Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics
Abstract
:1. Introduction
- (1)
- Broadband UV-Vis absorption. Melanins present interesting optical properties and in particular eumelanin but also PDA show a broad monotonic absorption band in the UV-Vis spectrum up to near-infrared (NIR) [6,31]. Although the origin of this typical absorption band is still debated, and objective of recent investigation based on ultrafast transient absorption (UF TA) techniques, the broad optical absorption is at the basis of the natural function of melanosomes that is imparting coloration and protecting from the potential dangerous effect of the solar radiation. The photo-protecting action is strongly related to the photophysical properties of melanin derivatives that absorb light very efficiently, producing excited states that deactivate mostly through ultra-fast non-radiative processes, without the production of any long-lived, potentially reactive transient species. As a consequence, eumelanin is photochemically very poorly reactive and it transforms light energy into heat. If the intensity of input radiation is moderate, like in the case of sunlight exposure, the heat produced can be dissipated by biological tissues safely, without causing significant local temperature increase [32]. On the contrary, upon locally controlled and intense light stimulation, the heat generation can be exploited for producing acoustic signals for diagnostic as in PAI or even induce a local hyperthermia and kill cancer cells as in PTT [33].
- (2)
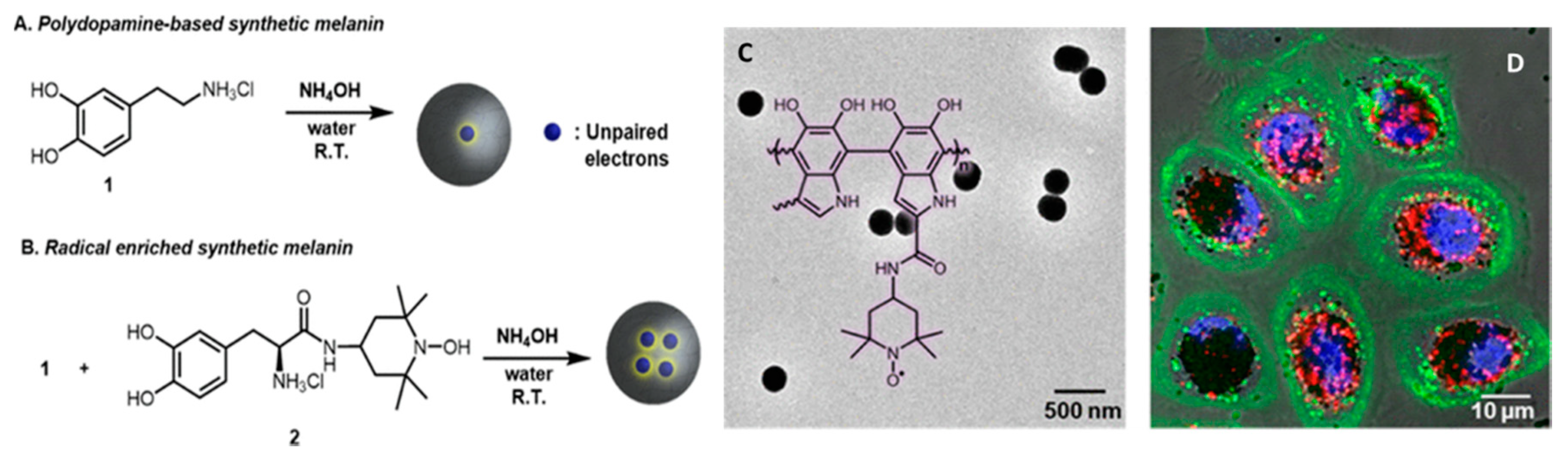
- Antioxidant and free radical scavenging activity. Melanin as well as artificial MNP, possessing various reductive functional groups as catechol, amine, and imine, exhibit a broad action of defense against multiple reactive oxygen and nitrogen species (RONS), including O2•−, H2O2, •OH, •NO, and ONOO−, that are generated, e.g., after solar exposure, as well as in diseases [34]. Radical-enriched artificial MNP have been even reported to be so efficient to work even as ionizing radiation-protector able to scavenge reactive oxygen species produced upon X-ray exposure [35].
- (3)
- (4)
- Drug loading capacity. The various π-conjugated structures, such as dihydroxyindole, indolequinone (see Figure 2), on the surface of melanin make it possible the binding with various aromatic structures via π–π stacking or other secondary interactions as hydrogen bonding and wan der Waals interactions. As an alternative, drugs can be also either linked through covalent bonds to the melanin surface or they can be physically encapsulated within the polymer matrix [41,42].
- (5)
- Easy functionalization. Also, in this case, the presence of numerous functional groups as catechol, o-quinone, amine, and imine, endows melanin with reactive sites for the formation of covalent bonds with various reactants and in particular with ligands for target recognition or “stealth”-polymer like PEG [9].
- (6)
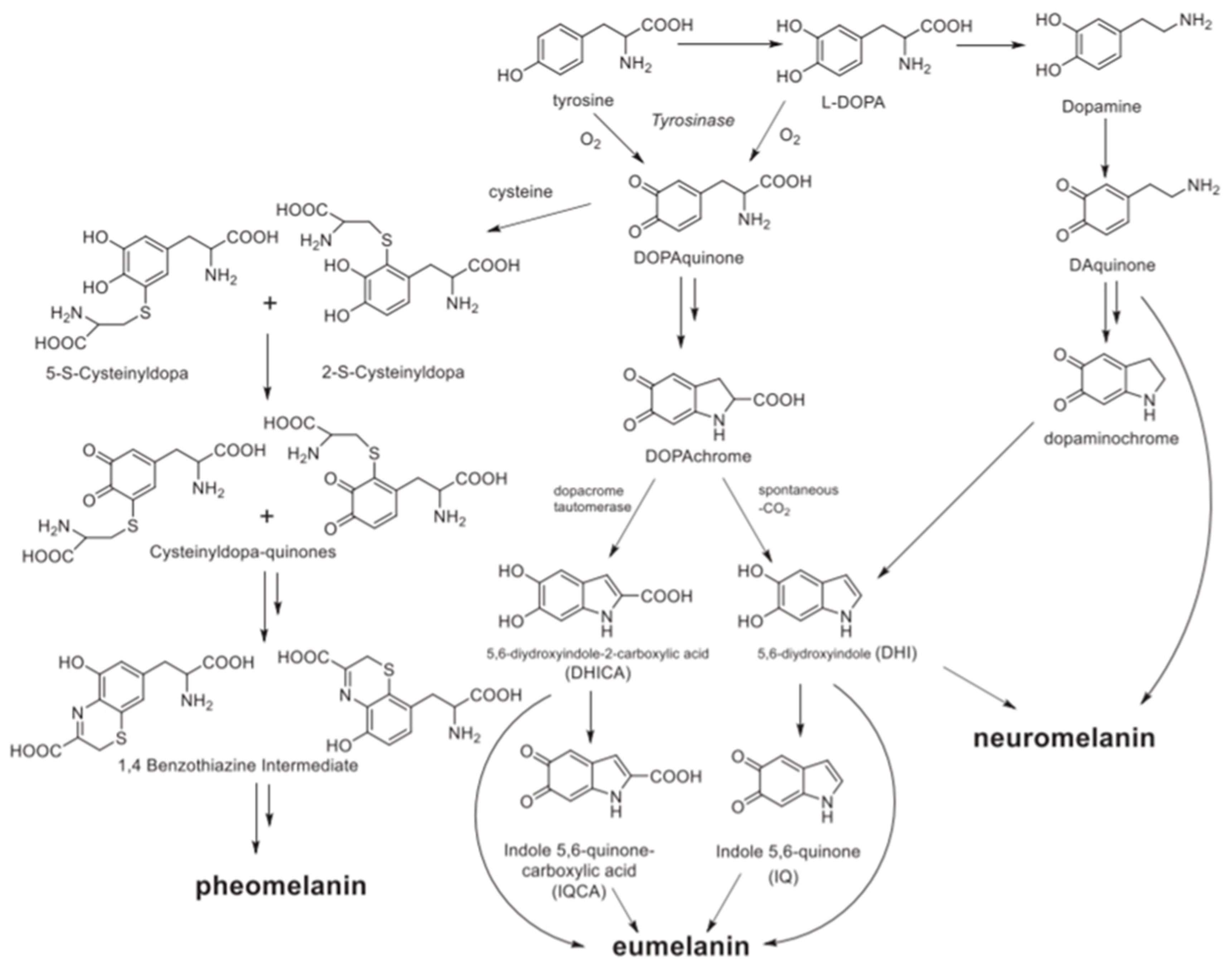
1.1. Melanin Types, Structure and Sources
1.1.1. Melanin Types and Structures
1.1.2. Natural versus Synthetic Sources
1.2. Preparation Methods, and Polymerization Mechanisms
1.2.1. Preparation Methods
1.2.2. Polymerization Mechanism
1.3. Physicochemical Properties
1.3.1. Photophysical and Photochemical Properties of MNP
1.3.2. Antioxidant (AOX) Activity
1.3.3. Metal Ion Chelating Ability and Redox Activities
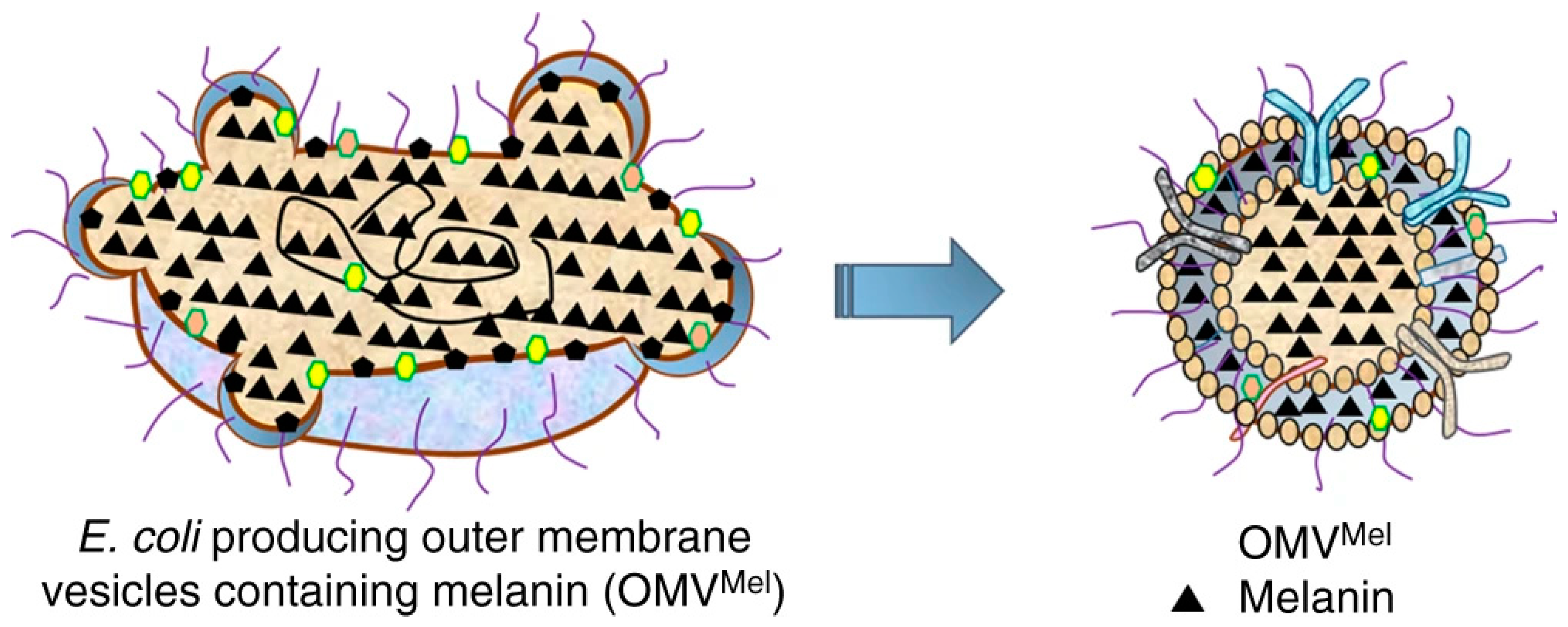
1.3.4. Functionalization and Coating: Use as a Bio-Template or as a Coating
2. MNP for Bio-Imaging
2.1. Fluorescence Imaging (FI)
2.2. Photoacoustic Imaging (PAI)
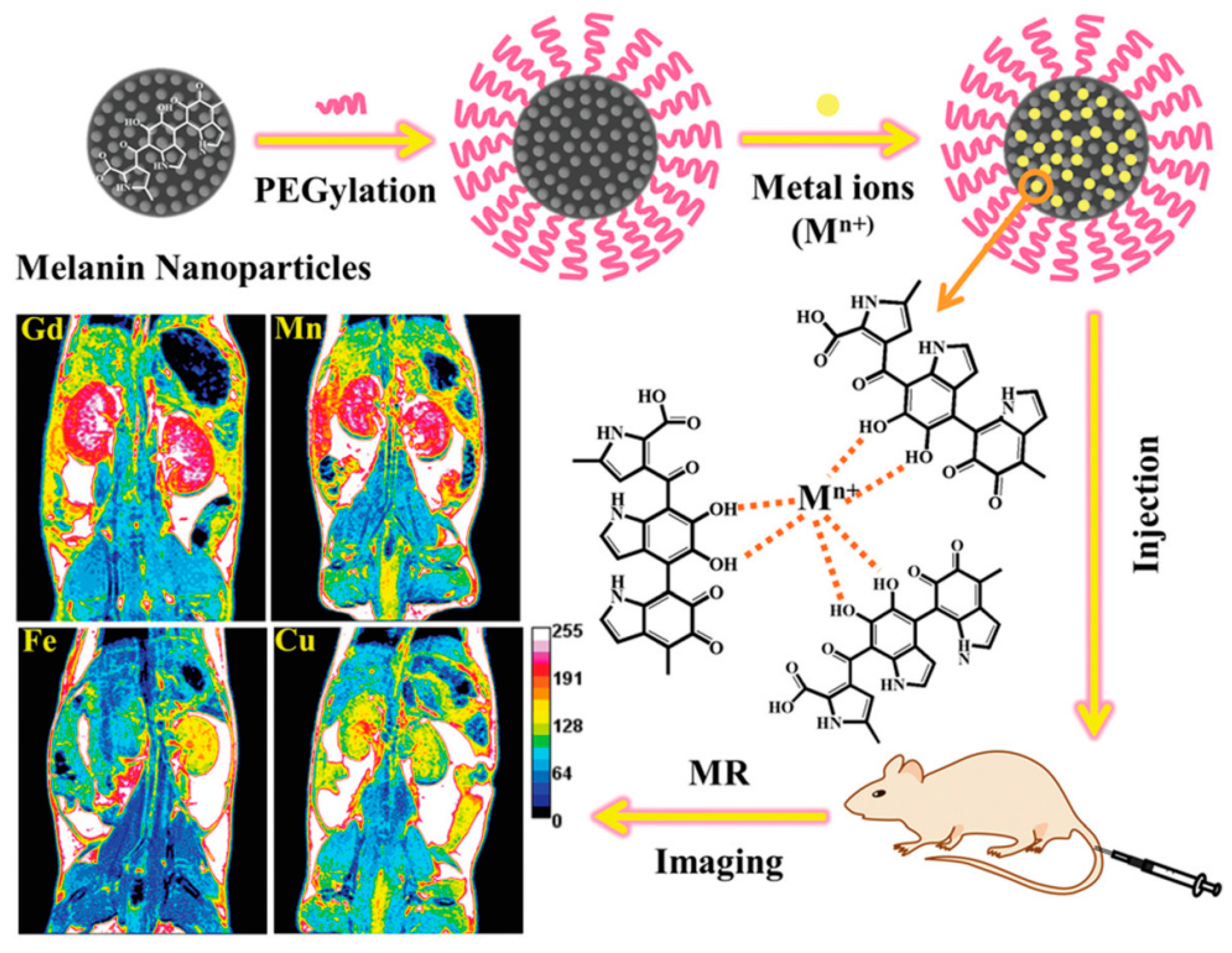
2.3. Magnetic Resonance Imaging (MRI)
2.4. Multimodal Imaging
3. MNP Based Therapy and Theranostics
3.1. Antioxidative Therapy
3.2. MNP as Nanocarriers for Simple Drug-Delivery
3.2.1. MNP for the Delivery of Non-Cancer Drugs
3.2.2. MNP for the Delivery of Anti-Cancer Drugs
3.3. Photothermal Therapy (PTT)
3.4. Synergistic PTT
3.4.1. PTT-Immunotherapy
3.4.2. PTT-Chemotherapy
3.4.3. PTT-Gene Therapy
3.4.4. Chemo-Gene-PTT
3.4.5. Chemo-PTT- Immunotherapy
3.5. Photodynamic Therapy (PDT) and Synergistic PDT-PTT
3.6. Antibacterial Infections
4. MNP and Nanocosmetics
4.1. MNP for Photoprotection
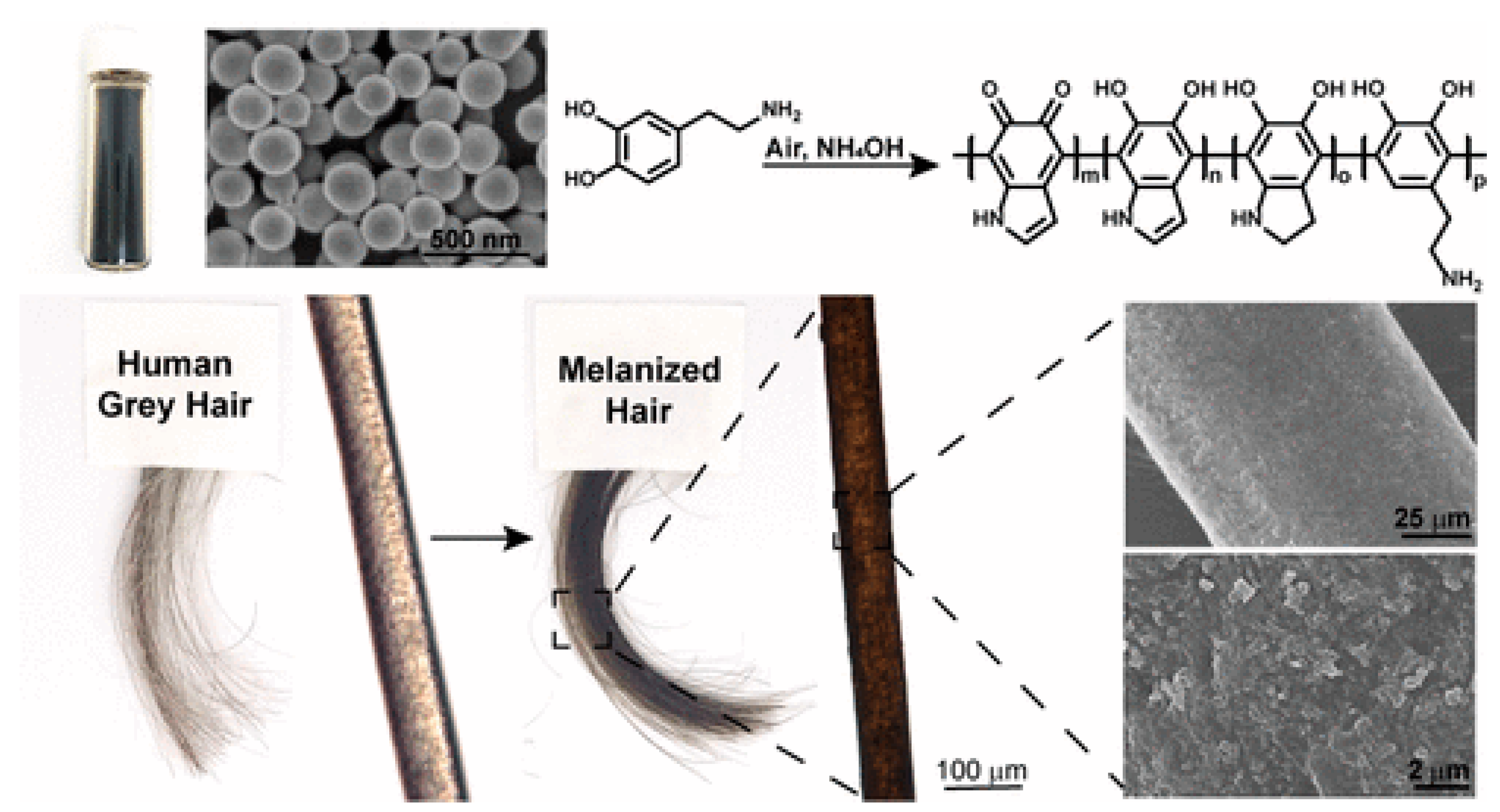
4.2. Hair Coloration
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wolfram, J.; Ferrari, M. Clinical cancer nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, J. Progress in the development of nanotheranostic systems. Theranostics 2016, 6, 915–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Lin, R.; Li, H.-J.; He, W.; Du, J.-Z.; Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, A.C.; Haugen, H.J. Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Zhang, L.; Sun, Y. Nanotechnology applied to overcome tumor drug resistance. J. Control. Release 2012, 162, 45–55. [Google Scholar] [CrossRef]
- Jie Kai, T.; Yip, L.X.; Tan, E.; Santitewagun, S.; Prasath, A.; Ke, P.; Ho, H.; Leong, D. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48, 5381–5407. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurbain, I.; Geerts, W.J.C.; Boudier, T.; Marco, S.; Verkleij, A.J.; Marks, M.S.; Raposo, G. Electron tomography of early melanosomes: Implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc. Natl. Acad. Sci. USA 2008, 105, 19726–19731. [Google Scholar] [CrossRef] [Green Version]
- Wasmeier, C.; Hume, A.N.; Bolasco, G.; Seabra, M.C. Melanosomes at a glance. J. Cell Sci. 2008, 121, 3995–3999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, X.Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoçi, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Peng, F.; Setyawati, M.; Jie Kai, T.; Ding, X.; Wang, J.; Nga, M.; Ho, H.; Leong, D. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef]
- Lapidot, S.; Meirovitch, S.; Sharon, S.; Heyman, A.; Kaplan, D.L.; Shoseyov, O. Clues for biomimetics from natural composite materials. Nanomedicine 2012, 7, 1409–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Ige, O.O.; Umoru, L.E.; Aribo, S. Natural Products: A Minefield of Biomaterials. Int. Sch. Res. Netw. ISRN Mater. Sci. 2012, 2012, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-Containing Polydopamine Nanomaterials: Catalysis, Energy, and Theranostics. Small 2020, 16, 1–21. [Google Scholar] [CrossRef]
- Seagle, B.L.L.; Rezai, K.A.; Kobori, Y.; Gasyna, E.M.; Rezael, K.A.; Norris, J.R. Melanin photoprotection in the human retinal pigment epithelium and its correlation with light-induced cell apoptosis. Proc. Natl. Acad. Sci. USA 2005, 102, 8978–8983. [Google Scholar] [CrossRef] [Green Version]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications—Cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef]
