Langmuir Films of Perfluorinated Fatty Alcohols: Evidence of Spontaneous Formation of Solid Aggregates at Zero Surface Pressure and Very Low Surface Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecules and Sample Preparation
2.2. Atomic Force Microscopy (AFM)
2.3. Grazing Incidence X-ray Diffraction (GIXD)
2.4. Simulation Details
3. Results
3.1. Thermodynamic Study
3.2. Grazing Incidence X-ray Diffraction Results
3.3. Atomic Force Microscopy Results
3.4. Molecular Dynamics Simulations Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwan, J.; Kaya, M.; Borden, M.A.; Dayton, P.A. Theranostic Oxygen Delivery Using Ultra-sound and Microbubbles. Theranostics 2012, 2, 1174–1184. [Google Scholar] [CrossRef] [Green Version]
- Riess, G.; Le Blanc, M. Solubility and Transport Phenomena in Perfluorochemicals Relevant to Blood Substitution and other Biomedical Applications. Pure Appl. Chem. 1982, 54, 2383–2406. [Google Scholar] [CrossRef]
- Tsagogiorgas, C.; Anger, F.; Beck, G.; Breedijk, A.; Yard, B.; Hoeger, S. Impact of different emulsifiers on biocompatibility and inflammatory potential of Perfluorohexyloctane (F6H8) emulsions for new intravenous drug delivery systems. Drug Des. Dev. Ther. 2019, 13, 2097–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rychak, J.J.; Klibanov, A.L. Nucleic acid delivery with microbubbles and ultrasound. Adv. Drug Deliv. Rev. 2014, 72, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P. Fluorine in medical microbubbles—Methodologies implemented for engineering and investigating fluorocarbon-based microbubbles. J. Fluor Chem. 2015, 177, 19–28. [Google Scholar] [CrossRef]
- Riess, J.G. Fluorocarbon-based injectable gaseous microbubbles for diagnosis and therapy. Curr. Opin. Coll. Interface Sci. 2003, 8, 259–266. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated Surfactants: Synthesis, Properties, Applications, 1st ed.; Marcel Dekker: New York, NY, USA, 1994; ISBN 978-0824790110. [Google Scholar]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Kaganer, V.M.; Möhwald, H.; Dutta, P. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 1999, 71, 779–819. [Google Scholar] [CrossRef] [Green Version]
