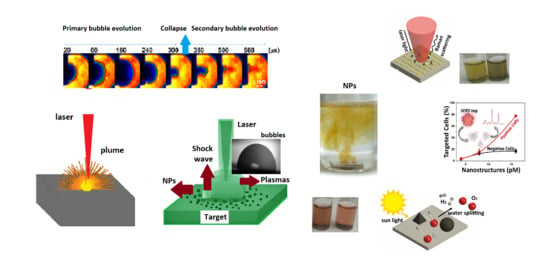
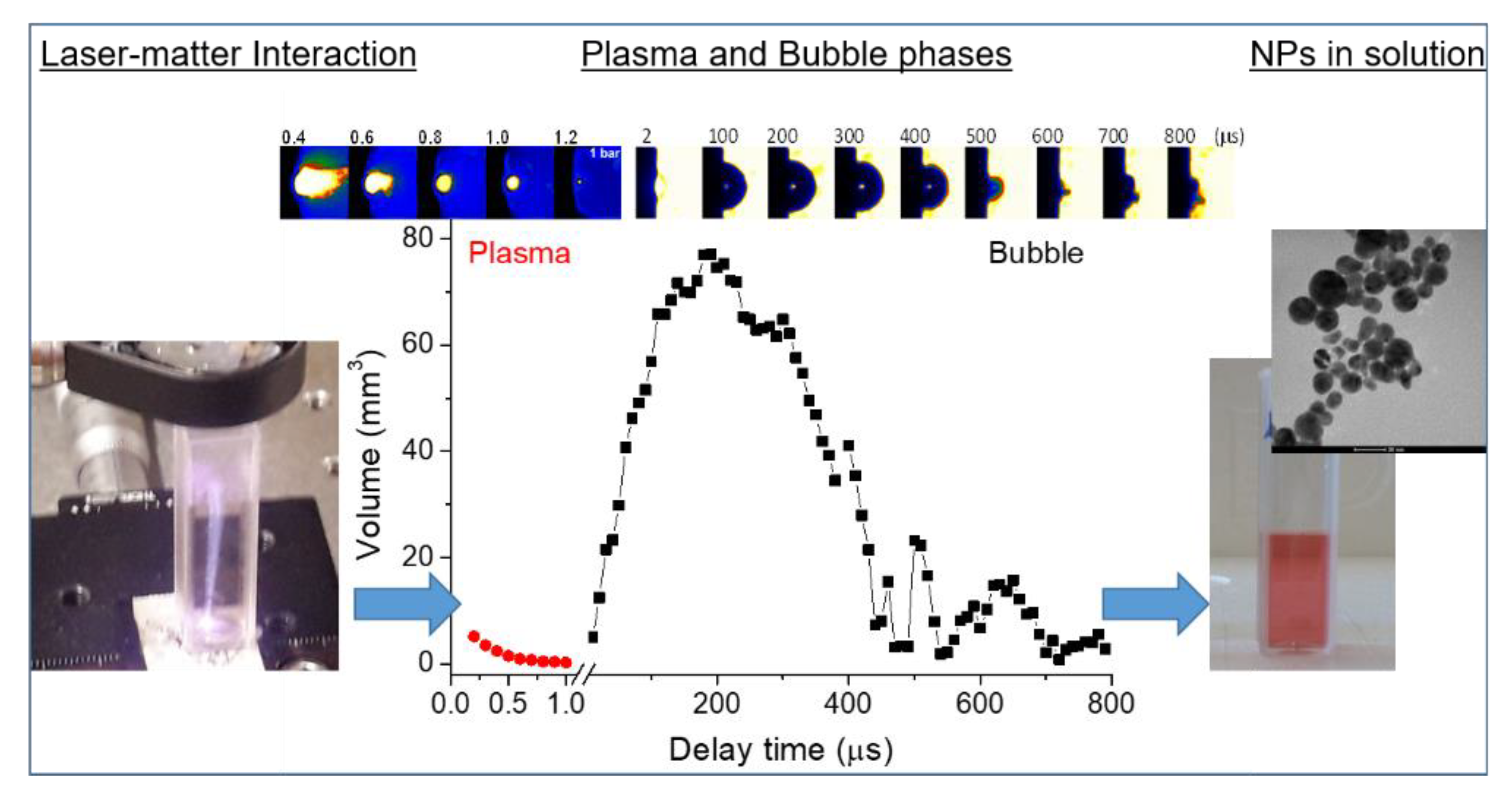
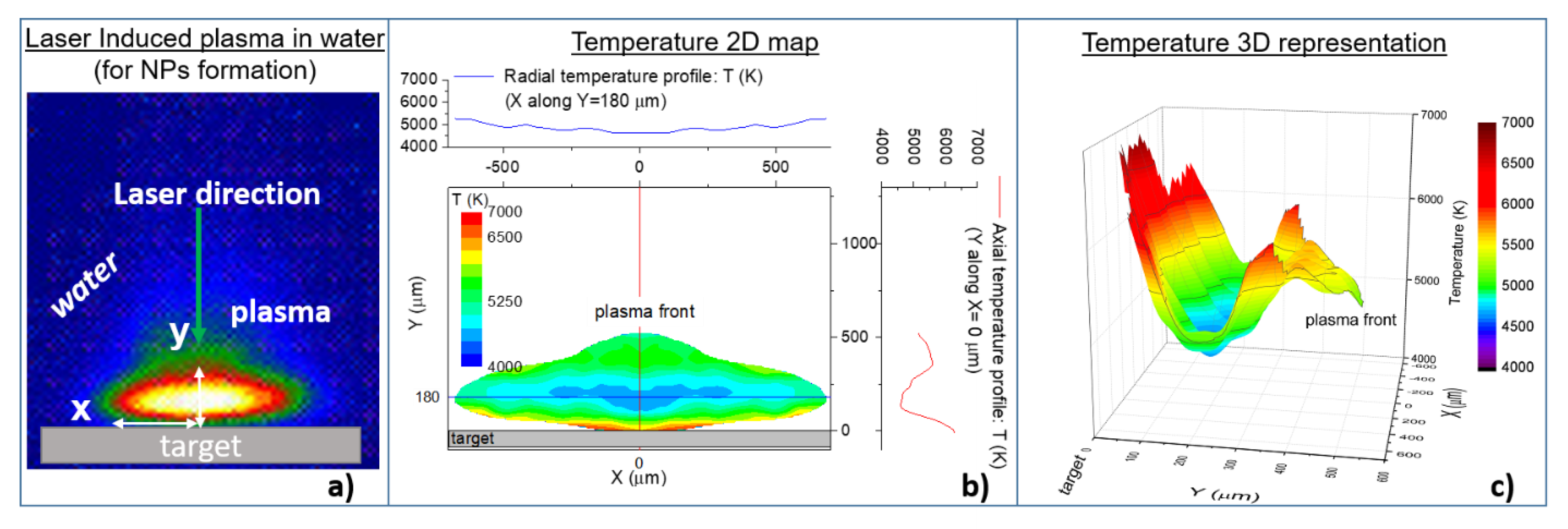
Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications
Abstract
:1. Introduction
2. LAL Synthesis Methods: Principle, Process Parameters and Liquids Effects on Nanoparticle Formation
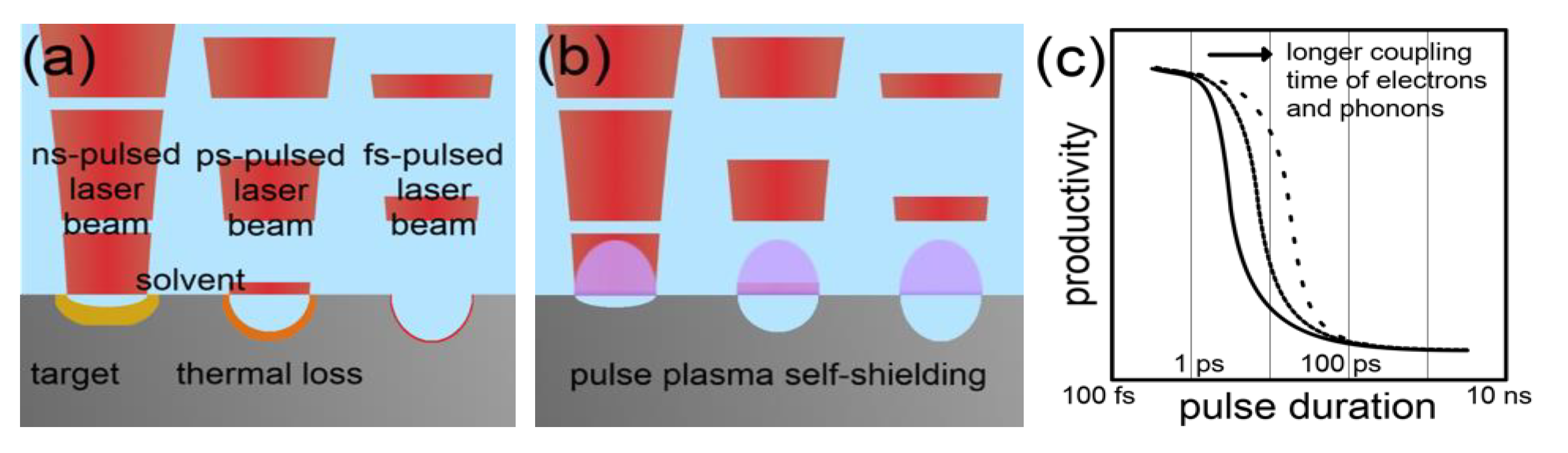
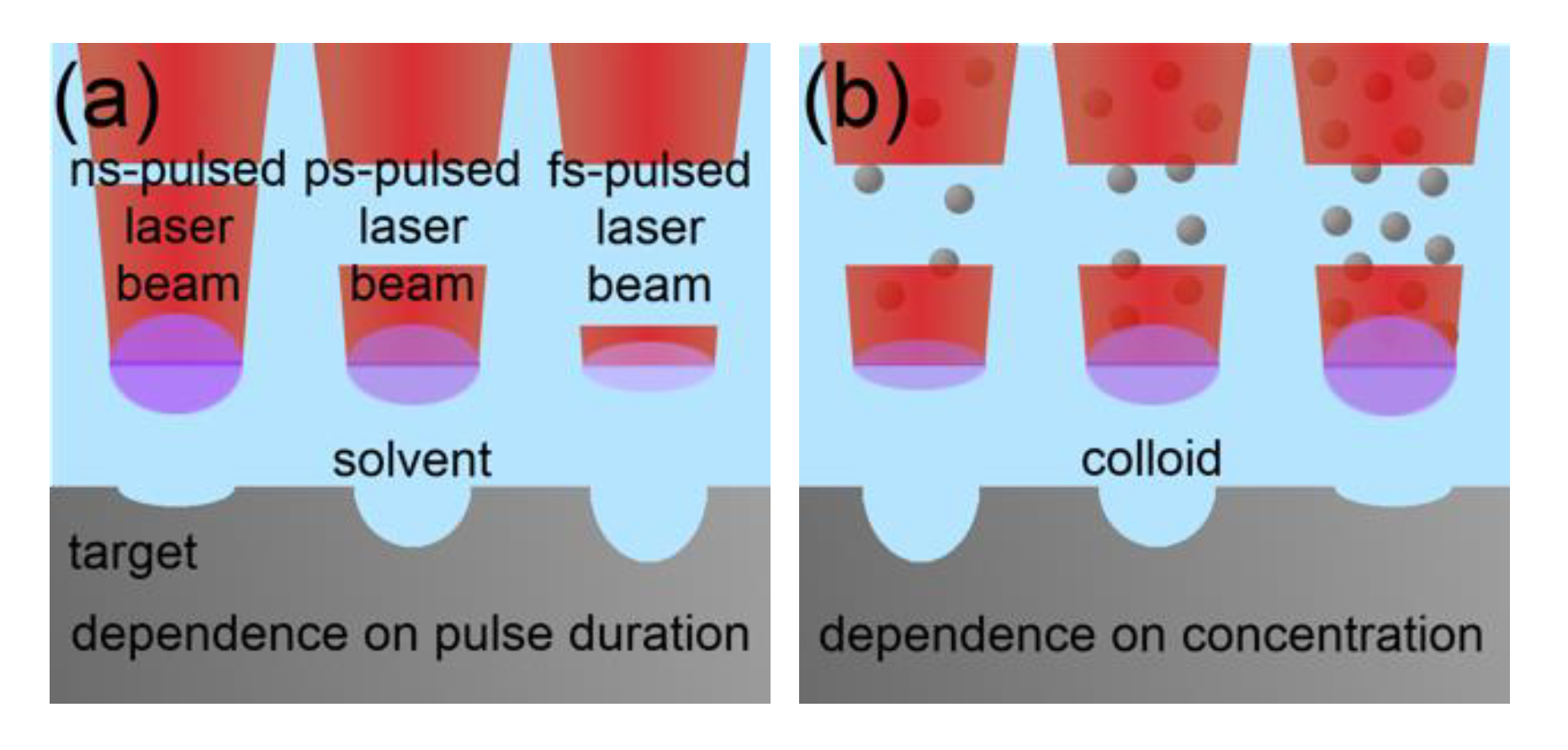
2.1. Mechanism in ns-Pulsed Laser Ablation in