Synthesis and Characterization of Molten Salt Nanofluids for Thermal Energy Storage Application in Concentrated Solar Power Plants—Mechanistic Understanding of Specific Heat Capacity Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Material Characterization
- ⬝
- Heat the container with sample on hot plate at 400 °C;
- ⬝
- Once the sample melt, remove them from hot plate and keep stirring/scratching the sample using the spatula to prevent them agglomerating in the container;
- ⬝
- Load ~20 mg of sample in the aluminum pan;
- ⬝
- Heat the pan on hot plate at 400 °C for few seconds until the sample melt, then quickly seal the pan with lid;
- ⬝
- Put the newly-prepared sample pan in a furnace and heat at 550 °C for half hour;
- ⬝
- Remove the pan from the furnace. Wait it cool down and take to the SEM room;
- ⬝
- When examining sample in SEM facility, remove the lid and place the sample pan in SEM chamber quickly to avoid absorption of moisture from the ambient.
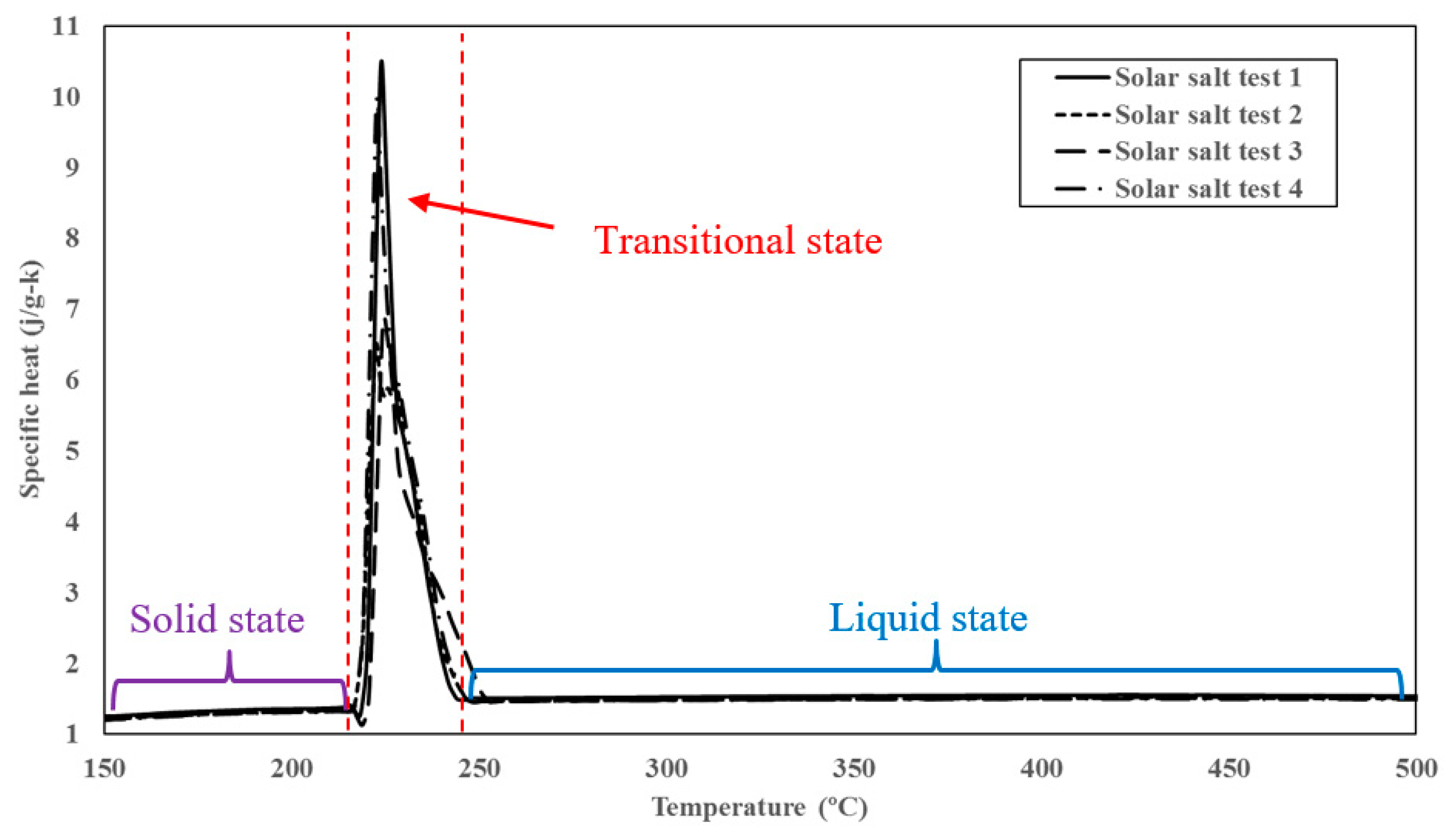
2.3. Specific Heat Capacity Measurement
3. Results
3.1. Thermophysical Properties
3.2. Material Morphology
4. Discussion
4.1. Mechanistic Understanding of Specific Heat Capacity Enhancement—The Role of Compressed Layer at Nanoparticle Surface
4.2. Mechanistic Understanding of Specific Heat Capacity Enhancement—The Role of Secondary Nanostructures
4.3. Mechanistic Understanding of the Effect of Nanoparticle Concentration on Specific Heat Capacity Enhancement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richter, C.; Teske, S.; Short, R. Concentrating Solar Power Global Outlook 09. Available online: https://www.greentechmedia.com/images/wysiwyg/News/Greenpeace_concentratingsolarpower2009.pdf (accessed on 26 July 2006).