- Goldmann, M.; Nassoy, P.; Rondelez, F. Search for perfectly ordered dense monolayers. Physica A 1993, 200, 688–695. [Google Scholar] [CrossRef]
- Acero, A.A.; Li, M.; Lin, B.; Rice, S.A.; Goldmann, M.; Azouz, I.B.; Goudot, A.; Rondelez, F. Molecular packing in water supported monolayers of F(CF2)11COOH and F(CF2)10CH2COOH. J. Chem. Phys. 1993, 99, 7214–7220. [Google Scholar] [CrossRef]
- Fontaine, P.; Filipe, E.J.M.; Fauré, M.-C.; Rego, T.; Taßler, S.; Alves, A.C.; Silva, G.M.C.; Morgado, P.; Goldmann, M. Structure of Langmuir Monolayers of Perfluorinated Fatty Acids: Evidence of a New 2D Smectic C Phase. Molecules 2019, 24, 3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoder, N.C.; Kalsani, V.; Schuy, S.; Vogel, R.; Janshoff, A.; Kumar, K. Nanoscale Patterning in Mixed Fluorocarbon–Hydrocarbon Phospholipid Bilayers. J. Am. Chem. Soc. 2007, 129, 9037–9043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krafft, M.P.; Goldmann, M. Monolayers made from fluorinated amphiphiles. Curr. Opin. Coll. Interface Sci. 2003, 8, 243–250. [Google Scholar] [CrossRef]
- Paczesny, J.; Niton, P.; Zywocinski, A.; Sozanski, K.; Holyst, R.; Fialkowski, M.; Kieffer, R.; Glettner, B.; Tschierske, C.; Pociecha, D.; et al. Stable, ordered multilayers of partially fluorinated bolaamphiphiles at the air-water interface. Soft Matter. 2012, 8, 5262–5272. [Google Scholar] [CrossRef]
- Paczesny, J.; Sozanski, K.; Zywocinski, A.; Holyst, R.; Glettner, B.; Kieffer, R.; Tschierske, C.; Nikiforov, K.; Pociecha, D.; Gorecka, E. Spontaneous self-assembly of partially fluorinated bolaamphiphiles into ordered layered structures. Phys. Chem. Chem. Phys. 2012, 14, 14365–14373. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P. Modern Fluoroorganic Chemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004; ISBN 978-3527331666. [Google Scholar]