- Meng, S.; Kaxiras, E. Mechanisms for Ultrafast Nonradiative Relaxation in Electronically Excited Eumelanin Constituents | Elsevier Enhanced Reader. Biophys. J. 2008, 95, 4396–4402. [Google Scholar] [CrossRef] [Green Version]
- Tada, M.; Kohno, M.; Niwano, Y. Scavenging or Quenching Effect of Melanin on Superoxide Anion and Singlet Oxygen. JCBN J. Clin. Biochem. Nutr. 2010, 46, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Stritzker, J.; Kirscher, L.; Scadeng, M.; Deliolanis, N.C.; Morscher, S.; Symvoulidis, P.; Schaefer, K.; Zhang, Q.; Buckel, L.; Hess, M.; et al. Vaccinia virus-mediated melanin production allows MR and optoacoustic deep tissue imaging and laser-induced thermotherapy of cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 3316–3320. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.; Shawkey, M.D.; Dhinojwala, A. Bioinspired Melanin-Based Optically Active Materials. Adv. Opt. Mater. 2020, 2000932. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.; Yi, G.; Yoo, J.; Park, C.; Koo, H.; Choi, H.S. Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv. Drug Deliv. Rev. 2019, 138, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Mantanona, A.J.; Mao, H.; McCallum, N.C.; Jiao, Y.; Battistella, C.; Caponetti, V.; Zang, N.; Thompson, M.P.; Montalti, M.; et al. Radical-Enriched Artificial Melanin. Chem. Mater. 2020, 32, 5759–5767. [Google Scholar] [CrossRef]
- Chen, A.; Sun, J.; Liu, S.; Li, L.; Peng, X.; Ma, L.; Zhang, R. The effect of metal ions on endogenous melanin nanoparticles used as magnetic resonance imaging contrast agents. Biomater. Sci. 2020, 8, 379–390. [Google Scholar] [CrossRef]
- Xu, W.; Sun, J.; Li, L.; Peng, X.; Zhang, R.; Wang, B. Melanin-manganese nanoparticles with ultrahigh efficient clearance: In vivo for tumor-targeting T 1 magnetic resonance imaging contrast agent. Biomater. Sci. 2018, 6, 207–215. [Google Scholar] [CrossRef]
- Ju, K.Y.; Lee, J.W.; Im, G.H.; Lee, S.; Pyo, J.; Park, S.B.; Lee, J.H.; Lee, J.K. Bio-inspired, melanin-like nanoparticles as a highly efficient contrast agent for T 1-weighted magnetic resonance imaging. Biomacromolecules 2013, 14, 3491–3497. [Google Scholar] [CrossRef]
- Addisu, K.D.; Hailemeskel, B.Z.; Mekuria, S.L.; Andrgie, A.T.; Lin, Y.C.; Tsai, H.C. Bioinspired, Manganese-Chelated Alginate-Polydopamine Nanomaterials for Efficient in Vivo T1-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2018, 10, 5147–5160. [Google Scholar] [CrossRef]
- Wang, Z.; Carniato, F.; Xie, Y.; Huang, Y.; Li, Y.; He, S.; Zang, N.; Rinehart, J.D.; Botta, M.; Gianneschi, N.C. High Relaxivity Gadolinium-Polydopamine Nanoparticles. Small 2017, 13, 1701830. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, Y.; Duan, Y. Application of polydopamine in tumor targeted drug delivery system and its drug release behavior. J. Control. Release 2018, 290, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Wang, Z.; Hao, S.; He, H.; Wan, Y.; Zhu, C.; Sun, L.P.; Cheng, G.; Zheng, S.Y. Mitochondria-Targeting Polydopamine Nanoparticles to Deliver Doxorubicin for Overcoming Drug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 16793–16802. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S.S.; et al. Transferring biomarker into molecular probe: Melanin nanoparticle as a naturally active platform for multimodality imaging. J. Am. Chem. Soc. 2014, 136, 15185–15194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Z.-Y.; Feng, H.-Y.; Bu, L.-H. Melanin-based nanomaterials: The promising nanoplatforms for cancer diagnosis and therapy. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102211. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Fu, L.-H.; Xu, H.; Wang, T.-F.; Lin, J.; Huang, P. Melanin/polydopamine-based nanomaterials for biomedical applications. Sci. China Chem. 2019, 62, 162–188. [Google Scholar] [CrossRef]
- Park, J.; Moon, H.; Hong, S. Recent advances in melanin-like nanomaterials in biomedical applications: A mini review. Biomater. Res. 2019, 23, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Yang, Y.; Liu, Y.; Pan, J.; Wang, J.; Man, F.; Zhang, W.; Liu, G. Melanin-Like Nanomaterials for Advanced Biomedical Applications: A Versatile Platform with Extraordinary Promise. Adv. Sci. 2020, 7, 1903129. [Google Scholar] [CrossRef] [Green Version]
- Cordero, R.J.B.; Casadevall, A. Melanin. Curr. Biol. 2020, 30, R142–R143. [Google Scholar] [CrossRef]
- Xie, W.; Pakdel, E.; Liang, Y.; Kim, Y.J.; Liu, D.; Sun, L.; Wang, X. Natural Eumelanin and Its Derivatives as Multifunctional Materials for Bioinspired Applications: A Review. Biomacromolecules 2019, 20, 4312–4331. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Hałdys, K.; Latajka, R. MedChemComm Thiosemicarbazones with tyrosinase inhibitory activity. Med. Chem. Commun. 2019, 10, 378. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of Eumelanin and Pheomelanin and Its Pathophysiological Implications. Photochem. Photobiol. 2018, 94, 409–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A.; et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucca, F.A.; Basso, E.; Cupaioli, F.A.; Ferrari, E.; Sulzer, D.; Casella, L.; Zecca, L. Neuromelanin of the human substantia Nigra: An update. Neurotox. Res. 2014, 25, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Lindquist, N.G. Melanin and neuromelanin binding of drugs and chemicals: Toxicological implications. Arch Toxicol. 2016, 3, 1883–1891. [Google Scholar] [CrossRef]
- Turick, C.E.; Knox, A.S.; Becnel, J.M.; Ekechukwu, A.A.; Millike, C.E. Properties and Function of Pyomelanin. Biopolymers 2010. [Google Scholar] [CrossRef] [Green Version]
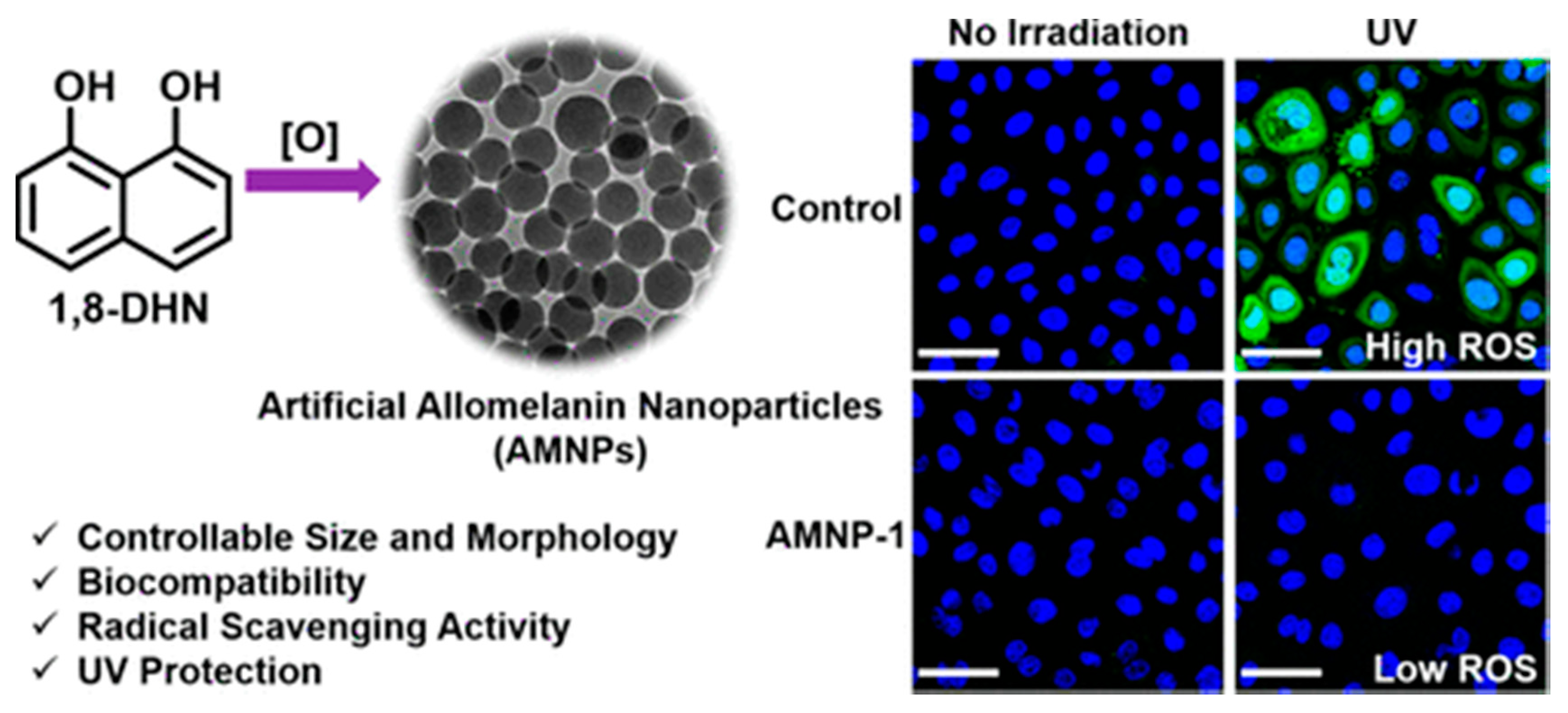
- Zhou, X.; McCallum, N.C.; Hu, Z.; Cao, W.; Gnanasekaran, K.; Feng, Y.; Stoddart, J.F.; Wang, Z.; Gianneschi, N.C. Artificial Allomelanin Nanoparticles. ACS Nano 2019, 13, 10980–10990. [Google Scholar] [CrossRef]
- Chu, M.; Hai, W.; Zhang, Z.; Wo, F.; Wu, Q.; Zhang, Z.; Shao, Y.; Zhang, D.; Jin, L.; Shi, D. Melanin nanoparticles derived from a homology of medicine and food for sentinel lymph node mapping and photothermal in vivo cancer therapy. Biomaterials 2016, 91, 182–199. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhou, Z.; Tan, L.; Wang, X.; Zheng, Y.; Han, Y.; Chen, D.F.; Yeung, K.W.K.; Cui, Z.; et al. Lysozyme-Assisted Photothermal Eradication of Methicillin-Resistant Staphylococcus aureus Infection and Accelerated Tissue Repair with Natural Melanosome Nanostructures. ACS Nano 2019, 13, 11153–11167. [Google Scholar] [CrossRef]