Liquid for the Production of Metallic Nanoparticles
2.2. Upscaling of Laser Synthesis of Colloids
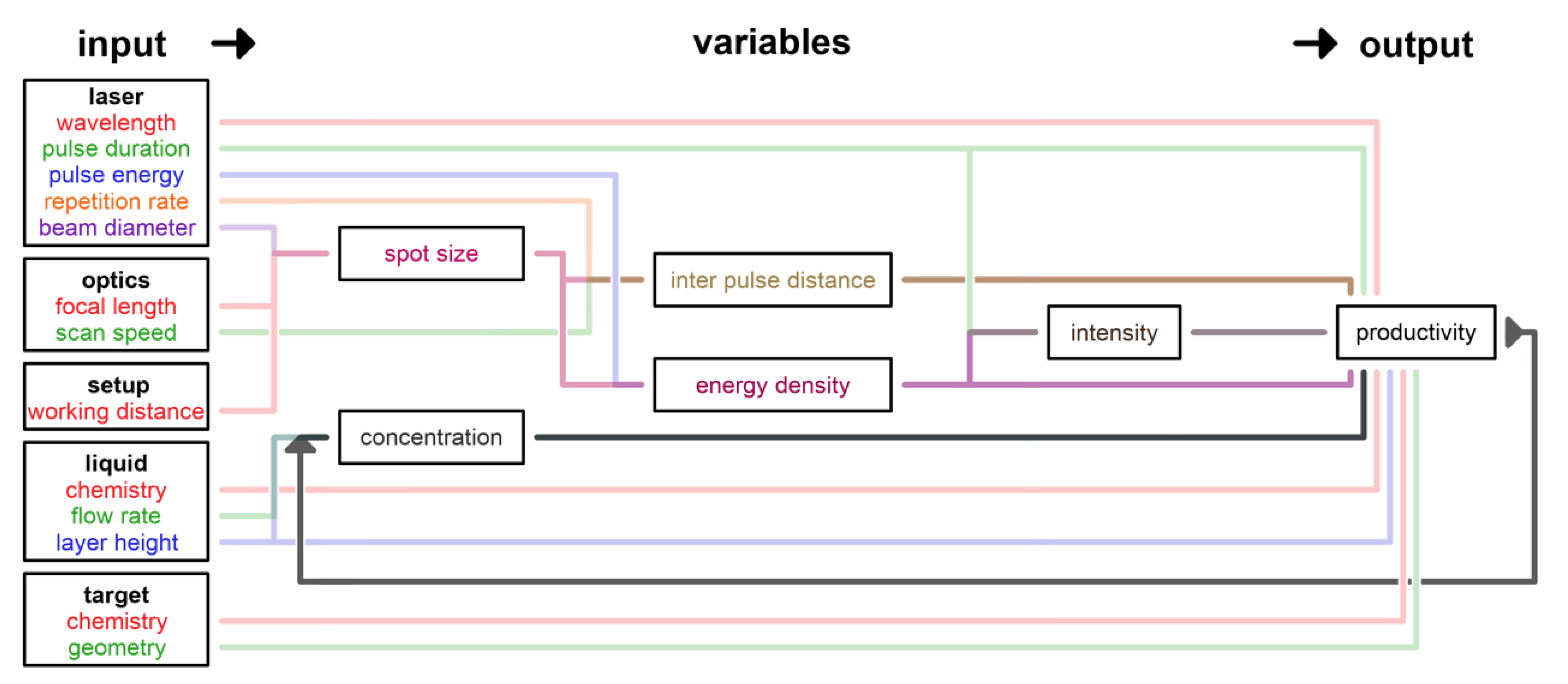