- Tsao, J.; Lewis, N.; Crabtree, G. Solar Faqs; US Department of Energy: Washington, DC, USA, 2006; Volume 13.
- International Energy Agency. Key World Energy Statistics; International Energy Agency: Paris, France, 2015.
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Zhang, M.; Reddy, R. Evaluation of ionic liquids as heat transfer materials in thermal storage systems. Mater. Sci. Technol. AIST 2007, 2, 1061. [Google Scholar]
- Kearney, D.; Herrmann, U.; Nava, P.; Kelly, B.; Mahoney, R.; Pacheco, J.; Cable, R.; Potrovitza, N.; Blake, D.; Price, H. Assessment of a molten salt heat transfer fluid in a parabolic trough solar field. J. Sol. Energy Eng. 2003, 125, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Reddy, R.G. Novel Molten Salts Thermal Energy Storage for Concentrating Solar Power Generation; University of Alabama: Tuscaloosa, AL, USA, 2013. [Google Scholar]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Solar Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Murphy, C.; Sun, Y.; Cole, W.J.; Maclaurin, G.J.; Mehos, M.S.; Turchi, C.S. The Potential Role of Concentrating Solar Power within the Context of DOE’s 2030 Solar Cost Targets; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2019.
- International Renewable Energy Agency. Renewable Power Generation Costs in 2019; IRENA: Abu Dhabi, UAE, 2020. [Google Scholar]
- Masuda, H.; Ebata, A.; Teramae, K. Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles. Dispersion of Al2O3, SiO2 and TiO2 ultra-fine particles. Netsu Bussei 1993, 7, 227–233. [Google Scholar] [CrossRef]
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab.: Lemont, IL, USA, 1995.
- Ueki, Y.; Fujita, N.; Kawai, M.; Shibahara, M. Molten salt thermal conductivity enhancement by mixing nanoparticles. Fusion Eng. Design 2018, 136, 1295–1299. [Google Scholar] [CrossRef]
- Madathil, P.K.; Balagi, N.; Saha, P.; Bharali, J.; Rao, P.V.; Choudary, N.V.; Ramesh, K. Preparation and characterization of molten salt based nanothermic fluids with enhanced thermal properties for solar thermal applications. Appl. Therm. Eng. 2016, 109, 901–905. [Google Scholar] [CrossRef]
- Ueki, Y.; Fujita, N.; Kawai, M.; Shibahara, M. Thermal conductivity of molten salt-based nanofluid. Aip Adv. 2017, 7, 055117. [Google Scholar] [CrossRef]
- Ma, B.; Banerjee, D. Experimental measurements of thermal conductivity of alumina nanofluid synthesized in salt melt. AIP Adv. 2017, 7, 115124. [Google Scholar] [CrossRef]
- Garg, J.; Poudel, B.; Chiesa, M.; Gordon, J.; Ma, J.; Wang, J.; Ren, Z.; Kang, Y.T.; Ohtani, H.; Nanda, J. Enhanced thermal conductivity and viscosity of copper nanoparticles in ethylene glycol nanofluid. J. Appl. Phys. 2008, 103, 074301. [Google Scholar] [CrossRef]
- Mishra, P.C.; Mukherjee, S.; Nayak, S.K.; Panda, A. A brief review on viscosity of nanofluids. Int. Nano Lett. 2014, 4, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Suganthi, K.S.; Anusha, N.; Rajan, K.S. Low viscous ZnO–propylene glycol nanofluid: A potential coolant candidate. J. Nanopart. Res. 2013, 15, 1986. [Google Scholar] [CrossRef]
- Namburu, P.; Kulkarni, D.; Dandekar, A.; Das, D. Experimental investigation of viscosity and specific heat of silicon dioxide nanofluids. Micro Nano Lett. 2007, 2, 67–71. [Google Scholar] [CrossRef]
- Zhou, S.-Q.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 093123. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Specific heat measurement of three nanofluids and development of new correlations. J. Heat Transf. 2009, 131, 071601. [Google Scholar] [CrossRef]
- Zhou, L.-P.; Wang, B.-X.; Peng, X.-F.; Du, X.-Z.; Yang, Y.-P. On the specific heat capacity of CuO nanofluid. Adv. Mech. Eng. 2010, 2, 172085. [Google Scholar] [CrossRef] [Green Version]