- Hong, S.; Zhang, Q.L.; Zheng, D.W.; Zhang, C.; Zhang, Y.; Ye, J.J.; Cheng, H.; Zhang, X.Z. Enzyme Mimicking Based on the Natural Melanin Particles from Human Hair. iScience 2020, 23, 100778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Deng, W.; Li, Y.; Huang, Z.; Meng, Y.; Xie, Q.; Ma, M.; Yao, S. Polymeric bionanocomposite cast thin films with in situ laccase-catalyzed polymerization of dopamine for biosensing and biofuel cell applications. J. Phys. Chem. B 2010, 114, 5016–5024. [Google Scholar] [CrossRef] [PubMed]
- Marjasvaara, A.; Torvinen, M.; Kinnunen, H.; Vainiotalo, P. Laccase-catalyzed polymerization of two phenolic compounds studied by matrix-assisted laser desorption/ionization time-of-flight and electrospray ionization fourier transform ion cyclotron resonance mass spectrometry with collision-induced dissociation ex. Biomacromolecules 2006, 7, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, T. Preparation and characterization of the dopamine film electrochemically deposited on a gold template and its applications for dopamine sensing in aqueous solution. Electrochim. Acta 2008, 53, 5725–5731. [Google Scholar] [CrossRef]
- Yu, F.; Chen, S.; Chen, Y.; Li, H.; Yang, L.; Chen, Y.; Yin, Y. Experimental and theoretical analysis of polymerization reaction process on the polydopamine membranes and its corrosion protection properties for 304 Stainless Steel. J. Mol. Struct. 2010, 982, 152–161. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Capozzi, V.; Perna, G.; Carmone, P.; Gallone, A.; Lastella, M.; Mezzenga, E.; Quartucci, G.; Ambrico, M.; Augelli, V.; Biagi, P.F.; et al. Optical and photoelectronic properties of melanin. Thin Solid Films 2006, 511–512, 362–366. [Google Scholar] [CrossRef]
- Kohl, F.R.; Grieco, C.; Kohler, B. Ultrafast spectral hole burning reveals the distinct chromophores in eumelanin and their common photoresponse. Chem. Sci. 2020, 11, 1248–1259. [Google Scholar] [CrossRef] [Green Version]
- Watt, A.A.R.; Bothma, J.P.; Meredith, P. The supramolecular structure of melanin. Soft Matter. 2009, 5, 3754–3760. [Google Scholar] [CrossRef]
- Chen, C.-T.; Ball, V.; de Almeida Gracio, J.J.; Singh, M.K.; Toniazzo, V.; Ruch, D.; Buehler, M.J. Self-Assembly of Tetramers of 5,6-Dihydroxyindole Explains the Primary Physical Properties of Eumelanin: Experiment, Simulation, and Design. ACS Nano 2013, 7, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.Y.; Fischer, M.C.; Warren, W.S. Understanding the Role of Aggregation in the Broad Absorption Bands of Eumelanin. ACS Nano 2018, 12, 12050–12061. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Yang, P.; Hu, J.; Wang, Z.; Li, Y. Size control synthesis of melanin-like polydopamine nanoparticles by tuning radicals. Polym. Chem. 2019, 10, 4194–4200. [Google Scholar] [CrossRef]
- Delparastan, P.; Malollari, K.G.; Lee, H.; Messersmith, P.B. Direct Evidence for the Polymeric Nature of Polydopamine. Angew. Chemie Int. Ed. 2019, 58, 1077–1082. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Chen, X.F.; Liu, X.H.; Wang, Z.; Li, Y.W. Recent developments in polydopamine fluorescent nanomaterials. Mater. Horizons 2020, 7, 746–761. [Google Scholar] [CrossRef]
- Meredith, P.; Riesz, J. Radiative Relaxation Quantum Yields for Synthetic Eumelanin. Photochem. Photobiol. 2004, 79, 211. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection versus photodamage: Updating an old but still unsolved controversy about melanin. Polym. Int. 2016, 65, 1276–1287. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, T.; Ma, P.; Bai, H.; Xie, Y.; Chen, M.; Dong, W. Simultaneous Enhancements of UV-Shielding Properties and Photostability of Poly(vinyl alcohol) via Incorporation of Sepia Eumelanin. ACS Sustain. Chem. Eng. 2016, 4, 2252–2258. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chemie Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic Building Blocks for the Assembly of Functional Materials. Angew. Chemie Int. Ed. 2019, 58, 1904–1927. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Molina, D.; Poseu, J.; Busqué, F.; Nador, F.; Mancebo-Aracil, J. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. 2018. [Google Scholar] [CrossRef]
- Mancebo-Aracil, J.; Casagualda, C.; Moreno-Villaécija, M.Á.; Nador, F.; García-Pardo, J.; Franconetti-García, A.; Busqué, F.; Alibés, R.; Esplandiu, M.J.; Ruiz-Molina, D.; et al. Bioinspired Functional Catechol Derivatives through Simple Thiol Conjugate Addition. Chem. A Eur. J. 2019, 25, 12367–12379. [Google Scholar] [CrossRef]
- Neto, A.I.; Vasconcelos, N.L.; Oliveira, S.M.; Ruiz-Molina, D.; Mano, J.F. High-Throughput Topographic, Mechanical, and Biological Screening of Multilayer Films Containing Mussel-Inspired Biopolymers. Adv. Funct. Mater. 2016, 26, 2745–2755. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Villaécija, M.-Á.; Sedó-Vegara, J.; Guisasola, E.; Baeza, A.; Regí, M.V.; Nador, F.; Ruiz-Molina, D. Polydopamine-like Coatings as Payload Gatekeepers for Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 7661–7669. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Delparastan, P.; Ney, M.R.; Gerst, M.; Messersmith, P.B. Cooperativity of Catechols and Amines in High-Performance Dry/Wet Adhesives. Angew. Chem. Int. Ed. 2020, 59, 16616–16624. [Google Scholar] [CrossRef]
- Lee, K.; Park, M.; Malollari, K.G.; Shin, J.; Winkler, S.M.; Zheng, Y.; Park, J.H.; Grigoropoulos, C.P.; Messersmith, P.B. Laser-induced graphitization of polydopamine leads to enhanced mechanical performance while preserving multifunctionality. Nat. Commun. 2020, 11, 4848. [Google Scholar] [CrossRef]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and Its Derivative Surface Chemistry in Material Science: A Focused Review for Studies at KAIST. Adv. Mater. 2020, 32, 1907505. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Ma, Y.; Zhou, F.; Hong, S.; Lee, H. Material-Independent Surface Chemistry beyond Polydopamine Coating. Acc. Chem. Res. 2019, 52, 704–713. [Google Scholar] [CrossRef]
- Park, H.K.; Park, J.H.; Lee, H.; Hong, S. Material-Selective Polydopamine Coating in Dimethyl Sulfoxide. ACS Appl. Mater. Interfaces 2020, 12, 49146–49154. [Google Scholar] [CrossRef]
- Liu, H.; Chu, C.; Liu, Y.; Pang, X.; Wu, Y.; Zhou, Z.; Zhang, P.; Zhang, W.; Liu, G.; Chen, X. Novel Intrapolymerization Doped Manganese-Eumelanin Coordination Nanocomposites with Ultrahigh Relaxivity and Their Application in Tumor Theranostics. Adv. Sci. 2018, 5, 1800032. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In Vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampazzo, E.; Boschi, F.; Bonacchi, S.; Juris, R.; Montalti, M.; Zaccheroni, N.; Prodi, L.; Calderan, L.; Rossi, B.; Becchi, S.; et al. Multicolor core/shell silica nanoparticles for in vivo and ex vivo imaging. Nanoscale 2012, 4, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalti, M.; Battistelli, G.; Cantelli, A.; Genovese, D. Photo-tunable multicolour fluorescence imaging based on self-assembled fluorogenic nanoparticles. Chem. Commun. 2014, 50, 5326. [Google Scholar] [CrossRef] [PubMed]