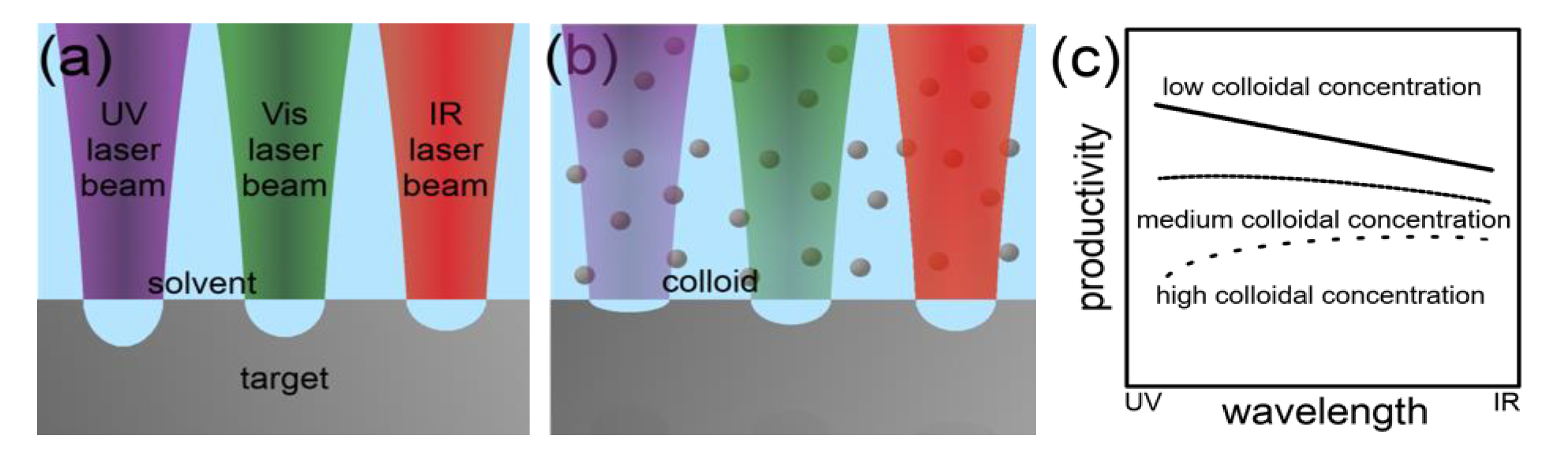
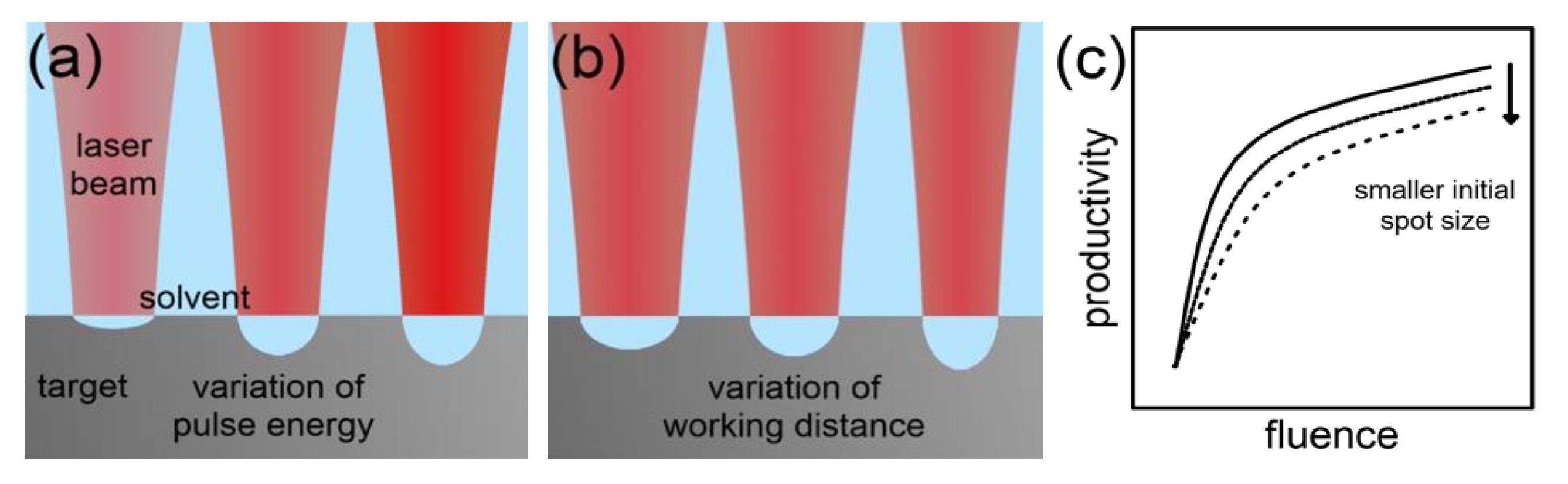
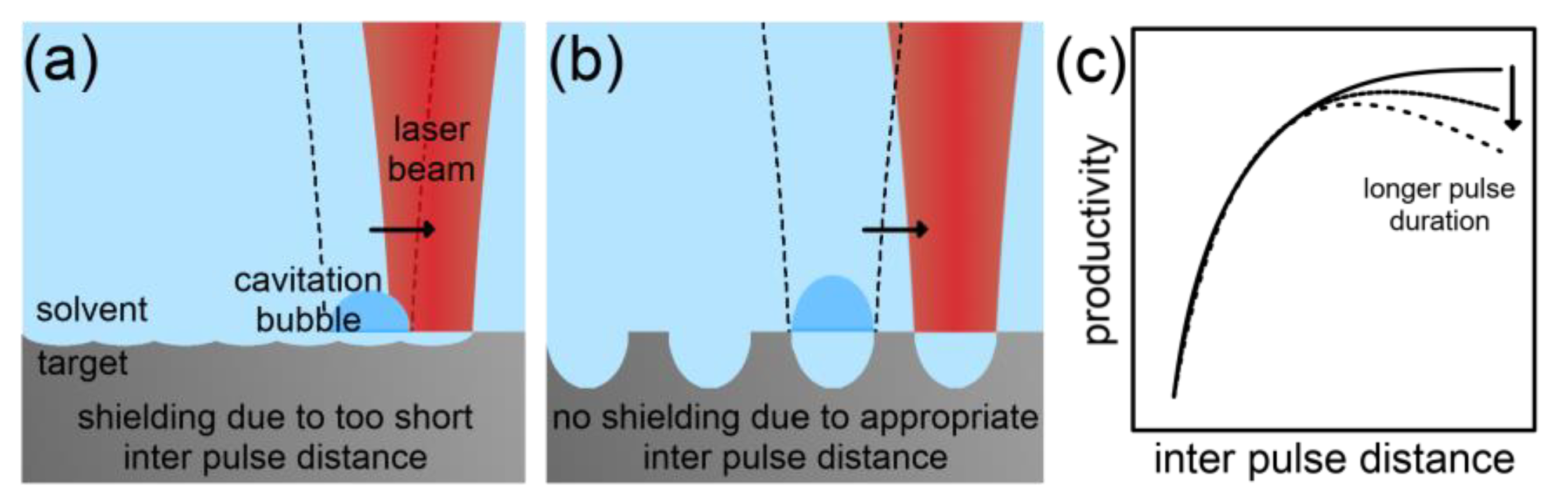
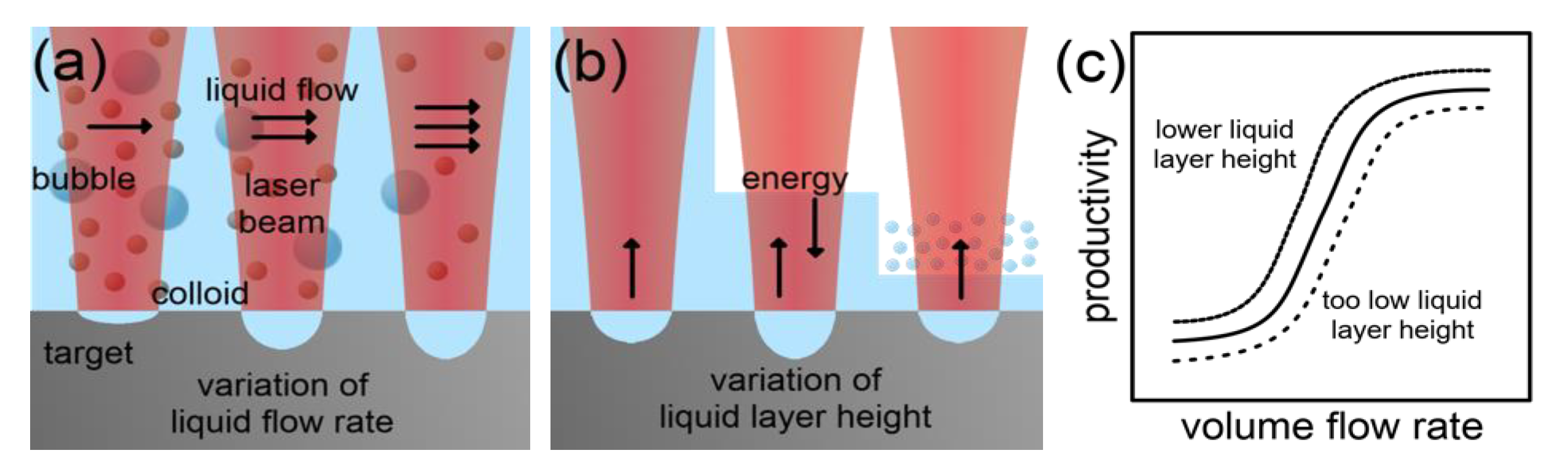