- Bunn, C.W.; Lowells, E.R. Structure of molecules and crystals of fluorocarbons. Nature 1954, 4429, 549–551. [Google Scholar] [CrossRef]
- Jang, S.S.; Blanco, M.; Goddard III, W.A.; Caldwell, G.; Ross, R.B. The Source of Helicity in Perfluorinated N-Alkanes. Macromolecules 2003, 36, 5331. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Lima, C.F.R.A.C.; Mendes, A.; Santos, L.M.N.B.F. Fluorination effect on the thermodynamic properties of long-chain hydrocarbons and alcohols. J. Chem. Thermodyn. 2016, 102, 378–385. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Morgado, P.; Haley, J.D.; Montoya, V.M.T.; McCabe, C.; Martins, L.F.G.; Filipe, E.J.M. Vapor pressure and liquid density of fluorinated alcohols: Experimental, simulation and GC-SAFT-VR predictions. Fluid Phase Equilib. 2016, 425, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Simons, J.H.; Dunlap, R.D. The Properties of n-Pentforane and Its Mixtures with n-Pentane. J. Chem. Phys. 1950, 18, 335–346. [Google Scholar] [CrossRef]
- Rowlinson, J.S.; Swinton, F.L. Liquids and Liquid Mixtures, 3rd ed.; Butterworth Scientific: London, UK, 1982. [Google Scholar]
- Siebert, E.M.D.; Knobler, C.M. Interaction virial coefficients in hydrocarbon-fluorocarbon mixtures. J. Phys. Chem. 1971, 75, 3863–3870. [Google Scholar] [CrossRef]
- Brode, S.; McDonald, I.R. Excess thermodynamic properties of liquid mixtures of methane and perfluoromethane. Mol. Phys. 1988, 65, 1007–1012. [Google Scholar] [CrossRef]
- Schoen, M.; Hoheisel, C.; Beyer, O. Liquid CH4, liquid CF4 and the partially miscible liquid mixture CH4/CF4. Mol. Phys. 1986, 58, 699–709. [Google Scholar] [CrossRef]
- Duce, C.; Tinè, M.R.; Lepori, L.; Matteoli, E. VLE and LLE of perfluoroalkane + alkane mixtures. Fluid Phase Equilib. 2002, 199, 197–212. [Google Scholar] [CrossRef]
- Schneider, G.M. High-pressure phase equilibria and spectroscopic investigations up to 200 MPa on fluid mixtures containing fluorinated compounds: A review. Fluid Phase Equilib. 2002, 199, 307–317. [Google Scholar] [CrossRef]