- Caponetti, V.; Trzcinski, J.W.; Cantelli, A.; Tavano, R.; Papini, E.; Mancin, F.; Montalti, M. Self-assembled biocompatible fluorescent nanoparticles for bioimaging. Front. Chem. 2019, 7, 168. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xu, L.; Feng, L.; Ji, Y.; Tao, L.; Li, S.; Wei, Y. Biocompatible polydopamine fluorescent organic nanoparticles: Facile preparation and cell imaging. Nanoscale 2012, 4, 5581. [Google Scholar] [CrossRef]
- Liu, M.; Ji, J.; Zhang, X.; Zhang, X.; Yang, B.; Deng, F.; Li, Z.; Wang, K.; Yang, Y.; Wei, Y. Self-polymerization of dopamine and polyethyleneimine: Novel fluorescent organic nanoprobes for biological imaging applications. J. Mater. Chem. B 2015, 3, 3476–3482. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jiang, R.; Liu, M.; Fu, L.; Zeng, G.; Wan, Q.; Mao, L.; Deng, F.; Zhang, X.; Wei, Y. Facile synthesis of polymeric fluorescent organic nanoparticles based on the self-polymerization of dopamine for biological imaging. Mater. Sci. Eng. C 2017, 77, 972–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.; Li, Y.; Hu, C.; Huang, Y.; He, Q.; Gao, H. Melanin-originated carbonaceous dots for triple negative breast cancer diagnosis by fluorescence and photoacoustic dual-mode imaging. J. Colloid Interface Sci. 2017, 497, 226–232. [Google Scholar] [CrossRef]
- Amin, D.R.; Sugnaux, C.; Lau, K.H.A.; Messersmith, P.B. Size control and fluorescence labeling of polydopamine melanin-Mimetic nanoparticles for intracellular imaging. Biomimetics 2017, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zou, Y.; Zhong, Y.; Liao, G.; Yu, C.; Xu, Z. Polydopamine-Based Tumor-Targeted Multifunctional Reagents for Computer Tomography/Fluorescence Dual-Mode Bioimaging-Guided Photothermal Therapy. ACS Appl. Bio. Mater. 2019, 2, 630–637. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.; Tan, X.; Zheng, A.; Zeng, Y.; Zhang, Z.; Zhang, X.; Liu, X. Sensitive fluorometric determination of glutathione using fluorescent polymer dots and the dopamine-melanin nanosystem. Microchim. Acta 2019, 186, 568. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, J.; Chen, X.; Chen, Y.; Hou, S.; Qian, H.; Zhang, L.; Tang, G.; Chen, Z.; Ping, Y.; et al. Mesoporous polydopamine with built-in plasmonic core: Traceable and NIR triggered delivery of functional proteins. Biomaterials 2020, 238, 119847. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Jiang, C.; Zhang, D.; Wang, Y.; Ren, X.; Ai, K.; Chen, X.; Lu, L. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017, 47, 124–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repenko, T.; Fokong, S.; De Laporte, L.; Go, D.; Kiessling, F.; Lammers, T.; Kuehne, A.J.C. Water-soluble dopamine-based polymers for photoacoustic imaging. Chem. Commun. 2015, 51, 6084–6087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, K.Y.; Kang, J.; Pyo, J.; Lim, J.; Chang, J.H.; Lee, J.K. PH-Induced aggregated melanin nanoparticles for photoacoustic signal amplification. Nanoscale 2016, 8, 14448–14456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Ji, Y.; Hu, X.; Cui, C.; Liu, H.; Tang, Y.; Qi, B.; Niu, Y.; Hu, X.; Yu, A.; et al. Cationic poly-l-lysine-encapsulated melanin nanoparticles as efficient photoacoustic agents targeting to glycosaminoglycans for the early diagnosis of articular cartilage degeneration in osteoarthritis. Nanoscale 2018, 10, 13471–13484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Zheng, Y.; Zhang, H.; Sun, J.H.; Tan, C.P.; He, L.; Zhang, W.; Ji, L.N.; Mao, Z.W. Delivery of Phosphorescent Anticancer Iridium(III) Complexes by Polydopamine Nanoparticles for Targeted Combined Photothermal-Chemotherapy and Thermal/Photoacoustic/Lifetime Imaging. Adv. Sci. 2018, 5, 1800581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gujrati, V.; Prakash, J.; Malekzadeh-Najafabadi, J.; Stiel, A.; Klemm, U.; Mettenleiter, G.; Aichler, M.; Walch, A.; Ntziachristos, V. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauffer, R.B. Paramagnetic Metal Complexes as Water Proton Relaxation Agents for NMR Imaging: Theory and Design. Chem. Rev. 1987, 87, 901–927. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; She, W.; Li, N.; Jiang, L.; Luo, K.; Gong, Q.; Gu, Z. A dendronized heparin–gadolinium polymer self-assembled into a nanoscale system as a potential magnetic resonance imaging contrast agent. Polym. Chem. 2016, 7, 2531–2541. [Google Scholar] [CrossRef]
- Cai, W.W.; Wang, L.J.; Li, S.J.; Zhang, X.P.; Li, T.T.; Wang, Y.H.; Yang, X.; Xie, J.; Li, J.D.; Liu, S.J.; et al. Effective tracking of bone mesenchymal stem cells in vivo by magnetic resonance imaging using melanin-based gadolinium3+ nanoparticles. J. Biomed. Mater. Res. Part A 2017, 105, 131–137. [Google Scholar] [CrossRef]
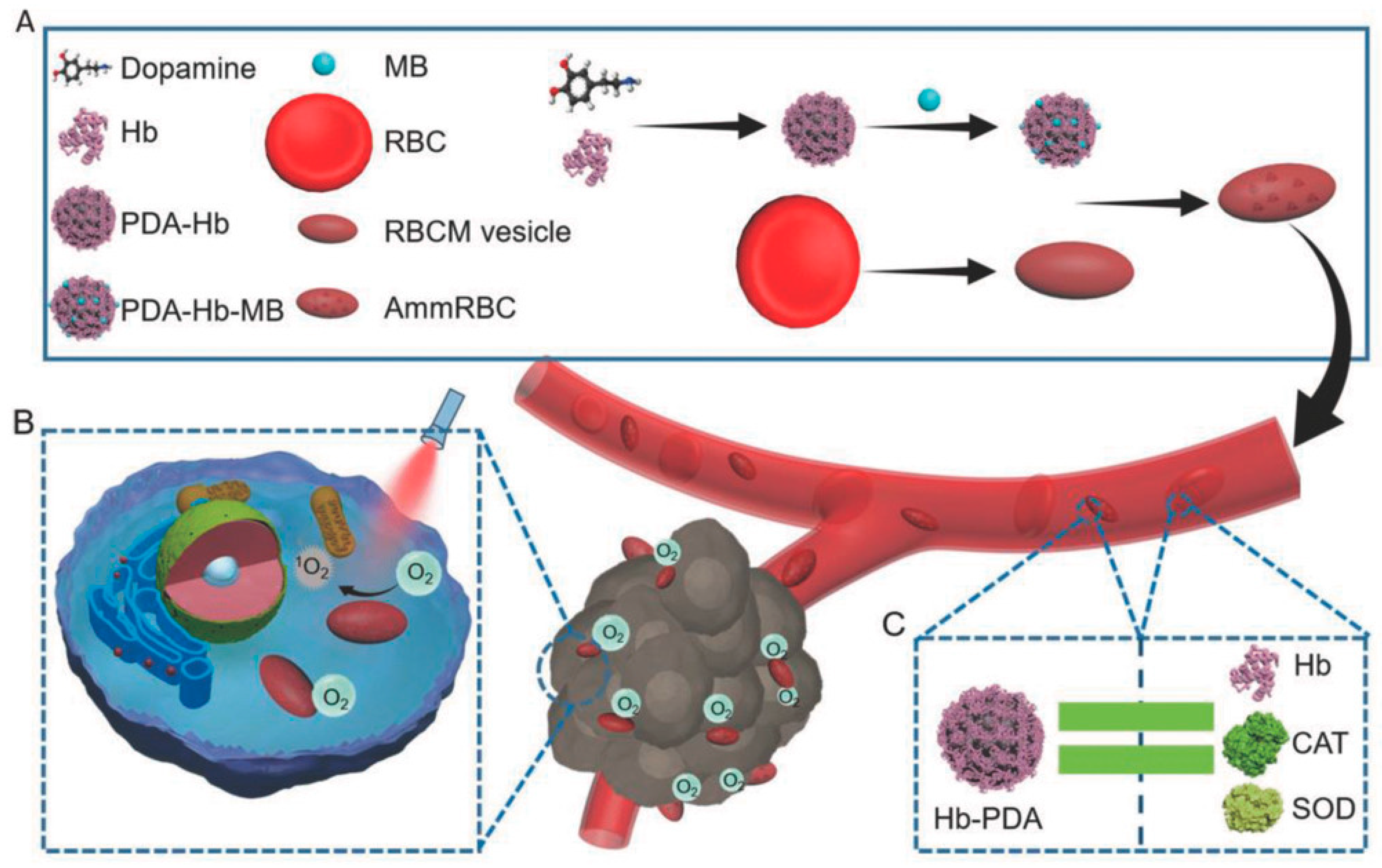
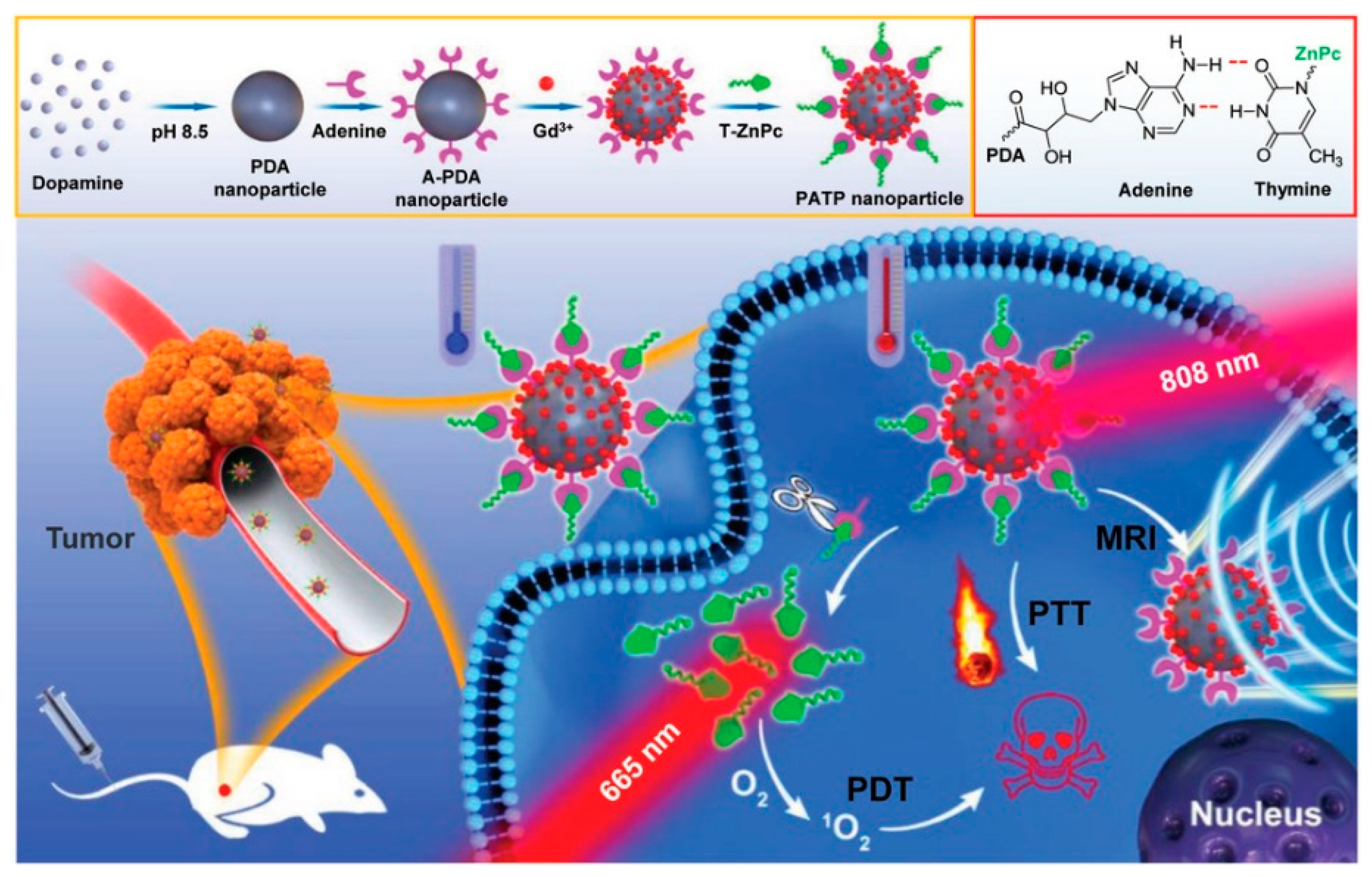
- Zhan, Q.; Shi, X.; Zhou, J.; Zhou, L.; Wei, S. Drug Delivery: Drug-Controlled Release Based on Complementary Base Pairing Rules for Photodynamic-Photothermal Synergistic Tumor Treatment. Small 2019, 15, 1970019. [Google Scholar] [CrossRef] [Green Version]
- Gale, E.M.; Atanasova, I.P.; Blasi, F.; Ay, I.; Caravan, P. A Manganese Alternative to Gadolinium for MRI Contrast. J. Am. Chem. Soc. 2015, 137, 15548–15557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Tian, X.M.; Yang, C.; Liu, P.; Luo, N.Q.; Liang, Y.; Li, H.B.; Chen, D.H.; Wang, C.X.; Li, L.; et al. Ultrahigh relaxivity and safe probes of manganese oxide nanoparticles for in vivo imaging. Sci. Rep. 2013, 3, 3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.C.; Lee, J.H.; Aoki, I.; Koretsky, A.P. Manganese-enhanced magnetic resonance imaging (MEMRI): Methodological and practical considerations. NMR Biomed. 2004, 17, 532–543. [Google Scholar] [CrossRef]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274. [Google Scholar] [CrossRef]
