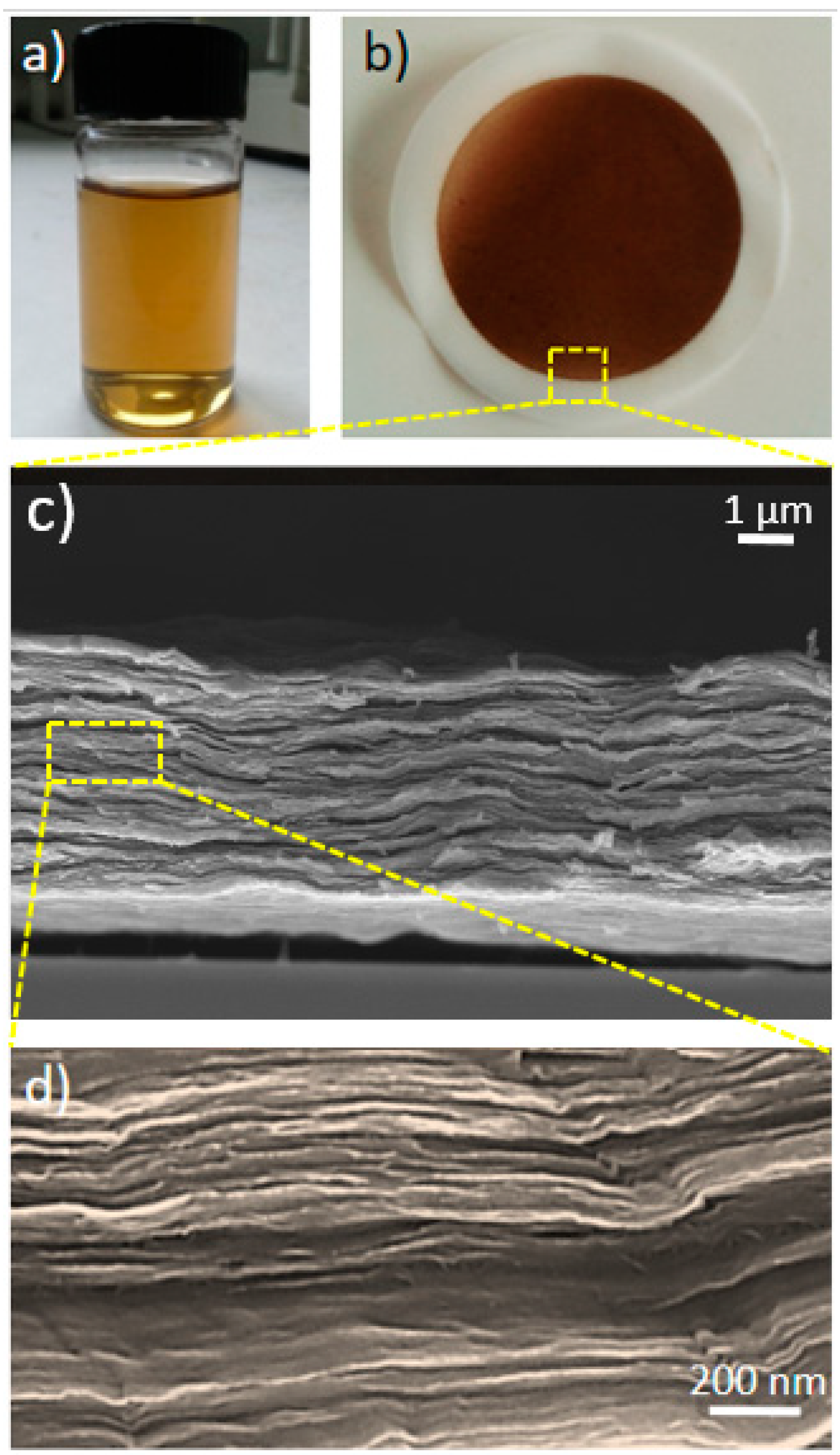
3.1. Membrane Fabrication
3.2. Membrane Characterization: Interlayer Distance, Z-potential, Surface Area
3.3. Water Permeation Measurements
3.4. Stability Test
3.5. Filtration Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tanugi, D.; Grossman, J.C. Mechanical Strength of Nanoporous Graphene as Desalination Membrane. Nano Lett. 2014, 14, 6171–6178. [Google Scholar] [CrossRef] [PubMed]
- Surwade, S.; Smirnov, S.; Vlassiouk, I.; Unocic, R.; Veith, G.; Dai, S.; Mahurin, S. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Gao, X.; Ma, Z.; Wang, X.; Wei, Y.; Gao, C. A review of graphene-based separation membrane: Materials, characteristics, preparation and applications. Desalination 2018, 437, 59–72. [Google Scholar] [CrossRef]
- Aghigh, A.; Alizadeh, V.; Wong, H.Y.; Islam, M.S.; Amin, N.; Zaman, M. Recent advances in utilization of graphene for filtration and desalination of water: A review. Desalination 2015, 365, 389–397. [Google Scholar] [CrossRef]
- Nicolai, A.; Sumpter, G.B.; Meunir, V. Tunable water desalination across graphene oxide framework membranes. Phys. Chem. Chem. Phys. 2014, 16, 8646–8654. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, R.; Chase-Woods, D.; Shin, Y.; Gotthold, D.W. Molecular Dynamics Simulations Reveal that Water Diffusion between Graphene Oxide Layer is Slow. Sci. Rep. 2016, 6, 29848. [Google Scholar]
- Lin, L.; Grossman, J.C. Atomistic understandings of reduced graphene oxide as an ultrathin-film nanoporous membrane for separation. Nat. Commun. 2016, 6, 8335. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Ma, J.; Ping, D.; Dong, X. Recent developments of graphene oxide-based membranes: A review. Membranes 2017, 7, 52. [Google Scholar]
- Jang, J.H.; Woo, J.Y.; Lee, J.; Han, C. Ultrathin graphene oxide membranes for water purification. In Proceedings of the 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO), Rome, Italy, 27–30 July 2015; pp. 212–215. [Google Scholar]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, K.A.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2015, 343, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Huang, L.; Wang, Y.; Du, Y.; Zhang, Z.; Wang, Y.; Kipper, M.; Belfiore, L.; Tang, J. Graphene oxide nanofiltration membranes containing silver nanoparticles: Tuning separation efficiency via nanoparticle size. Nanomaterials 2020, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, D.; Wang, C.; Liu, H.; Qu, L.; Li, H. A novel nanocomposite membrane combining BN nanosheets and GO for effective removal of antibiotic in water. Nanomaterials 2019, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Yun, J.; Seo, Y.; Byun, H.; Houa, J.; Kim, J. Mixed Dye Removal Efficiency of Electrospun Polyacrylonitrile–Graphene Oxide Composite Membranes. Polymers 2020, 12, 2009. [Google Scholar] [CrossRef]