- Tiznobaik, H.; Shin, D. Enhanced specific heat capacity of high-temperature molten salt-based nanofluids. Int. J. Heat Mass Transf. 2013, 57, 542–548. [Google Scholar] [CrossRef]
- Lu, M.-C.; Huang, C.-H. Specific heat capacity of molten salt-based alumina nanofluid. Nanoscale Res. Lett. 2013, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Qiao, G.; Lasfargues, M.; Alexiadis, A.; Ding, Y. Simulation and experimental study of the specific heat capacity of molten salt based nanofluids. Appl. Therm. Eng. 2017, 111, 1517–1522. [Google Scholar] [CrossRef]
- Ho, M.X.; Pan, C. Optimal concentration of alumina nanoparticles in molten Hitec salt to maximize its specific heat capacity. Int. J. Heat Mass Transf. 2014, 70, 174–184. [Google Scholar] [CrossRef]
- Andreu-Cabedo, P.; Mondragon, R.; Hernandez, L.; Martinez-Cuenca, R.; Cabedo, L.; Julia, J.E. Increment of specific heat capacity of solar salt with SiO2 nanoparticles. Nanoscale Res. Lett. 2014, 9, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 2013, 8, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercole, D.; Manca, O.; Vafai, K. An investigation of thermal characteristics of eutectic molten salt-based nanofluids. Int. Commun. Heat Mass Transf. 2017, 87, 98–104. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhanced specific heat of silica nanofluid. J. Heat Transf. 2011, 133, 024501. [Google Scholar] [CrossRef]
- El Far, B.; Rizvi, S.M.M.; Nayfeh, Y.; Shin, D. Investigation of heat capacity and viscosity enhancements of binary carbonate salt mixture with SiO2 nanoparticles. Int. J. Heat Mass Transf. 2020, 156, 119789. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; González-Fernández, L.; Grosu, Y.; Zaki, A.; Igartua, J.M.; Faik, A. Shape effect of Al2O3 nanoparticles on the thermophysical properties and viscosity of molten salt nanofluids for TES application at CSP plants. Appl. Therm. Eng. 2020, 169, 114942. [Google Scholar] [CrossRef]
- Grosu, Y.; Nithiyanantham, U.; González-Fernández, L.; Faik, A. Preparation and characterization of nanofluids based on molten salts with enhanced thermophysical properties for thermal energy storage at concentrate solar power. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2019; p. 200021. [Google Scholar]
- Navarrete, N.; Hernández, L.; Vela, A.; Mondragón, R. Influence of the production method on the thermophysical properties of high temperature molten salt-based nanofluids. J. Mol. Liq. 2020, 302, 112570. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M.; Torre, L. Heat capacity of nanofluids for solar energy storage produced by dispersing oxide nanoparticles in nitrate salt mixture directly at high temperature. Solar Energy Mater. Solar Cells 2017, 167, 60–69. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-T.; Wang, X.; Ma, C.-F. Experimental study on thermophysical properties of molten salt nanofluids prepared by high-temperature melting. Solar Energy Mater. Solar Cells 2019, 191, 209–217. [Google Scholar] [CrossRef]
- Luo, Y.; Du, X.; Awad, A.; Wen, D. Thermal energy storage enhancement of a binary molten salt via in-situ produced nanoparticles. Int. J. Heat Mass Transf. 2017, 104, 658–664. [Google Scholar] [CrossRef]
- Lasfargues, M.; Bell, A.; Ding, Y. In situ production of titanium dioxide nanoparticles in molten salt phase for thermal energy storage and heat-transfer fluid applications. J. Nanopart. Res. 2016, 18, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Cheng, X.; Li, Y.; Yu, G.; Xu, K.; Li, G. Effect of in-situ synthesized nano-MgO on thermal properties of NaNO3-KNO3. Solar Energy 2018, 160, 208–215. [Google Scholar] [CrossRef]