- Guan, Q.; Guo, R.; Huang, S.; Zhang, F.; Liu, J.; Wang, Z.; Yang, X.; Shuai, X.; Cao, Z. Mesoporous polydopamine carrying sorafenib and SPIO nanoparticles for MRI-guided ferroptosis cancer therapy. J. Control. Release 2020. [Google Scholar] [CrossRef] [PubMed]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Duan, X.; Guan, Q.; Liu, J.; Yang, X.; Zhang, F.; Huang, P.; Shen, J.; Shuai, X.; Cao, Z. Mesoporous Polydopamine Carrying Manganese Carbonyl Responds to Tumor Microenvironment for Multimodal Imaging-Guided Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Lemaster, J.E.; Wang, Z.; Hariri, A.; Chen, F.; Hu, Z.; Huang, Y.; Barback, C.V.; Cochran, R.; Gianneschi, N.C.; Jokerst, J.V. Gadolinium Doping Enhances the Photoacoustic Signal of Synthetic Melanin Nanoparticles: A Dual Modality Contrast Agent for Stem Cell Imaging. Chem. Mater. 2018, 31, 251–259. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Choe, J.W.; Pu, K.; Devulapally, R.; Bachawal, S.; Machtaler, S.; Chowdhury, S.M.; Luong, R.; Tian, L.; Khuri-Yakub, B.; et al. Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. J. Control. Release 2015, 203, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef]
- Wang, W.; Jing, T.; Xia, X.; Tang, L.; Huang, Z.; Liu, F.; Wang, Z.; Ran, H.; Li, M.; Xia, J. Melanin-loaded biocompatible photosensitive nanoparticles for controlled drug release in combined photothermal-chemotherapy guided by photoacoustic/ultrasound dual-modality imaging. Biomater. Sci. 2019, 7, 4060–4074. [Google Scholar] [CrossRef]
- Dai, Y.; Xiao, H.; Liu, J.; Yuan, Q.; Ma, P.; Yang, D.; Li, C.; Cheng, Z.; Hou, Z.; Yang, P.; et al. In Vivo Multimodality Imaging and Cancer Therapy by Near-Infrared Light-Triggered trans-Platinum Pro-Drug-Conjugated Upconverison Nanoparticles. J. Am. Chem. Soc. 2013, 135, 18920–18929. [Google Scholar] [CrossRef]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Li, C.; Dai, Y.; Yang, G.; Lin, J. A Yolk-like Multifunctional Platform for Multimodal Imaging and Synergistic Therapy Triggered by a Single Near-Infrared Light. ACS Nano 2015, 9, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, L.; Li, X.; Zhang, L.; Li, L.; Zhang, X.; Wang, C. Co-delivery of hydrophilic/hydrophobic drugs by multifunctional yolk-shell nanoparticles for hepatocellular carcinoma theranostics. Chem. Eng. J. 2020, 389, 124416. [Google Scholar] [CrossRef]
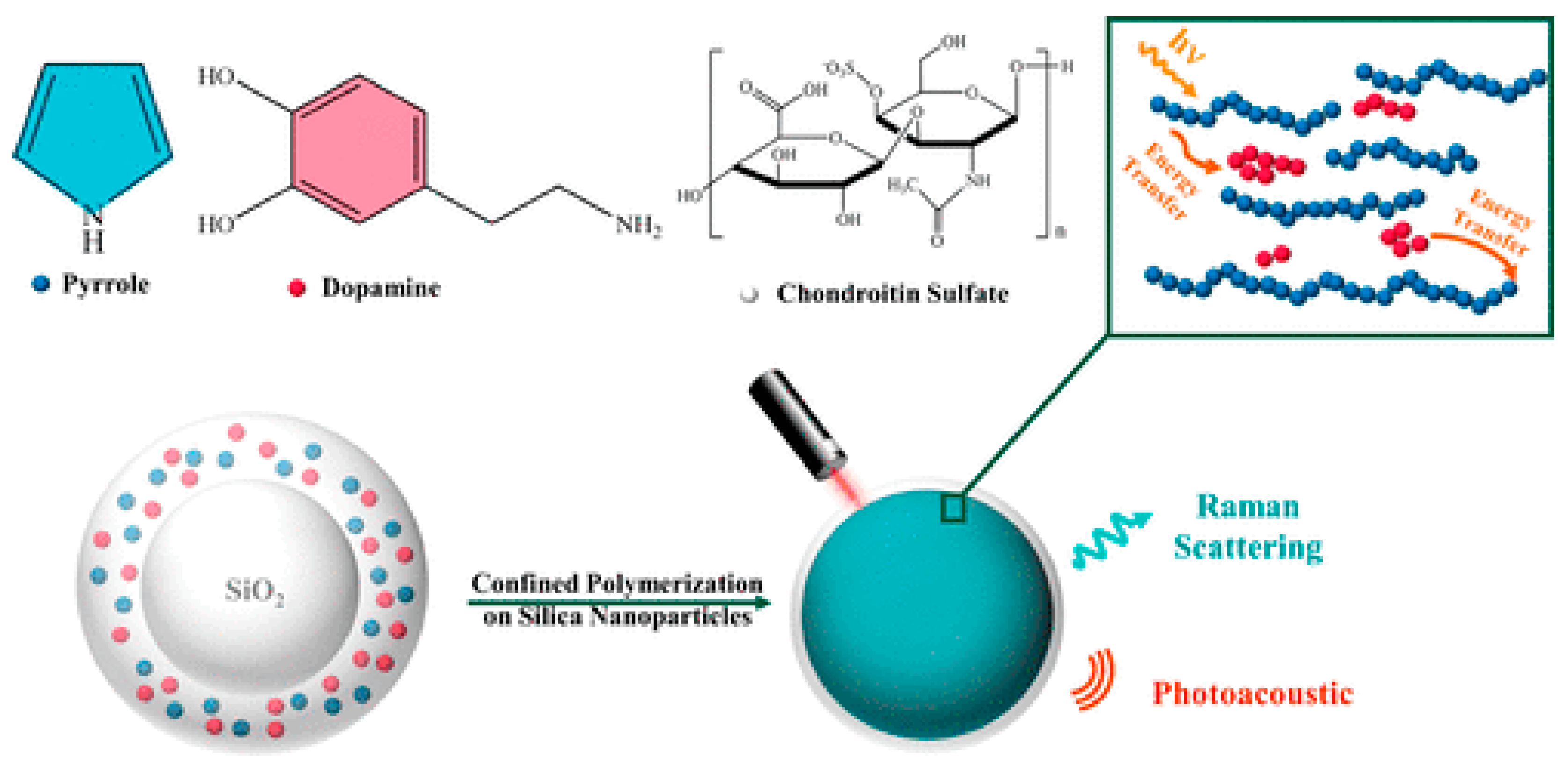
- Lin, Q.; Yang, Y.; Ma, Y.; Zhang, R.; Wang, J.; Chen, X.; Shao, Z. Bandgap Engineered Polypyrrole-Polydopamine Hybrid with Intrinsic Raman and Photoacoustic Imaging Contrasts. Nano Lett. 2018, 18, 7485–7493. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Zhu, Z.L.; Zhang, W.L.; Yin, Y.J.; Tang, Y.L.; Liang, X.H.; Zhang, L. Light stimulus responsive nanomedicine in the treatment of oral squamous cell carcinoma. Eur. J. Med. Chem. 2020, 199, 112394. [Google Scholar] [CrossRef]
- Aguilar, T.A.F.; Navarro, B.C.H.; Pérez, J.A.M. Endogenous Antioxidants: A Review of their Role in Oxidative Stress. In A Master Regulator of Oxidative Stress—The Transcription Factor Nrf2; InTech: Bergharen, The Netherlands, 2016. [Google Scholar]
- Liu, Y.; Shi, J. Antioxidative nanomaterials and biomedical applications. Nano Today 2019, 27, 146–177. [Google Scholar] [CrossRef]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, D.; Rosenkrans, Z.T.; Ehlerding, E.B.; Ni, D.; Qi, C.; Kutyreff, C.J.; Barnhart, T.E.; Engle, J.W.; Huang, P.; et al. A Melanin-Based Natural Antioxidant Defense Nanosystem for Theranostic Application in Acute Kidney Injury. Adv. Funct. Mater. 2019, 29, 1904833. [Google Scholar] [CrossRef]
- Zhao, H.; Zeng, Z.; Liu, L.; Chen, J.; Zhou, H.; Huang, L.; Huang, J.; Xu, H.; Xu, Y.; Chen, Z.; et al. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale 2018, 10, 6981–6991. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Pang, J.; Chen, J.; Wang, H.; Xie, Q.; Jiang, Y. Polydopamine-Mediated Carrier with Stabilizing and Self-Antioxidative Properties for Polyphenol Delivery Systems. Ind. Eng. Chem. Res. 2018, 57, 590–599. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, M.; Zeng, Y.; Wu, L.; Wang, Q.; Han, X.; Liu, X.; Liu, J. Chlorin e6 Conjugated Poly(dopamine) Nanospheres as PDT/PTT Dual-Modal Therapeutic Agents for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 8176–8187. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, C.; Xiao, X. Polydopamine modified TiO2 nanotube arrays as a local drug delivery system for ibuprofen. J. Drug Deliv. Sci. Technol. 2020, 56, 101537. [Google Scholar] [CrossRef]
- Lim, E.B.; Vy, T.A.; Lee, S.W. Comparative release kinetics of small drugs (ibuprofen and acetaminophen) from multifunctional mesoporous silica nanoparticles. J. Mater. Chem. B 2020, 8, 2096–2106. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Mohandas, A.; Jayakumar, R. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surfaces B Biointerfaces 2019, 177, 41–49. [Google Scholar] [CrossRef]
- Sardoiwala, M.N.; Srivastava, A.K.; Kaundal, B.; Karmakar, S.; Choudhury, S.R. Recuperative effect of metformin loaded polydopamine nanoformulation promoting EZH2 mediated proteasomal degradation of phospho-α-synuclein in Parkinson’s disease model. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102088. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Marcelo, G.; Martín Del Valle, E.M. Polydopamine nanoparticles kill cancer cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef] [Green Version]
- Tao, C.; Chen, T.; Liu, H.; Su, S. Preparation and adsorption performance research of large-volume hollow mesoporous polydopamine microcapsules. MRS Commun. 2019, 9, 744–749. [Google Scholar] [CrossRef]
- Tran, H.Q.; Bhave, M.; Xu, G.; Sun, C.; Yu, A. Synthesis of polydopamine hollow capsules via a polydopamine mediated silica water dissolution process and its application for enzyme encapsulation. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ozlu, B.; Kabay, G.; Bocek, I.; Yilmaz, M.; Piskin, A.K.; Shim, B.S.; Mutlu, M. Controlled release of doxorubicin from polyethylene glycol functionalized melanin nanoparticles for breast cancer therapy: Part I. Production and drug release performance of the melanin nanoparticles. Int. J. Pharm. 2019, 570, 118613. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, D.; Qi, X.; Guo, Y.; Yue, F.; Wang, X.; Han, M. Polydopamine-based surface modification of paclitaxel nanoparticles for osteosarcoma targeted therapy Recent citations. Nanotechnology 2019, 30, 255101. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Millot, N.; Maurizi, L.; Lizard, G.; Kumar, R. Taurine-Conjugated Mussel-Inspired Iron Oxide Nanoparticles with an Elongated Shape for Effective Delivery of Doxorubicin into the Tumor Cells. ACS Omega 2020, 5, 16165–16175. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wei, Z.; Song, C.; Tang, C.; Han, W.; Dong, X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2019, 48, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging: Via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Jeong, C.; Kim, W.J. Synergistic nanomedicine by combined gene and photothermal therapy. Adv. Drug Deliv. Rev. 2016, 98, 99–112. [Google Scholar] [CrossRef]