- Sun, P.; Ma, R.; Deng, H.; Song, Z.; Zhen, Z.; Wang, K.; Sasaki, T.; Xu, Z.; Zhu, H. Intrinsic high water/ion selectivity of graphene oxide lamellar membranes in concentration gradient-driven diffusion. Chem. Sci. 2016, 7, 6899–6994. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nanofiltration Membrane Intercalated by Carbon Nanotubes. Acs Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef]
- Alnoor, O.; Laoui, T.; Ibrahim, A.; Kafiah, F.; Nadhreen, G.; Akhtar, S.; Khan, Z. Graphene Oxide-Based Membranes for Water Purification Applications: Effect of Plasma Treatment on the Adhesion and Stability of the Synthesized Membranes. Membranes 2020, 10, 292. [Google Scholar] [CrossRef]
- Nakagawa, K.; Araya, S.; Kunimatsu, M.; Yoshioka, T.; Shintani, T.; Kamio, E.; Matsuyama, H. Fabrication of Stacked Graphene Oxide Nanosheet Membranes Using Triethanolamine as a Crosslinker and Mild Reducing Agent for Water Treatment. Membranes 2018, 8, 130. [Google Scholar] [CrossRef]
- Lawler, J. Incorporation of Graphene-Related Carbon Nanosheets in Membrane Fabrication for Water Treatment: A Review. Membranes 2016, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.C.; Chen, Y.-C.; Yang, H.-X.; Yen, C.-H. Performance and mechanisms for the removal of phthalates and pharmaceuticals from aqueous solution by graphene-containing ceramic composite tubular membrane coupled with the simultaneous electrocoagulation and electrofiltration process. Chemosphere 2016, 155, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hang, L.; Wang, L.; Qiao, Y.; Tang, C.; Jung, C.; Yoon, Y.; Li, S.; Yu, M. Ultrafiltration Membranes with Structure-Optimized Graphene-Oxide Coatings for Antifouling Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1400433. [Google Scholar] [CrossRef]
- Zinadini, S.; Vatanpour, V.; Zinatizadeh, A.A.; Rahimi, Z.; Kian, M. Preparation and characterization of antifouling graphene oxide/polyethersulfone ultrafiltration membrane: Application in MBR for diary wastewater treatment. J. Water Process Eng. 2015, 7, 280–284. [Google Scholar] [CrossRef]
- Ranjan, P.; Agrawal, S.; Sinha, A.; Rao, T.R.; Thakur, A.D. A Low-Cost Non-explosive Synthesis of Graphene Oxide for Scalable Applications. Sci. Rep. 2018, 8, 12007. [Google Scholar] [CrossRef]
- Le, T.X.H.; Dumée, L.F.; Lacour, S.; Rivallin, M.; Yi, Z.; Kong, L.; Bechelany, M.; Cretin, M. Hybrid graphene-decorated metal hollow fibre membrane reactors for efficient electro-Fenton—Filtration co-processes. J. Membr. Sci. 2019, 587, 117182. [Google Scholar]
- Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Gassara, S.; Cambedouzou, J.; Bechelany, M.; Miele, P. Development of novel h-BNNS/PVA porous membranes via Pickering emulsion templating. Green Chem. 2018, 20, 4319–4329. [Google Scholar] [CrossRef]