- Morgado, P.; Black, J.; Lewis, J.B.; Iacovella, C.R.; McCabe, C.; Martins, L.F.G.; Filipe, E.J.M. Viscosity of liquid systems involving hydrogenated and fluorinated substances: Liquid mixtures of (hexane + perfluorohexane). Fluid Phase Equilib. 2013, 358, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Handa, T.; Mukerjee, P. Surface tensions of nonideal mixtures of fluorocarbons and hydrocarbons and their interfacial tensions against water. J. Phys. Chem. 1981, 85, 3916–3920. [Google Scholar] [CrossRef]
- Morgado, P.; Martins, L.F.G.; Filipe, E.J.M. From nano-emulsions to phase separation: Evidence of nanosegregation in (Alkane + Perfluoroalkane) mixtures using 129Xe NMR Spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 3742–3751. [Google Scholar] [CrossRef]
- Morgado, P.; Barras, J.; Filipe, E.J.M. From nano-seggregation to mesophases: Probing the liquid structure of perfluoroalkylalkanes with 129Xe NMR spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 14736–14747. [Google Scholar] [CrossRef]
- Morgado, P.; Bonifácio, R.P.; Martins, L.F.G.; Filipe, E.J.M. Probing the Structure of Liquids with 129Xe NMR Spectroscopy: N-Alkanes, Cycloalkanes, and Branched Alkanes. J. Phys. Chem. B 2013, 117, 9014–9024. [Google Scholar] [CrossRef] [Green Version]
- Morgado, P.; Garcia, A.R.; Martins, L.F.G.; Ilharco, L.M.; Filipe, E.J.M. Alkane Coiling in Perfluoroalkane Solutions: A New Primitive Solvophobic Effect. Langmuir 2017, 33, 11429–11435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgado, P.; Garcia, A.R.; Ilharco, L.M.; Marcos, J.; Anastácio, M.; Martins, L.F.G.; Filipe, E.J.M. Liquid mixtures involving hydrogenated and fluorinated alcohols: Thermodynamics, spectroscopy, and simulation. J. Phys. Chem. B 2016, 120, 10091–10105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, O.; Yamamoto, S.K.; Lee, S.; Sugihara, G. Mixed Monolayer Properties of Tetradecanoic Acid with n-Perfluorocarboxylic Acids with 10, 12, 14, 16, and 18 Carbon Atoms. J. Coll. Interface Sci. 1996, 184, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Imae, T.; Takeshita, T.; Kato, M. Phase Separation in Hybrid Langmuir–Blodgett Films of Per-fluorinated and Hydrogenated Amphiphiles. Examination by Atomic Force Microscopy. Langmuir 2000, 16, 612–621. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tanaka, K.; Azumi, R.; Kondo, Y.; Yoshino, N. Structure of Phase-Separated Langmuir—Blodgett Films of Hydrogenated and Perfluorinated Carboxylic Acids Investigated by IR Spectroscopy, AFM, and FFM. Langmuir 2003, 19, 2802–2807. [Google Scholar] [CrossRef]
- Rontu, N.; Vaida, V. Miscibility of Perfluorododecanoic Acid with Organic Acids at the Air–Water Interface. J. Phys. Chem. C 2007, 111, 9975–9980. [Google Scholar] [CrossRef]
- Qaqish, S.E.; Paige, M.F. Structural and Compositional Mapping of a Phase-Separated Langmuir–Blodgett Monolayer by Atomic Force Microscopy. Langmuir 2007, 23, 2582–2587. [Google Scholar] [CrossRef]
- Christensen, S.; Lanke, U.D.; Haines, B.; Qaqish, S.E.; Paige, M.F.; Urquhart, S.G. Structural and compositional mapping of a phase-separated Langmuir–Blodgett monolayer by X-ray photo-electron emission microscopy. J. Electron Spectros. Relat. Phenom. 2008, 162, 107–114. [Google Scholar] [CrossRef]
- Rehman, J.; Araghi, H.Y.; He, A.; Paige, M.F. Morphology and composition of structured, phase-separated behenic acid–perfluorotetradecanoic acid monolayer films. Langmuir 2016, 32, 5341–5349. [Google Scholar] [CrossRef]
- Takiue, T.; Vollhardt, D. Miscibility of alkanol and fluoroalkanol in Langmuir film at the air/water interface. Coll. Surf. A Physicochem. Eng. Asp. 2002, 798, 797–804. [Google Scholar] [CrossRef]
- Sowah-Kuma, D.; Paige, M.F. The influence of surfactant head group on miscibility in mixed hydrocarbon-perfluorocarbon monolayers. Coll. Surf. A Physicochem. Eng. Asp. 2018, 556, 157–166. [Google Scholar] [CrossRef]
- Gamboa, A.L.S.; Filipe, E.J.M.; Brogueira, P. Nanoscale Pattern Formation in Langmuir-Blodgett Films of a Semifluorinated Alkane and a Polystyrene-Poly(Ethylene Oxide) Diblock Copolymer. Nano Lett. 2002, 2, 1083–1086. [Google Scholar] [CrossRef]