- Sidharth Shibu, E.; Hamada, M.; Murase, N.; Biju, V. Invited review Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 53–72. [Google Scholar] [CrossRef]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-Infrared Resonant Nanoshells for Combined Optical Imaging and Photothermal Cancer Therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2015, 81, 114–124. [Google Scholar] [CrossRef]
- Liopo, A.; Su, R.; Oraevsky, A.A. Melanin nanoparticles as a novel contrast agent for optoacoustic tomography. Photoacoustics 2015, 3, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Zhang, S.; Zhang, N.; Wang, Y.; Zhong, J.; Sun, X.; Qi, Y.; Chen, X.; Li, Z.; Li, Y. Tailoring Synthetic Melanin Nanoparticles for Enhanced Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 42671–42679. [Google Scholar] [CrossRef]
- Sun, J.; Xu, W.; Li, L.; Fan, B.; Peng, X.; Qu, B.; Wang, L.; Li, T.; Li, S.; Zhang, R. Ultrasmall endogenous biopolymer nanoparticles for magnetic resonance/photoacoustic dual-modal imaging-guided photothermal therapy. Nanoscale 2018, 10, 10584–10595. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Sun, F.; Zhang, Y.; Yang, Z.; Liu, P.; Zou, Y.; Yu, Y.; Tong, F.; Yi, C.; Yang, S.; et al. Polydopamine-mediated bio-inspired synthesis of copper sulfide nanoparticles for T 1-weighted magnetic resonance imaging guided photothermal cancer therapy. Colloids Surfaces B Biointerfaces 2018, 173, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, X.; Liu, Y.; Ye, Y.; Yu, J.; Chen, Q.; Wang, J.; Zhang, Y.; Hu, Q.; Kang, Y.; et al. Shape-controlled synthesis of liquid metal nanodroplets for photothermal therapy. Nano Res. 2019, 12, 1313–1320. [Google Scholar] [CrossRef]
- Kong, C.; Hao, M.; Chen, X.; Zhao, X.; Wang, Y.; Li, J.; Gao, Y.; Zhang, H.; Yang, B.; Jiang, J. NF-κB inhibition promotes apoptosis in androgen-independent prostate cancer cells by the photothermal effect: Via the IκBα/AR signaling pathway. Biomater. Sci. 2019, 7, 2559–2570. [Google Scholar] [CrossRef]
- Colombo, L.L.; Vanzulli, S.I.; Blázquez-Castro, A.; Terrero, C.S.; Stockert, J.C. Photothermal effect by 808-nm laser irradiation of melanin: A proof-of-concept study of photothermal therapy using B16-F10 melanotic melanoma growing in BALB/c mice. Biomed. Opt. Express 2019, 10, 2932. [Google Scholar] [CrossRef]
- Jiang, Q.; Luo, Z.; Men, Y.; Yang, P.; Peng, H.; Guo, R.; Tian, Y.; Pang, Z.; Yang, W. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials 2017, 143, 29–45. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, N.; Yu, W.; Xu, H.; Li, X.; Li, M.; Peng, C.; Wang, Q.; Zhu, M.; Chen, Z. In situ growth of Au nanoparticles on natural melanin as biocompatible and multifunctional nanoagent for efficient tumor theranostics. J. Mater. Chem. B 2019, 7, 133–142. [Google Scholar] [CrossRef]
- Kim, M.A.; Yoon, S.D.; Kim, E.M.; Jeong, H.J.; Lee, C.M. Natural melanin-loaded nanovesicles for near-infrared mediated tumor ablation by photothermal conversion. Nanotechnology 2018, 29, 415101. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, D.; Wang, D.; Yao, Y.; Yang, K.; Wang, Z.; Deng, L.; Chen, Y. Bioinspired multifunctional melanin-based nanoliposome for photoacoustic/magnetic resonance imaging-guided efficient photothermal ablation of cancer. Theranostics 2018, 8, 1591–1606. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Wang, L.; Zhang, Q.; Cheng, Y. Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy. Biomaterials 2019, 203, 63–72. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-S.; Peng, S.-J.; Li, G.-F.; Zhao, Y.-X.; Meng, X.-Y.; Yu, X.-R.; Li, Z.-H.; Chen, J.-M. Polydopamine Nanoparticles for Deep Brain Ablation via Near-Infrared Irradiation. ACS Biomater. Sci. Eng. 2019, 6, 664–672. [Google Scholar] [CrossRef]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy—Strategies and perspectives. J. Control. Release 2016, 240, 489–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Li, Z.; Song, W.; Rubinstein, M.; Liu, D. Recent updates in cancer immunotherapy: A comprehensive review and perspective of the 2018 China Cancer Immunotherapy Workshop in Beijing. J. Hematol. Oncol. 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Wang, X.; Tian, X.; Qin, W.; Wang, X.; Liang, J.; Zhang, H.; Leng, X. Polydopamine as the Antigen Delivery Nanocarrier for Enhanced Immune Response in Tumor Immunotherapy. ACS Biomater. Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Chen, W.; Qin, M.; Chen, X.; Wang, Q.; Zhang, Z.; Sun, X. Combining photothermal therapy and immunotherapy against melanoma by polydopamine-coated Al2O3 nanoparticles. Theranostics 2018, 8, 2229–2241. [Google Scholar] [CrossRef]
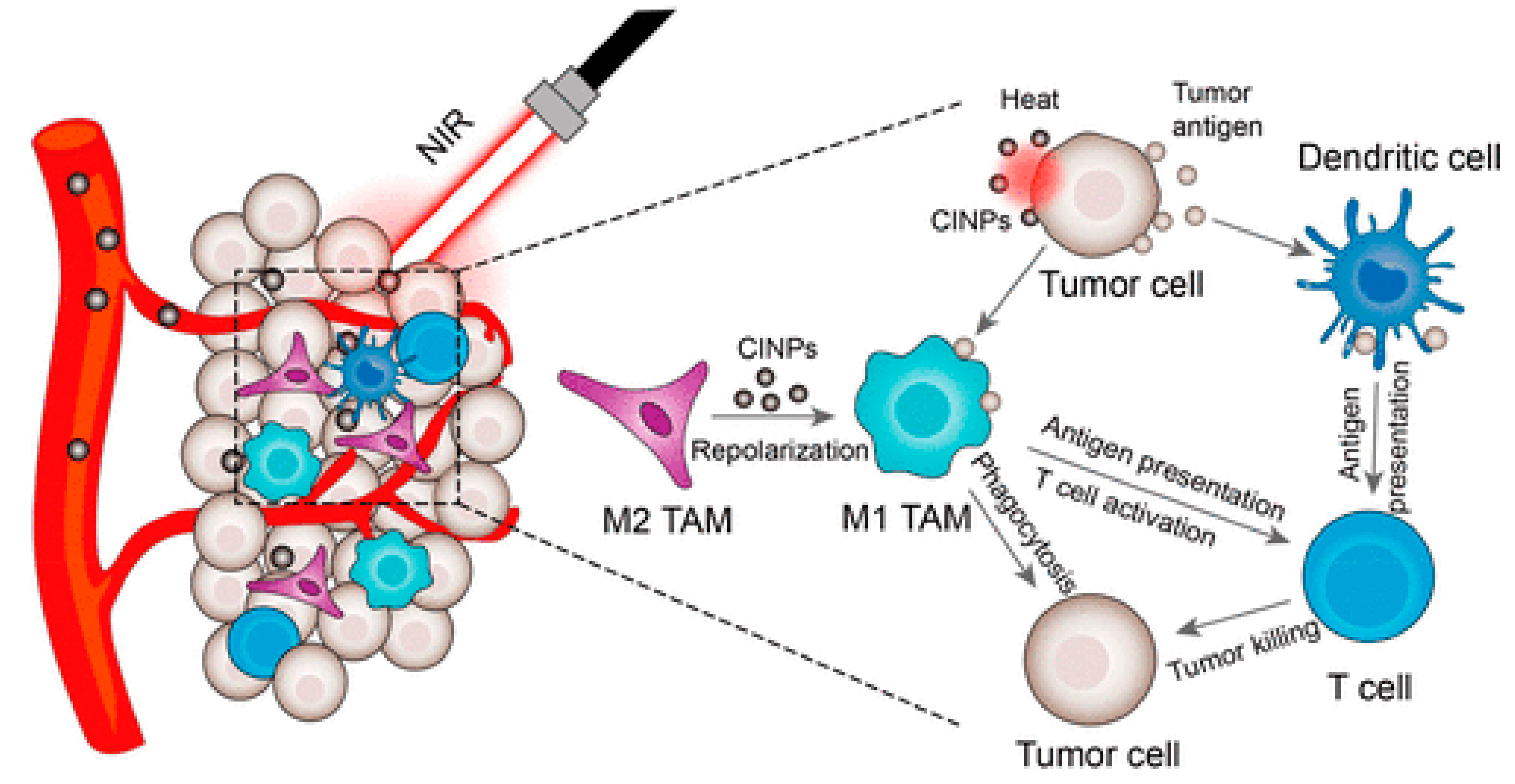
- Rong, L.; Zhang, Y.; Li, W.-S.; Su, Z.; Fadhil, I.; Zhang, C. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials 2019, 225, 119515. [Google Scholar] [CrossRef]
- Deng, R.H.; Zou, M.Z.; Zheng, D.; Peng, S.Y.; Liu, W.; Bai, X.F.; Chen, H.S.; Sun, Y.; Zhou, P.H.; Zhang, X.Z. Nanoparticles from Cuttlefish Ink Inhibit Tumor Growth by Synergizing Immunotherapy and Photothermal Therapy. ACS Nano 2019, 13, 8618–8629. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Pan, W.; Li, N.; Tang, B.; Li, R. Photothermal therapy-induced immunogenic cell death based on natural melanin nanoparticles against breast cancer. Chem. Commun 2020, 56, 1389. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, C.; Zhang, X.; Hu, Q.; Zhang, Y.; Liu, Q.; Wen, D.; Milligan, J.; Bellotti, A.; Huang, L.; et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol. 2017, 2, eaan5692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coccia, M.; Wang, L. Path-breaking directions of nanotechnology-based chemotherapy and molecular cancer therapy. Technol. Forecast. Soc. Chang. 2014, 94, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wang, S.; Chen, K.; Zhao, Y.; Ma, X.; Wang, L. Doxorubicin-Loaded Melanin Particles for Enhanced Chemotherapy in Drug-Resistant Anaplastic Thyroid Cancer Cells. J. Nanomater. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhao, X.; Guo, H. Synergic highly effective photothermal-chemotherapy with platinum prodrug linked melanin-like nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Ding, F.; Chen, Z.; Zhang, R.; Li, C.; Xu, Y.; Zhang, Y.; Ni, R.; Li, X.; Yang, G.; et al. Melanin-dot–mediated delivery of metallacycle for NIR-II/photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 16729–16735. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, Q.; He, X.; Chen, H.; Zou, Y.; Li, Y.; Lin, K.; Cai, X.; Xiao, J.; Zhang, Q.; et al. Multifunctional melanin-like nanoparticles for bone-targeted chemo-photothermal therapy of malignant bone tumors and osteolysis. Biomaterials 2018, 183, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wu, J.; Jia, Z.; Tang, P.; Sheng, J.; Xie, C.; Liu, C.; Gan, D.; Hu, D.; Zheng, W.; et al. An Injectable, Bifunctional Hydrogel with Photothermal Effects for Tumor Therapy and Bone Regeneration. Macromol. Biosci. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, N.; Yuan, W. NIR/Thermoresponsive Injectable Self-Healing Hydrogels Containing Polydopamine Nanoparticles for Efficient Synergistic Cancer Thermochemotherapy. Cite This ACS Appl. Mater. Interfaces 2020, 12, 9131. [Google Scholar] [CrossRef]