- Castro, C.; Cocuzza, M.; Lamberti, A.; Laurenti, M.; Pedico, A.; Pirri, C.F.; Rocca, V.; Borello, E.S.; Scaltrito, L.; Serazio, C.; et al. Graphene-Based Membrane Technology: Reaching Out to the Oil and Gas Industry. Geofluids 2018, 2018, 7026426. [Google Scholar] [CrossRef]
- Reddy, C.M.; Quinn, J.G. GC-MS analysis of total petroleum hydrocarbons and polycyclic aromatic hydrocarbons in seawater samples after the North Cape oil spill. Mar. Pollut. Bull. 1999, 38, 126–135. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Zhou, J.; Wang, Y.; Liang, J.; Zhang, X.; Chang, Q.; Song, L. The improved oil/water separation performance of graphene oxide modified Al2O3 microfiltration membrane. J. Membr. Sci. 2015, 476, 200–204. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, W.; Liu, Y.; Qin, C.; Meng, M.; Jiang, Y.; Qiu, J.; Peng, J. A mussel inspired highly stable graphene oxide membrane for efficient oil-in-water emulsions separation. Sep. Purif. Technol. 2018, 199, 37–46. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, X.; Xue, Q.; He, D.; Zhu, L.; Guo, Q. Antifouling hydrolyzed polyacrylonitrile/graphene oxide membrane with spindle-knotted structure for highly effective separation of oil-water emulsion. J. Membr. Sci. 2017, 532, 38–46. [Google Scholar] [CrossRef]
- Gebreslase, G.A.; Bousquet, G.; Bouyer, D. Review on Membranes for the Filtration of Aqueous Based Solution: Oil in Water Emulsion. J. Membr. Sci. Technol. 2018, 8, 1000188. [Google Scholar] [CrossRef]
- Kocherginsky, N.M.; Tan, C.L.; Lu, W.F. Demulsification of water-in-oil emulsions via filtration through a hydrophilic polymer membrane. J. Membr. Sci. 2003, 220, 117–128. [Google Scholar] [CrossRef]
- Zeiger, C.; Kumberg, J.; Vullers, F.; Worgull, M.; Holscher, H.; Kavalenka, M.N. Selective filtration of oil/water mixtures with bioinspired porous membranes. Rsc Adv. 2017, 7, 32806–32811. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G. Cross flow microfiltration of oil-water emulsions using clay based ceramic membrane support and TiO2 composite membrane. Egypt. J. Pet. 2017, 26, 679–694. [Google Scholar] [CrossRef]
- Milic, J.K.; Petrinic, I.; Gorsek, A.; Simonic, M. Ultrafiltration of oil-in-water emulsion by using ceramic membrane: Taguchi experimental design approach. Cent. Eur. J. Chem. 2014, 12, 242–249. [Google Scholar]
- Nasiri, M.; Jafari, I. Produced Water from Oil-Gas Plants: A Short Review on Challenges and Opportunities. Period. Polytech. Chem. Eng. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Tang, Y.; Paul, D.R.; Chung, T.S. Free-standing graphene oxide thinfilms assembled by a pressurized ultrafiltration method for dehydration of ethanol. J. Membr. Sci. 2014, 458, 199–208. [Google Scholar] [CrossRef]
- Solomon, B.R.; Hyder, M.N.; Varanasi, K.K. Separating Oil-Water Nanoemulsions using Flux-Enhanced Hierarchical Membranes. Sci. Rep. 2014, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [PubMed]