- Zhang, G.; Marie, P.; Maaloum, M.; Muller, P.; Benoit, N.; Krafft, M.P. Occurrence, Shape, and Dimensions of Large Surface Hemimicelles Made of Semifluorinated Alkanes. Elongated versus Circular Hemimicelles. Pit- and Tip-Centered Hemimicelles. J. Am. Chem. Soc. 2005, 127, 10412–10419. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, P.; Goldmann, M.; Muller, P.; Fauré, M.-C.; Konovalov, O.; Krafft, M.P. Direct Evidence for Highly Organized Networks of Circular Surface Micelles of Surfactant at the Air-Water Interface. J. Am. Chem. Soc. 2005, 127, 512–513. [Google Scholar] [CrossRef] [PubMed]
- Bardin, L.; Fauré, M.-C.; Filipe, E.J.M.; Fontaine, P.; Goldmann, M. Highly organized crystal-line monolayer of a semi-fluorinated alkane on a solid substrate obtained by spin-coating. Thin Solid Films 2010, 519, 414–416. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Morgado, P.; Lourenço, P.; Goldmann, M.; Filipe, E.J.M. Spontaneous self-assembly and structure of perfluoroalkylalkane surfactant hemimicelles by molecular dynamics simulations. Proc. Natl. Acad. Sci. USA 2019, 116, 14868–14873. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, S.; Takeuchi, Y.; Endo, A. Nanometer-Sized Domains in Langmuir–Blodgett Films for Patterning SiO2. Langmuir 2010, 26, 6161–6163. [Google Scholar] [CrossRef]
- Kato, T.; Kameyama, M.; Ehara, M.; Iimura, K. Monodisperse Two-Dimensional Nanometer Size Clusters of Partially Fluorinated Long-Chain Acids. Langmuir 1998, 14, 1786–1798. [Google Scholar] [CrossRef]
- Matsumoto, M.; Watanabe, S.; Tanaka, K.; Kimura, H.; Kasahara, M.; Shibata, H.; Azumi, R.; Sa-kai, H.; Abe, M.; Kondo, Y.; et al. Control of Two-Dimensional Nanopatterns by Adjusting Inter-molecular Interactions. Adv. Mater. 2007, 19, 3668–3671. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996; ISBN 978-0750628396. [Google Scholar]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Fontaine, P.; Ciatto, G.; Aubert, N.; Goldmann, M. Soft Interfaces and Resonant Investigation on Undulator Source: A Surface X-ray Scattering Beamline to Study Organic Molecular Films at the SOLEIL Synchrotron. Sci. Adv. Mater. 