- Obiweluozor, F.O.; Emechebe, G.A.; Tiwari, A.P.; Kim, J.Y.; Park, C.H.; Kim, C.S. Short duration cancer treatment: Inspired by a fast bio-resorbable smart nano-fiber device containing NIR lethal polydopamine nanospheres for effective chemo–photothermal cancer therapy. Int. J. Nanomed. 2018, 13, 6375–6390. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wu, J.; Bremner, D.H.; Niu, S.; Li, Y.; Zhang, X.; Xie, X.; Zhu, L.M. A multifunctional nanoplatform based on MoS2-nanosheets for targeted drug delivery and chemo-photothermal therapy. Colloids Surfaces B Biointerfaces 2020, 185, 110585. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, W.; Zhang, H.; Zhang, T.T.; Chen, Z.; Yuan, S.; Peng, P.; Xiao, M.; Xu, L. Photothermally triggered cytosolic drug delivery of glucose functionalized polydopamine nanoparticles in response to tumor microenvironment for the GLUT1-targeting chemo-phototherapy. J. Control. Release 2020, 317, 232–245. [Google Scholar] [CrossRef] [PubMed]
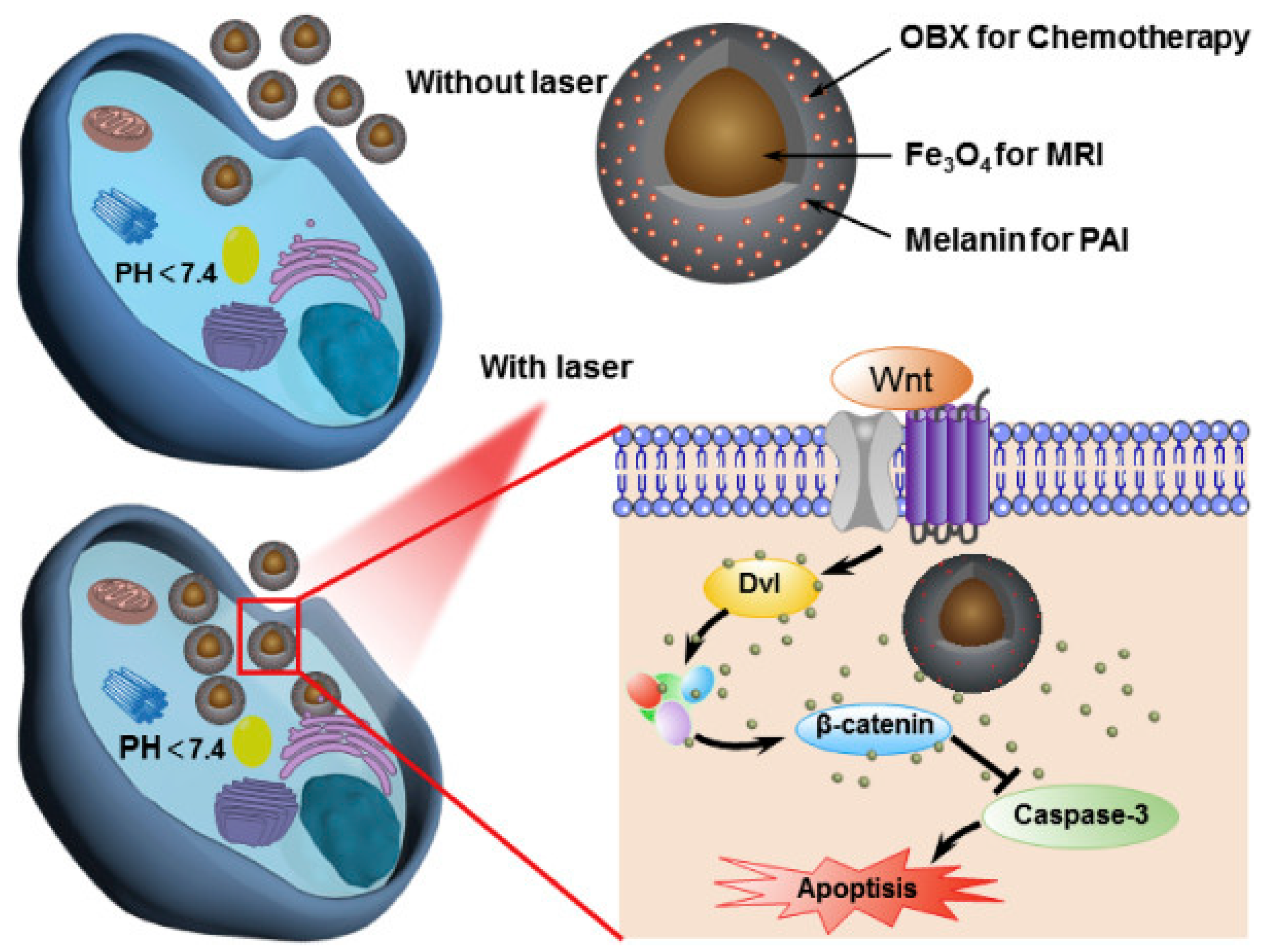
- Feng, T.; Zhou, L.; Wang, Z.; Li, C.; Zhang, Y.; Lin, J.; Lu, D.; Huang, P. Dual-stimuli responsive nanotheranostics for mild hyperthermia enhanced inhibition of Wnt/β-catenin signaling. Biomaterials 2020, 232, 119709. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Qu, H.; Wu, D.; Zhu, C.; Yang, Y.; Jin, X.; Zheng, J.; Shi, X.; Yan, X.; Wang, Y. Platelet-camouflaged nanococktail: Simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics 2018, 8, 2683–2695. [Google Scholar] [CrossRef]
- Chen, X.; Tong, R.; Liu, B.; Liu, H.; Feng, X.; Ding, S.; Lei, Q.; Tang, G.; Wu, J.; Fang, W. Duo of (-)-epigallocatechin-3-gallate and doxorubicin loaded by polydopamine coating ZIF-8 in the regulation of autophagy for chemo-photothermal synergistic therapy. Biomater. Sci. 2020, 8, 1380–1393. [Google Scholar] [CrossRef]
- Liu, S.; Pan, J.; Liu, J.; Ma, Y.; Qiu, F.; Mei, L.; Zeng, X.; Pan, G. Dynamically PEGylated and Borate-Coordination-Polymer-Coated Polydopamine Nanoparticles for Synergetic Tumor-Targeted, Chemo-Photothermal Combination Therapy. Small 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, J.; Chen, F.; Liu, J.; Cai, K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale 2017, 9, 8781–8790. [Google Scholar] [CrossRef]
- Wang, J.; Chai, J.; Liu, L.; Cui, Z.; Duan, D.; Shi, R.; Zhang, Y. Dual-functional melanin-based nanoliposomes for combined chemotherapy and photothermal therapy of pancreatic cancer. RSC Adv. 2019, 9, 3012–3019. [Google Scholar] [CrossRef] [Green Version]
- Saw, P.E.; Song, E.W. siRNA therapeutics: A clinical reality. Sci. China Life Sci. 2020, 63, 485–500. [Google Scholar] [CrossRef]
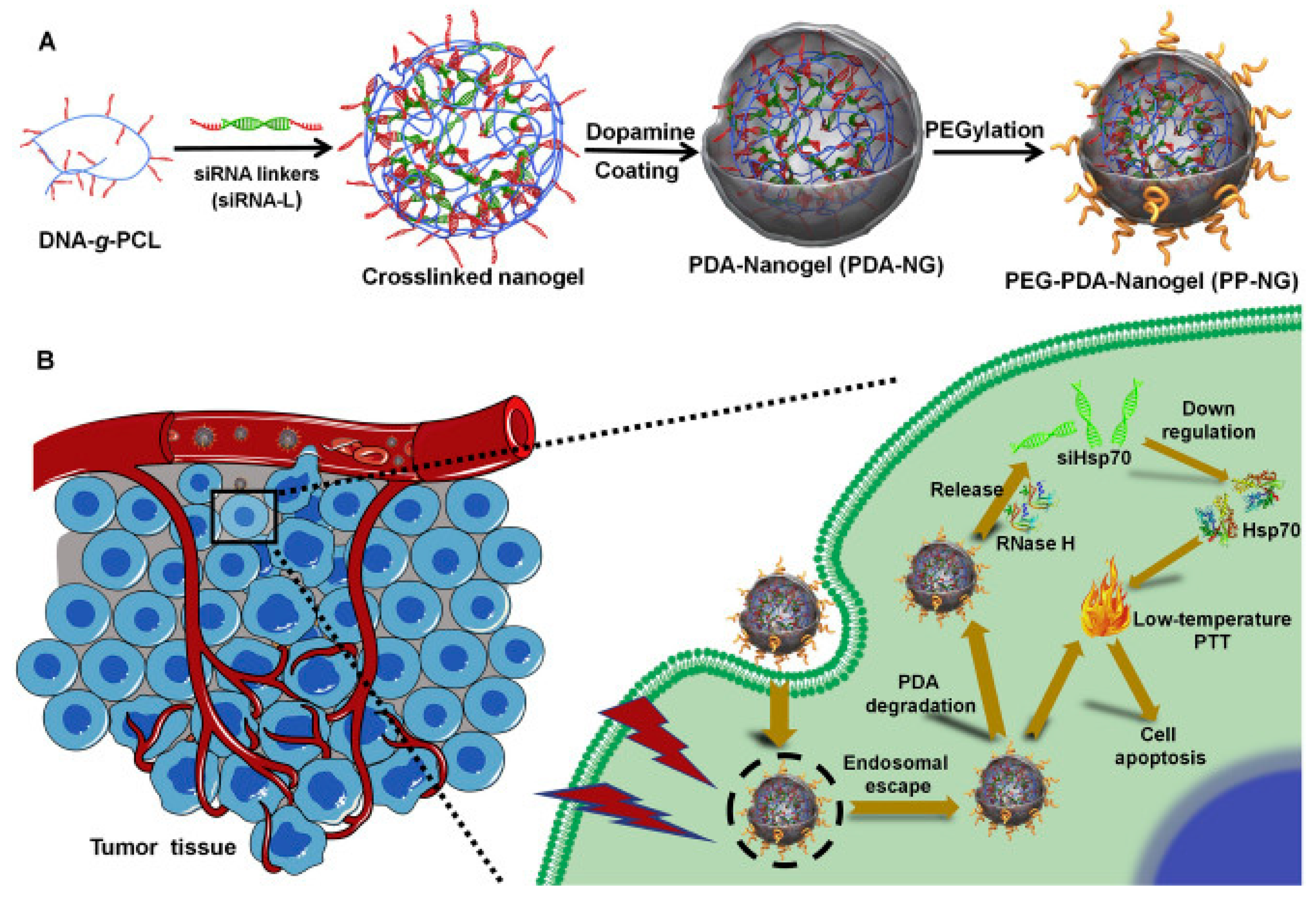
- Ding, F.; Gao, X.; Huang, X.; Ge, H.; Xie, M.; Qian, J.; Song, J.; Li, Y.; Zhu, X.; Zhang, C. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy. Biomaterials 2020, 245. [Google Scholar] [CrossRef]
- Yang, X.; Fan, B.; Gao, W.; Li, L.; Li, T.; Sun, J.; Peng, X.; Li, X.; Wang, Z.; Wang, B.; et al. Enhanced endosomal escape by photothermal activation for improved small interfering RNA delivery and antitumor effect. Int. J. Nanomed. 2018, 13, 4333–4344. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.; Li, J.; Yan, S.; Zhang, H.; Zhang, W.; Zhang, F.; Jiang, J. SiRNA Delivery with Stem Cell Membrane-Coated Magnetic Nanoparticles for Imaging-Guided Photothermal Therapy and Gene Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3895–3905. [Google Scholar] [CrossRef]
- Fan, B.; Yang, X.; Li, X.; Lv, S.; Zhang, H.; Sun, J.; Li, L.; Wang, L.; Qu, B.; Peng, X.; et al. Photoacoustic-imaging-guided therapy of functionalized melanin nanoparticles: Combination of photothermal ablation and gene therapy against laryngeal squamous cell carcinoma. Nanoscale 2019, 11, 6285–6296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, Q.; Li, X.; Wang, Y. pH-responsive polydopamine nanoparticles for photothermally promoted gene delivery. Mater. Sci. Eng. C 2020, 108, 110396. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Yang, Y.; Zhang, C.; Zhao, N.; Xu, F.-J. NIR-Responsive Polycationic Gatekeeper-Cloaked Hetero-Nanoparticles for Multimodal Imaging-Guided Triple-Combination Therapy of Cancer. Small 2017, 13, 1603133. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Gao, N.; Liu, G.; Tao, W.; Xiao, X.; Jiang, L.; Liu, Z.; Zeng, X.; Mei, L. A Multifunctional Nanoplatform against Multidrug Resistant Cancer: Merging the Best of Targeted Chemo/Gene/Photothermal Therapy. Adv. Funct. Mater. 2017, 27, 1–15. [Google Scholar] [CrossRef]
- Shim, G.; Park, J.; Kim, M.-G.; Yang, G.; Lee, Y.; Oh, Y.-K. Noncovalent tethering of nucleic acid aptamer on DNA nanostructure for targeted photo/chemo/gene therapies. Nanomed. Nanotechnol. Biol. Med. 2019, 24, 102053. [Google Scholar] [CrossRef]
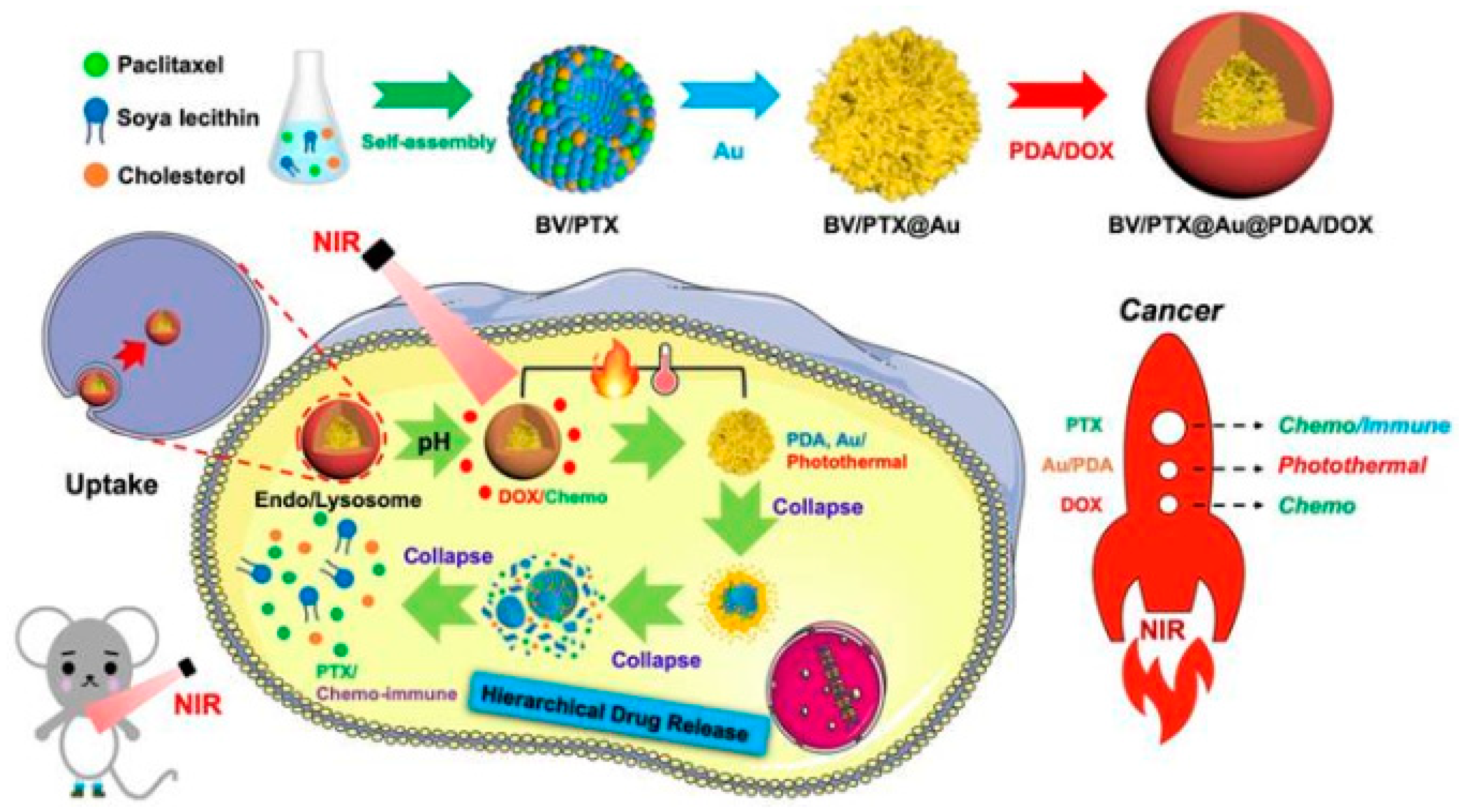
- He, Y.; Cong, C.; Li, X.; Zhu, R.; Li, A.; Zhao, S.; Li, X.; Cheng, X.; Yang, M.; Gao, D. Nano-drug system based on hierarchical drug release for deep localized/systematic cascade tumor therapy stimulating antitumor immune responses. Theranostics 2019, 9, 2897–2909. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.-S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- McFarland, S.A.; Mandel, A.; Dumoulin-White, R.; Gasser, G. Metal-based photosensitizers for photodynamic therapy: The future of multimodal oncology? Curr. Opin. Chem. Biol. 2020, 56, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Chinna Ayya Swamy, P.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Girotti, A.W.; Fahey, J.M. Upregulation of pro-tumor nitric oxide by anti-tumor photodynamic therapy. Biochem. Pharmacol. 2019, 176, 113750. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Lin, J.; Fu, L.H.; Huang, P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018, 47, 357–403. [Google Scholar] [CrossRef] [PubMed]