- Chong, J.Y.; Wang, B.; Mattevi, C.; Li, K. Dynamic microstructure of graphene oxide membranes and the permeation flux. J. Membr. Sci. 2018, 549, 358–392. [Google Scholar]
- Wei, Y.; Zhang, Y.; Gao, X.; Yuan, Y.; Su, B.; Gao, C. Declining flux and narrowing nanochannels under wrinkles of compacted graphene oxide nanofiltration membranes. Carbon 2016, 108, 568–575. [Google Scholar]
- Tsou, C.; An, Q.; Lo, S.; De Guzman, M.; Hung, W.; Hu, C.; Lee, K.; Lai, J. Effect of microstructure of graphene oxide fabricated through different self-assembly techniques on 1-butanol dehydration. J. Membr. Sci. 2015, 477, 93–100. [Google Scholar]
- Chong, J.Y.; Aba, N.F.D.; Wang, B.; Mattevi, C.; Li, K. UV-Enhanced Sacrificial Layer Stabilised Graphene Oxide Hollow Fibre Membranes for Nanofiltration. Sci. Rep. 2015, 5, 15799. [Google Scholar]
- Neir, R.R.; Wu, H.A.; Jayaram, N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water Through Helium-Leak-Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar]
- Rezania, B.; Severin, N.; Talyzin, A.V.; Rabe, J. Hydration of Bilayered Graphene Oxide. Nano Lett. 2014, 14, 3993–3998. [Google Scholar]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar]
- Kipling, J.J.; Wilson, R.B. Adsorption of Methylene Blue in the determination of surface areas. J. Appl. Chem. 1960, 10, 109–113. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyes, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphene oxide. Carbon 2007, 45, 1558–1565. [Google Scholar]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Zuttel, A.; Sudan, P.; Mauron, P.; Wenger, P. Model for the hydrogen adsorption on carbon nanostructures. Appl. Phys. A 2004, 78, 941–946. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaria, R.; Menendez, R.; Corma, A.; Garcia, H. Surface Area Measurement of Graphene Oxide in Aqueous Solutions. Langmuir 2013, 29, 13443–13448. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Sheath, P.; Martin, S.T.; Shinde, D.B.; Shaibani, M.; Banarjee, C.; Tkacz, R.; Bhattacharyya, D.; Majumder, M. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat. Commun. 2016, 7, 10891. [Google Scholar] [CrossRef] [PubMed]
- Sanemasa, I.; Araki, M.; Deguchi, T.; Nagai, H. Solubility Measurements of Benzene and the Alkylbenzenes in Water by Making Use of Solute Vapor. Bull. Chem. Soc. Jpn. 1982, 55, 1054–1062. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, M.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Xu, Z.; Zhu, H. Selective Ion Penetration of Graphene Oxide Membranes. Acs Nano 2012, 7, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Stenutz, R. Tables of Properties of Chemical Compounds. Available online: http://www.stenutz.eu/chem/index.php (accessed on 1 October 2020).