2014, 6, 2312–2316. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Pereira, L.A.M.; Martins, L.F.G.; Ascenso, J.R.; Morgado, P.; Ramalho, J.P.P.; Filipe, E.J.M. Diffusion Coefficients of Fluorinated Surfactants in Water: Experimental Results and Prediction by Computer Simulation. J. Chem. Eng. Data 2014, 59, 3151–3159. [Google Scholar] [CrossRef] [Green Version]
- Chitra, R.; Smith, P.E. A comparison of the properties of 2,2,2-trifluoroethanol and 2,2,2-trifluoroethanol/water mixtures using different force fields. J. Chem. Phys. 2001, 115, 5521–5530. [Google Scholar] [CrossRef]
- Watkins, E.K.; Jorgensen, W.L. Perfluoroalkanes: Conformational Analysis and Liquid-State Properties from ab Initio and Monte Carlo Calculations. J. Phys. Chem. A 2001, 105, 4118–4125. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Hess, B. P-LINCS: A Parallel Linear Constraint Solver for Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Broniatowski, M.; Dynarowicz-Łatka, P. Semifluorinated Chains at the Air/Water Interface: Studies of the Interaction of a Semifluorinated Alkane with Fluorinated Alcohols in Mixed Langmuir Monolayers. Langmuir 2006, 22, 2691–2696. [Google Scholar] [CrossRef] [PubMed]
- Broniatowski, M.; Dynarowicz-Łatka, P. Interactions of a Fluoroaryl Surfactant with Hydrogenated, Partially Fluorinated, and Perfluorinated Surfactants at the Air/Water Interface. Langmuir 2006, 22, 6622–6628. [Google Scholar] [CrossRef] [PubMed]
- Qaqish, S.E.; Paige, M.F. Mechanistic Insight into Domain Formation and Growth in a Phase-Separated Langmuir—Blodgett Monolayer. Langmuir 2007, 23, 10088–10094. [Google Scholar] [CrossRef]
- Qaqish, S.E.; Paige, M.F. Characterization of domain growth kinetics in a mixed perfluorocarbon-hydrocarbon Langmuir—Blodgett monolayer. J. Coll. Interface Sci. 2008, 325, 290–293. [Google Scholar] [CrossRef]
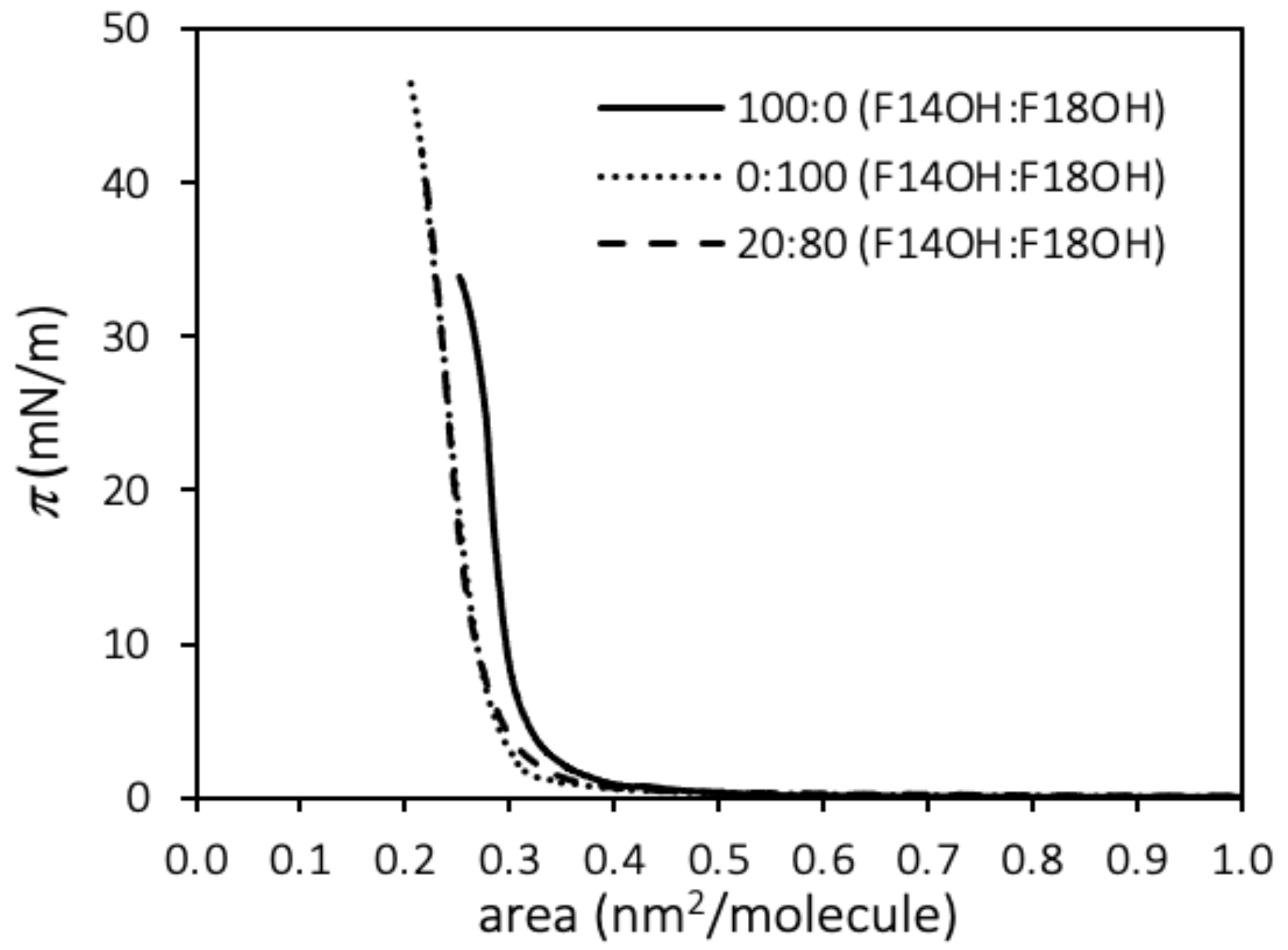
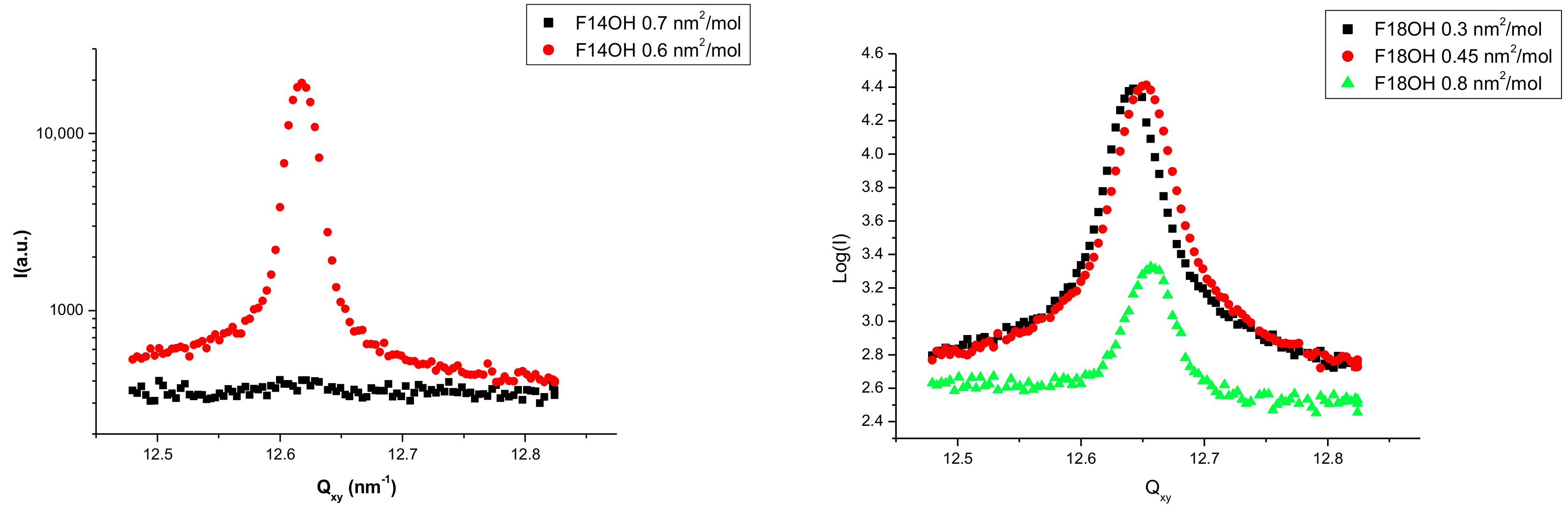