- Konan, Y.N.; Gurny, R.; Allémann, E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2002, 66, 89–106. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, Z.; Li, Z. Polydopamine-Based Nanocarriers for Photosensitizer Delivery. Front. Chem. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef]
- Feng, C.; Zhu, D.; Chen, L.; Lu, Y.; Liu, J.; Kim, N.Y.; Liang, S.; Zhang, X.; Lin, Y.; Ma, Y.; et al. Targeted Delivery of Chlorin e6 via Redox Sensitive Diselenide-Containing Micelles for Improved Photodynamic Therapy in Cluster of Differentiation 44-Overexpressing Breast Cancer. Front. Pharmacol. 2019, 10, 369. [Google Scholar] [CrossRef]
- Liu, W.L.; Liu, T.; Zou, M.Z.; Yu, W.Y.; Li, C.X.; He, Z.Y.; Zhang, M.K.; Liu, M.D.; Li, Z.H.; Feng, J.; et al. Aggressive Man-Made Red Blood Cells for Hypoxia-Resistant Photodynamic Therapy. Adv. Mater. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Liu, W.; Liu, Y.; Tang, Y.; Teng, Z.; Zhang, C.; Wang, S.; Lu, G. Photosensitizer-loaded biomimetic platform for multimodal imaging-guided synergistic phototherapy. RSC Adv. 2018, 8, 32200–32210. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guo, Y.; Hu, J.; Li, W.; Kang, Y.; Cao, Y.; Liu, H. Development of Multifunctional Polydopamine Nanoparticles As a Theranostic Nanoplatform against Cancer Cells. Langmuir 2018, 34, 9516–9524. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, W.; Xu, Z.; Wang, F. An intelligent ZIF-8-gated polydopamine nanoplatform for in vivo cooperatively enhanced combination phototherapy. Chem. Sci. 2020, 11, 1649–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batul, R.; Bhave, M.; Mahon, J.P.; Yu, A. Polydopamine Nanosphere with In-Situ Loaded Gentamicin and Its Antimicrobial Activity. Molecules 2020, 25, 2090. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Yeroslavsky, G.; Lavi, R.; Alishaev, A.; Rahimipour, S. Sonochemically-Produced Metal-Containing Polydopamine Nanoparticles and Their Antibacterial and Antibiofilm Activity. Langmuir 2016, 32, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, J.; Lee, Y.M.; Park, J.; Kim, W.J. Polydopamine Hollow Nanoparticle Functionalized with N-diazeniumdiolates as a Nitric Oxide Delivery Carrier for Antibacterial Therapy. Adv. Healthc. Mater. 2016, 5, 2019–2024. [Google Scholar] [CrossRef]
- Fasciani, C.; Silvero, M.J.; Anghel, M.A.; Argüello, G.A.; Becerra, M.C.; Scaiano, J.C. Aspartame-stabilized gold-silver bimetallic biocompatible nanostructures with plasmonic photothermal properties, antibacterial activity, and long-term stability. J. Am. Chem. Soc. 2014, 136, 17394–17397. [Google Scholar] [CrossRef] [Green Version]
- Calvaresi, M.; Arnesano, F.; Bonacchi, S.; Bottoni, A.; Calò, V.; Conte, S.; Falini, G.; Fermani, S.; Losacco, M.; Montalti, M.; et al. C60@Lysozyme: Direct observation by nuclear magnetic resonance of a 1:1 fullerene protein adduct. ACS Nano 2014, 8, 1871–1877. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Sitek, A.; Rosset, I.; Żądzińska, E.; Kasielska-Trojan, A.; Neskoromna-Jędrzejczak, A.; Antoszewski, B. Skin color parameters and Fitzpatrick phototypes in estimating the risk of skin cancer: A case-control study in the Polish population. J. Am. Acad. Dermatol. 2016. [Google Scholar] [CrossRef]
- Wu, X.S.; Masedunskas, A.; Weigert, R.; Copeland, N.G.; Jenkins, N.A.; Hammer, J.A. Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc. Natl. Acad. Sci. USA 2012, 109, E2101–E2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bino, S.; Bernerd, F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br. J. Dermatol. 2013, 169, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vij, M.; Grover, R.; Gotherwal, V.; Wani, N.A.; Joshi, P.; Gautam, H.; Sharma, K.; Chandna, S.; Gokhale, R.S.; Rai, R.; et al. Bioinspired Functionalized Melanin Nanovariants with a Range of Properties Provide Effective Color Matched Photoprotection in Skin. Biomacromolecules 2016, 17, 2912–2919. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.; Allen, M.C.; Deheyn, D.D.; Yue, X.; Zhao, J.; Gianneschi, N.C.; Shawkey, M.D.; Dhinojwala, A. Bio-inspired structural colors produced via self-assembly of synthetic melanin nanoparticles. ACS Nano 2015, 9, 5454–5460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Li, Y.; Hu, Z.; Yue, X.; Proetto, M.T.; Jones, Y.; Gianneschi, N.C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. ACS Cent. Sci. 2017, 3, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, D.; Dai, T.; Xu, P.; Wu, P.; Zou, Y.; Yang, P.; Hu, J.; Li, Y.; Cheng, Y. Skin Pigmentation-Inspired Polydopamine Sunscreens. Adv. Funct. Mater. 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.N. The Red and the Black. Acc. Chem. Res. 2010, 43, 1452–1460. [Google Scholar] [CrossRef] [Green Version]
- Van Neste, D.; Tobin, D.J. Hair cycle and hair pigmentation: Dynamic interactions and changes associated with aging. Micron 2004, 35, 193–200. [Google Scholar] [CrossRef]
- Morel, O.J.X.; Christie, R.M. Current Trends in the Chemistry of Permanent Hair Dyeing. Chem. Rev. 2011, 111, 2537–2561. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Fautz, R.; Benech-Kieffer, F.; Toutain, H. Toxicity and human health risk of hair dyes. Food Chem. Toxicol. 2004, 42, 517–543. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, L.; Chiou, K.; Huang, J. Multifunctional Graphene Hair Dye. Chem 2018, 4, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Im, K.M.; Kim, T.W.; Jeon, J.R. Metal-Chelation-Assisted Deposition of Polydopamine on Human Hair: A Ready-to-Use Eumelanin-Based Hair Dyeing Methodology. ACS Biomater. Sci. Eng. 2017, 3, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.F.; Wang, X.Y.; Gao, J.B.; Xia, F. Rapid preparation of polydopamine coating as a multifunctional hair dye. RSC Adv. 2019, 9, 20492–20496. [Google Scholar] [CrossRef] [Green Version]
- Battistella, C.; McCallum, N.C.; Gnanasekaran, K.; Zhou, X.; Caponetti, V.; Montalti, M.; Gianneschi, N.C. Mimicking Natural Human Hair Pigmentation with Synthetic Melanin. ACS Cent. Sci. 2020, 6, 1179–1188. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. https://doi.org/10.3390/nano10112276
Mavridi-Printezi A, Guernelli M, Menichetti A, Montalti M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials. 2020; 10(11):2276. https://doi.org/10.3390/nano10112276
Chicago/Turabian StyleMavridi-Printezi, Alexandra, Moreno Guernelli, Arianna Menichetti, and Marco Montalti. 2020. "Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics" Nanomaterials 10, no. 11: 2276. https://doi.org/10.3390/nano10112276
APA StyleMavridi-Printezi, A., Guernelli, M., Menichetti, A., & Montalti, M. (2020). Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials, 10(11), 2276. https://doi.org/10.3390/nano10112276