- Kim, S.; Thiessen, A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound database. Nucleic Acids Res. 2016, 44, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Su, Y.; Chi, C.; Cherian, C.T.; Huang, K.; Kravets, V.G.; Wang, F.C.; Zhang, J.C.; Pratt, A.; Grigorenko, A.N.; et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nat. Mater. 2017, 16, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wu, H.; Tao, L.; Qu, L.; Li, C. Enhanced stability and separation efficiency of graphene oxide membranes in organic solvent nanofiltration. J. Mater. Chem. A 2018, 40, 7. [Google Scholar] [CrossRef]



| Membrane | Solution | Concentration | Separation | Source |
|---|---|---|---|---|
| Al2O3/GO | Machine oil | 1 g/L | Crossflow | [31] |
| GO/polymers | Mineral oil, toluene, hexane, chloroform | 3.33 g/L | Vacuum | [32] |
| PAN/GO | Lubricating oil | 1 g/L | Crossflow | [33] |
| Polymeric | Kerosene | 50 g/L | Tangential flow | [35] |
| TiO2/ceramic | Crude oil | 200 mg/L | Crossflow | [37] |
| Al2O3/ZrO2 | Cutting oil | 5 g/L | Tangential flow | [38] |
| GO | H2O in EtOH | 0~100% | Pervaporation | [40] |
| Polymeric | H2O in hexadecane | 97% | Crossflow | [41] |
| GO | Toluene, methylcyclohexane | 1 mg/L | Crossflow | This work |
| Solution | Concentration | Effect |
|---|---|---|
| DI H2O | 100% | None |
| HCl | 0.01 M | None |
| HNO3 | 0.01 M | None |
| NaOH | 0.01 M | Damage |
| NaCl | 0.6 M | None |
| Toluene | 100 ppm | None |
| Ethylene glycol | 10% | None |
| Ethanol | 10% | None |
| Acetone | 10% | None |
| Molecule | Molecular Diameter (nm) | Dipole Moment (D) | Concentration | Rejection (%) |
|---|---|---|---|---|
| Toluene | 0.696 | 0.31 | 100 ppm | 84 ± 4 |
| Toluene | 0.696 | 0.31 | 1 ppm | 90 ± 2 |
| Methylcyclohexane | 0.740 | 0.00 | 1 ppm | 97 ± 1 |
| Methanol | 0.505 | 2.87 | 5~30% | <5 |
| Ethanol | 0.570 | 1.66 | 5~30% | <5 |
| Acetone | 0.615 | 2.69 | 5~30% | <5 |
| Ethylene glycol | 0.561 | 2.27 | 5~30% | <5 |
| Triethylene glycol | 0.751 | 2.99 | 5~30% | 20 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedico, A.; Fontana, M.; Bianco, S.; Kara, S.; Periolatto, M.; Carminati, S.; Pirri, C.F.; Tresso, E.; Lamberti, A. Graphene Oxide Membranes for Trace Hydrocarbon Contaminant Removal from Aqueous Solution. Nanomaterials 2020, 10, 2242. https://doi.org/10.3390/nano10112242
Pedico A, Fontana M, Bianco S, Kara S, Periolatto M, Carminati S, Pirri CF, Tresso E, Lamberti A. Graphene Oxide Membranes for Trace Hydrocarbon Contaminant Removal from Aqueous Solution. Nanomaterials. 2020; 10(11):2242. https://doi.org/10.3390/nano10112242
Chicago/Turabian StylePedico, Alessandro, Marco Fontana, Stefano Bianco, Seifeddine Kara, Monica Periolatto, Stefano Carminati, Candido Fabrizio Pirri, Elena Tresso, and Andrea Lamberti. 2020. "Graphene Oxide Membranes for Trace Hydrocarbon Contaminant Removal from Aqueous Solution" Nanomaterials 10, no. 11: 2242. https://doi.org/10.3390/nano10112242
APA StylePedico, A., Fontana, M., Bianco, S., Kara, S., Periolatto, M., Carminati, S., Pirri, C. F., Tresso, E., & Lamberti, A. (2020). Graphene Oxide Membranes for Trace Hydrocarbon Contaminant Removal from Aqueous Solution. Nanomaterials, 10(11), 2242. https://doi.org/10.3390/nano10112242