- Lasfargues, M.; Stead, G.; Amjad, M.; Ding, Y.; Wen, D. In Situ production of copper oxide nanoparticles in a binary molten salt for concentrated solar power plant applications. Materials 2017, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Banerjee, D. A review of nanofluid synthesis. In Advances in Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 135–176. [Google Scholar]
- Chen, L.-C.; Ho, C.-C. Submerged arc spray synthesis of TiO2 nanoparticles with desired form sphericity using process characterization and optimization. J. Nanosci. Nanotechnol. 2008, 8, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bang, I.C.; Onoe, J. Characteristic stability of bare Au-water nanofluids fabricated by pulsed laser ablation in liquids. Opt. Lasers Eng. 2009, 47, 532–538. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Siegel, N.P. Molten nitrate salt development for thermal energy storage in parabolic trough solar power systems. In Proceedings of the Energy Sustainability, Jacksonville, FL, USA, 10–14 August 2008; pp. 631–637. [Google Scholar]
- Ma, B.; Kumar, N.; Kuchibhotla, A.; Banerjee, D. Experimental Measurement of the Effect of Particle Concentration on the Specific Heat Capacity of Silica Nanofluids. In Proceedings of the 2018 17th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), San Diego, CA, USA, 29 May–1 June 2018; pp. 246–251. [Google Scholar]
- Nanda, K.; Maisels, A.; Kruis, F.; Fissan, H.; Stappert, S. Higher surface energy of free nanoparticles. Phys. Rev. Lett. 2003, 91, 106102. [Google Scholar] [CrossRef]
- Yao, Y.; Wei, Y.; Chen, S. Size effect of the surface energy density of nanoparticles. Surf. Sci. 2015, 636, 19–24. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Enhanced specific heat capacity of molten salt-based carbon nanotubes nanomaterials. J. Heat Transf. 2015, 137, 091013. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Jo, B. Anomalous increase in specific heat of binary molten salt-based graphite nanofluids for thermal energy storage. Appl. Sci. 2018, 8, 1305. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Banerjee, D. Enhanced specific heat capacity of nanomaterials synthesized by dispersing silica nanoparticles in eutectic mixtures. J. Heat Transf. 2013, 135, 032801. [Google Scholar] [CrossRef]
- Iwadate, Y.; Okada, I.; Kawamura, K. Density and heat capacity of molten sodium nitrite-potassium nitrate mixtures. J. Chem. Eng. Data 1982, 27, 288–290. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Gao, H.; Yu, Y. Experimental and theoretical studies of surface nitrate species on Ag/Al2O3 using DRIFTS and DFT. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Mondragón, R.; Juliá, J.E.; Cabedo, L.; Navarrete, N. On the relationship between the specific heat enhancement of salt-based nanofluids and the ionic exchange capacity of nanoparticles. Sci. Rep. 2018, 8, 7532. [Google Scholar] [CrossRef] [PubMed]
- Atkin, R.; Borisenko, N.; Drüschler, M.; Endres, F.; Hayes, R.; Huber, B.; Roling, B. Structure and dynamics of the interfacial layer between ionic liquids and electrode materials. J. Mol. Liq. 2014, 192, 44–54. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [Green Version]
- Jurado, L.A.; Kim, H.; Arcifa, A.; Rossi, A.; Leal, C.; Spencer, N.D.; Espinosa-Marzal, R.M. Irreversible structural change of a dry ionic liquid under nanoconfinement. Phys. Chem. Chem. Phys. 2015, 17, 13613–13624. [Google Scholar] [CrossRef]