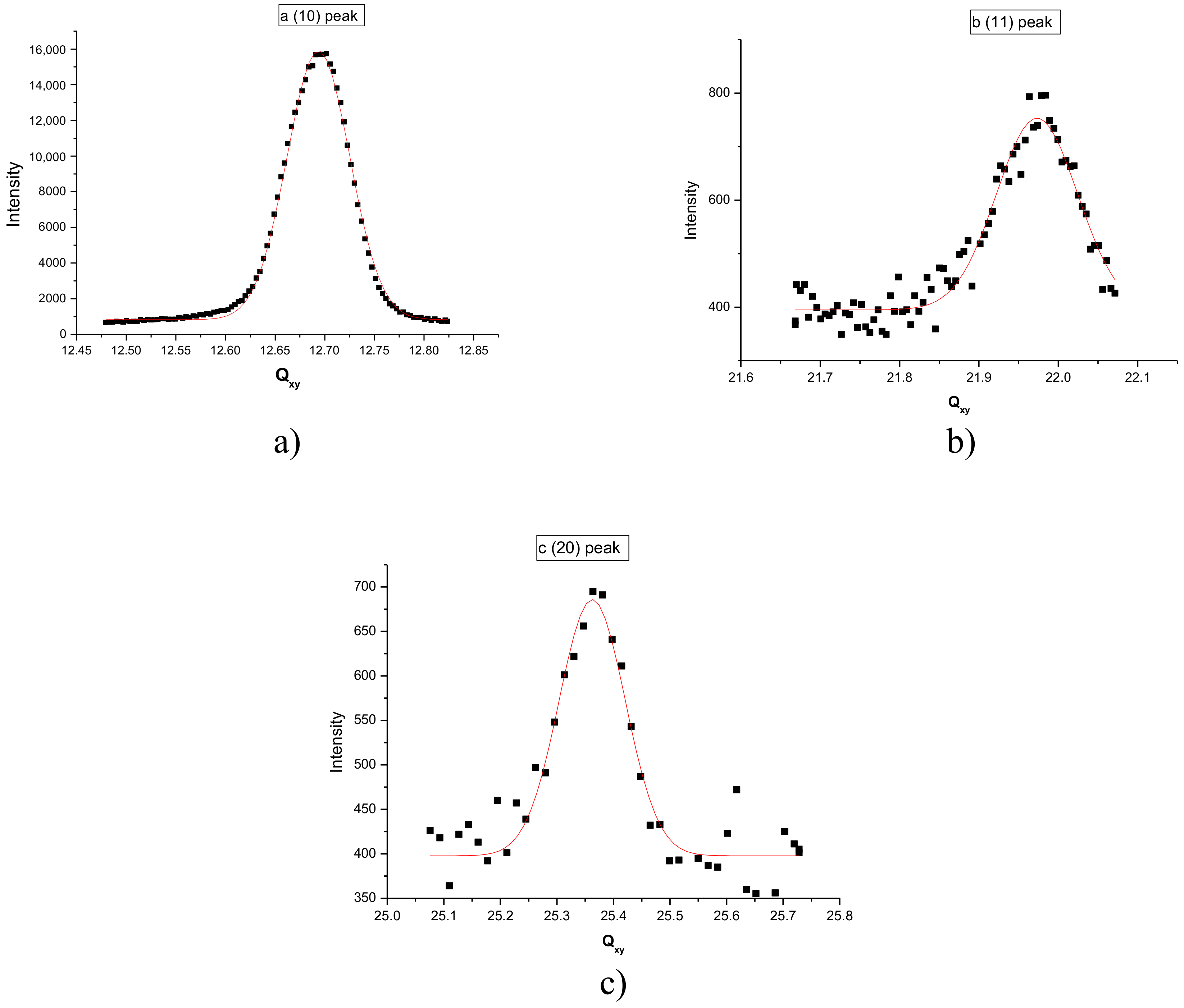



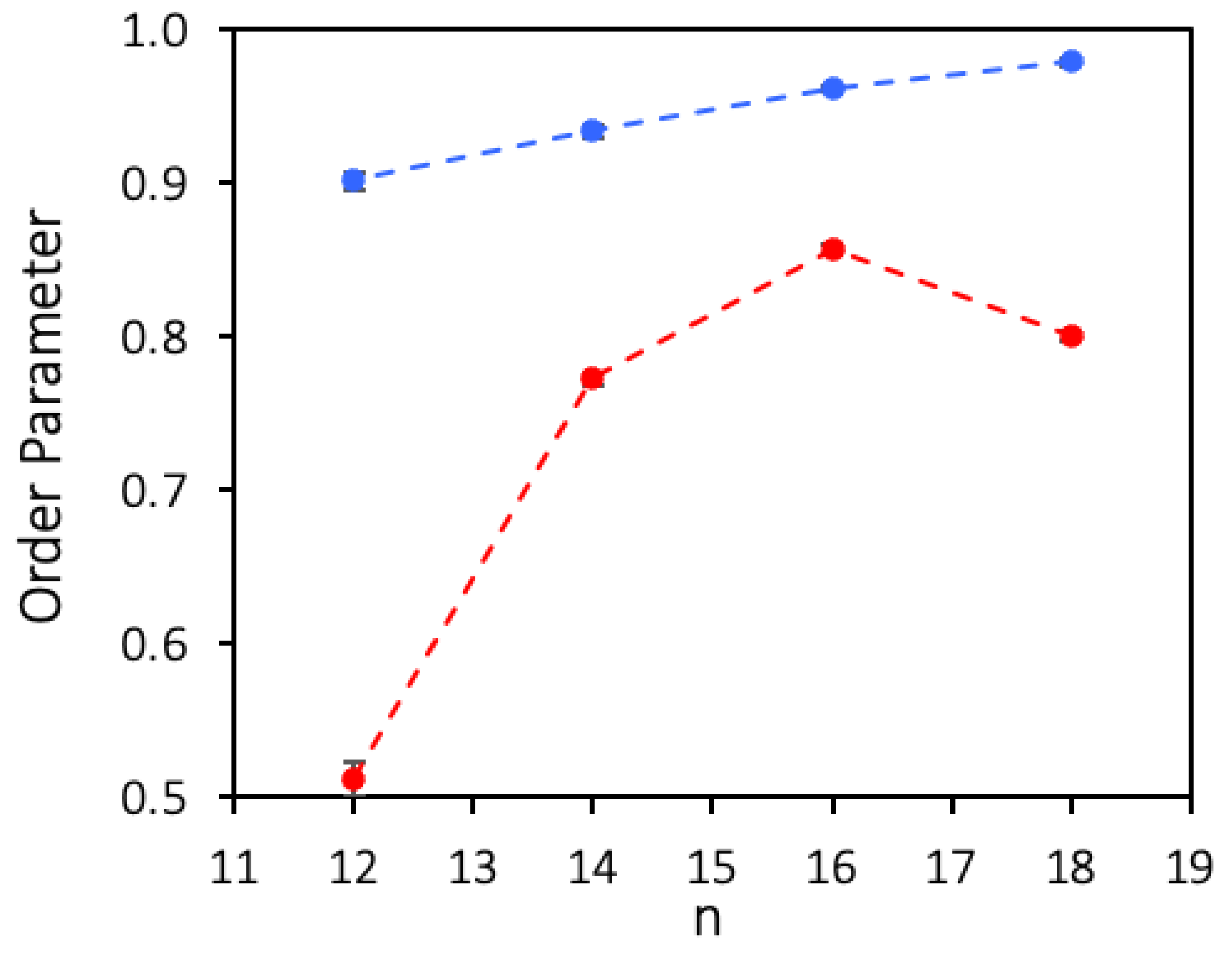
| Molecule | Limit Molecular Area (Å2/molecule) |
|---|---|
| F18OH | 27.6 ± 0.9 |
| F14OH | 31.2 ± 0.4 |
| (20:80) F14OH + F18OH | 27.0 ± 0.4 |
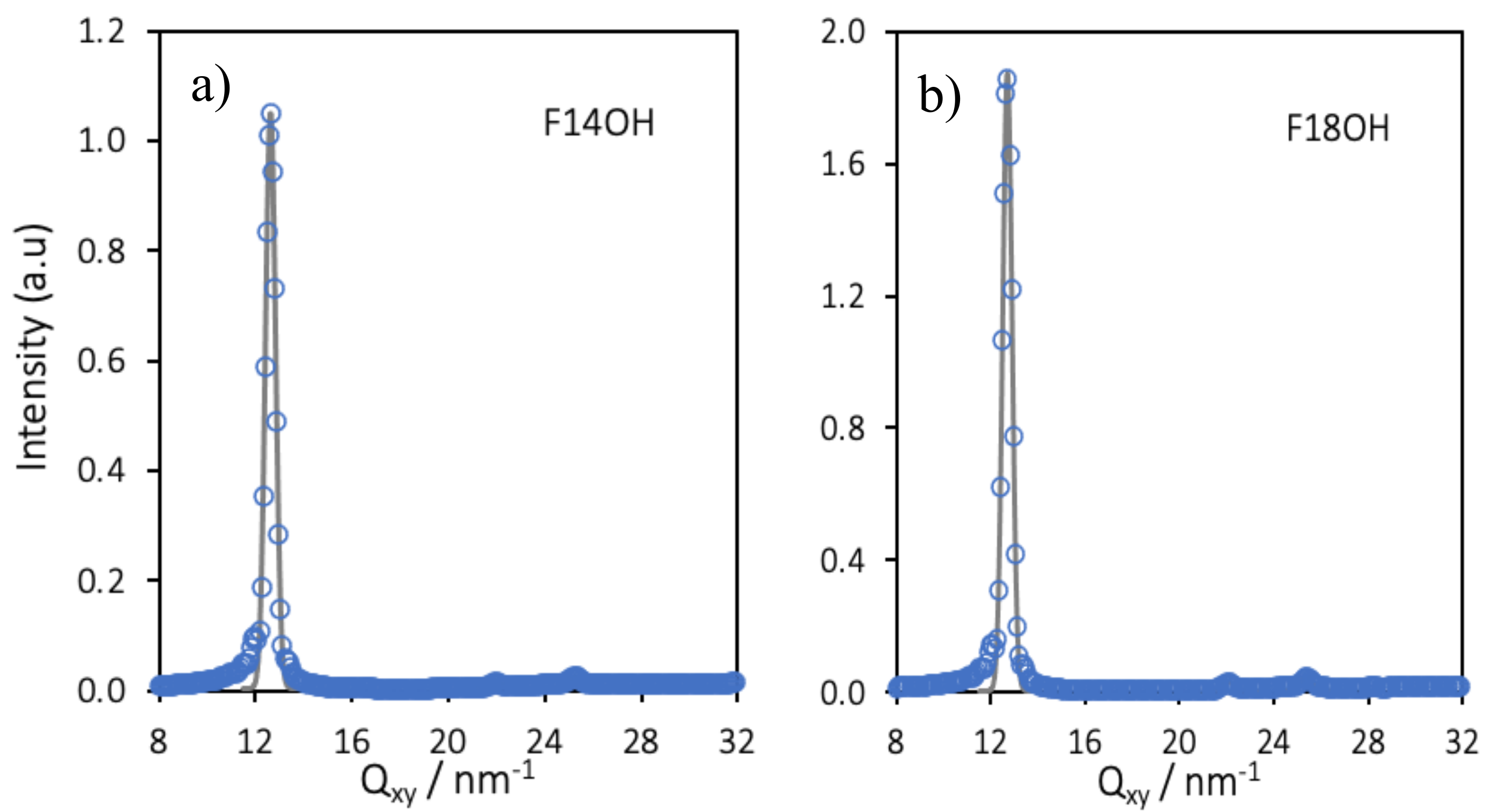
| Molecule | A (nm2/molecule) | Qxy (nm−1) | FWHM (nm−1) | Hexagonal Lattice Parameter (nm) | Coherence Length (nm) |
|---|---|---|---|---|---|
| F14OH | 0.60 | 12.62 | 0.020 | 0.575 | >320 |
| F18OH | 0.45 | 12.65 | 0.029 | 0.5753 | 300 |
| Peak Indexation | Peak Position (nm−1) | FWHM (nm−1) |
|---|---|---|
| (10) | 12.69 | 0.06 |
| (11) | 21.97 | 0.1 |
| (20) | 25.36 | 0.08 |
| Peak | Peak Position (nm−1) | |
|---|---|---|
| F18OH | F14OH | |
| Peak 1 | 12.69 | 12.62 |
| Peak 2 | 22.06 | 21.98 |
| Peak 3 | 25.40 | 25.25 |
| Peak 2/Peak 1 | 1.738 | 1.741 |
| Peak 3/Peak 1 | 2.001 | 2.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.; Nova, D.; Teixeira, M.; Cardoso, V.; Morgado, P.; Nunes, B.; Colaço, R.; Fauré, M.-C.; Fontaine, P.; Goldmann, M.; et al. Langmuir Films of Perfluorinated Fatty Alcohols: Evidence of Spontaneous Formation of Solid Aggregates at Zero Surface Pressure and Very Low Surface Density. Nanomaterials 2020, 10, 2257. https://doi.org/10.3390/nano10112257
Silva P, Nova D, Teixeira M, Cardoso V, Morgado P, Nunes B, Colaço R, Fauré M-C, Fontaine P, Goldmann M, et al. Langmuir Films of Perfluorinated Fatty Alcohols: Evidence of Spontaneous Formation of Solid Aggregates at Zero Surface Pressure and Very Low Surface Density. Nanomaterials. 2020; 10(11):2257. https://doi.org/10.3390/nano10112257
Chicago/Turabian StyleSilva, Pedro, Duarte Nova, Miguel Teixeira, Vitória Cardoso, Pedro Morgado, Bruno Nunes, Rogério Colaço, Marie-Claude Fauré, Philippe Fontaine, Michel Goldmann, and et al. 2020. "Langmuir Films of Perfluorinated Fatty Alcohols: Evidence of Spontaneous Formation of Solid Aggregates at Zero Surface Pressure and Very Low Surface Density" Nanomaterials 10, no. 11: 2257. https://doi.org/10.3390/nano10112257