- Anaredy, R.S.; Shaw, S.K. Long-range ordering of ionic liquid fluid films. Langmuir 2016, 32, 5147–5154. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Banerjee, D.; Shin, D. Effect of formation of “long range” secondary dendritic nanostructures in molten salt nanofluids on the values of specific heat capacity. Int. J. Heat Mass Transf. 2015, 91, 342–346. [Google Scholar] [CrossRef]










| Raw Material Mass (g) for Synthesis | Final Product Mass (g) | |||||
|---|---|---|---|---|---|---|
| Target Nanoparticle Concentration | NaNO3 | KNO3 | Al(NO3)3·9H2O | Solar Salt | Al2O3 | Total |
| 0.5% | 20.895 | 13.93 | 1.287 | 34.825 | 0.175 | 35 |
| 1.0% | 20.790 | 13.86 | 2.575 | 34.650 | 0.350 | 35 |
| 1.5% | 20.685 | 13.79 | 3.862 | 34.475 | 0.525 | 35 |
| Specific heat enhancement for solar salt nanofluid containing 0.5% alumina nanoparticles | |||||
| Test number | 300 °C | 400 °C | 500 °C | Average | STD |
| 1 | 37.9% | 17.6% | 11.4% | 20.8% | 7.0% |
| 2 | 28.2% | 13.2% | 9.7% | 15.9% | 6.0% |
| 3 | 42.5% | 18.1% | 11.1% | 20.8% | 8.0% |
| 4 | 26.3% | 11.3% | 6.6% | 13.6% | 5.1% |
| 5 | 39.7% | 19.5% | 13.1% | 22.1% | 7.2% |
| 6 | 39.3% | 15.8% | 8.5% | 18.9% | 7.9% |
| 7 | 30.6% | 16.6% | 10.1% | 17.7% | 6.2% |
| 8 | 28.1% | 11.3% | 6.0% | 13.8% | 6.3% |
| 9 | 32.0% | 12.5% | 7.6% | 15.3% | 7.2% |
| 10 | 36.0% | 13.9% | 7.0% | 15.7% | 7.8% |
| Average | 34.1% | 15.0% | 9.1% | ||
| Grand Avg | 17.4% | Grand STD | 7.5% | ||
| Specific heat enhancement for solar salt nanofluid containing 1.0% alumina nanoparticles | |||||
| Test number | 300 °C | 400 °C | 500 °C | Average | STD |
| 1 | 44.5% | 36.0% | 28.1% | 35.1% | 5.1% |
| 2 | 60.3% | 34.7% | 30.3% | 37.6% | 7.7% |
| 3 | 53.6% | 37.0% | 31.2% | 38.7% | 6.7% |
| 4 | 61.3% | 36.9% | 32.8% | 41.6% | 8.6% |
| 5 | 54.3% | 38.1% | 34.1% | 40.3% | 6.0% |
| 6 | 53.8% | 35.5% | 30.4% | 38.5% | 6.9% |
| 7 | 57.4% | 32.6% | 28.7% | 36.0% | 7.5% |
| 8 | 59.4% | 34.7% | 32.0% | 38.7% | 7.5% |
| 9 | 52.6% | 37.3% | 31.7% | 39.1% | 6.4% |
| 10 | 60.7% | 36.2% | 32.1% | 41.8% | 9.2% |
| Avg | 55.8% | 35.9% | 31.2% | ||
| Grand Avg | 38.8% | Grand STD | 7.5% | ||
| Specific heat enhancement for solar salt nanofluid containing 1.5% alumina nanoparticles | |||||
| Test number | 300 °C | 400 °C | 500 °C | Average | STD |
| 1 | 46.5% | 27.8% | 20.2% | 29.4% | 7.0% |
| 2 | 67.6% | 29.6% | 20.5% | 32.8% | 10.8% |
| 3 | 53.1% | 27.9% | 20.3% | 30.6% | 8.3% |
| 4 | 58.0% | 28.7% | 20.6% | 31.2% | 9.3% |
| 5 | 52.1% | 27.6% | 21.6% | 30.6% | 7.9% |
| 6 | 73.2% | 30.9% | 22.4% | 35.6% | 12.0% |
| 7 | 58.6% | 28.6% | 22.3% | 32.4% | 9.7% |
| 8 | 64.2% | 27.7% | 20.1% | 32.3% | 10.8% |
| 9 | 56.1% | 26.3% | 19.6% | 30.3% | 9.4% |
| 10 | 57.2% | 28.8% | 22.2% | 32.2% | 9.1% |
| Avg | 58.7% | 28.4% | 21.0% | ||
| Grand Avg | 31.8% | Grand STD | 9.7% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Shin, D.; Banerjee, D. Synthesis and Characterization of Molten Salt Nanofluids for Thermal Energy Storage Application in Concentrated Solar Power Plants—Mechanistic Understanding of Specific Heat Capacity Enhancement. Nanomaterials 2020, 10, 2266. https://doi.org/10.3390/nano10112266
Ma B, Shin D, Banerjee D. Synthesis and Characterization of Molten Salt Nanofluids for Thermal Energy Storage Application in Concentrated Solar Power Plants—Mechanistic Understanding of Specific Heat Capacity Enhancement. Nanomaterials. 2020; 10(11):2266. https://doi.org/10.3390/nano10112266
Chicago/Turabian StyleMa, Binjian, Donghyun Shin, and Debjyoti Banerjee. 2020. "Synthesis and Characterization of Molten Salt Nanofluids for Thermal Energy Storage Application in Concentrated Solar Power Plants—Mechanistic Understanding of Specific Heat Capacity Enhancement" Nanomaterials 10, no. 11: 2266. https://doi.org/10.3390/nano10112266