2.2.1. Scaling and Control Factors for Laser Ablation in Liquids
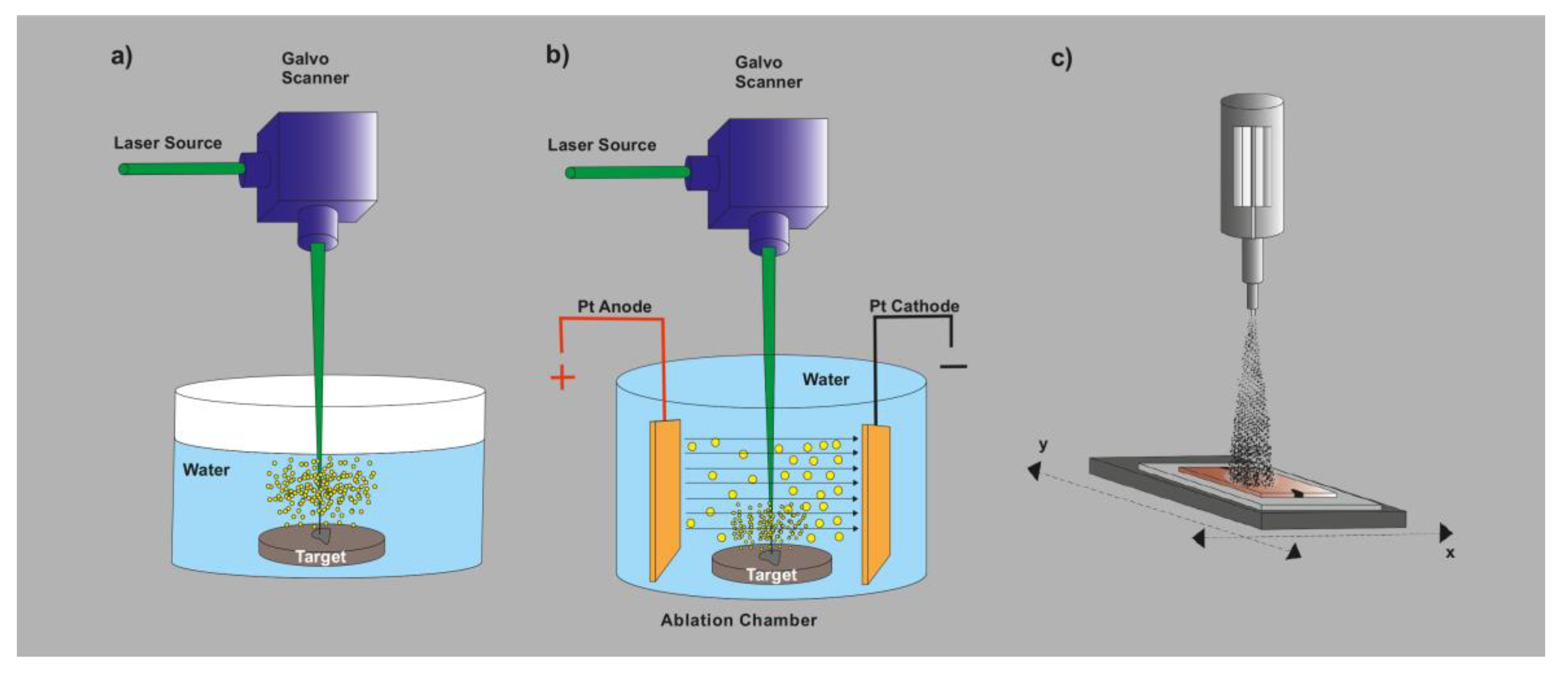
2.3. LAL-Based Techniques for Nanomaterials Synthesis and Processing
3. Promising Applications of LAL Nanostructures for Biotechnology Applications and for Organic Pollutants Degradation
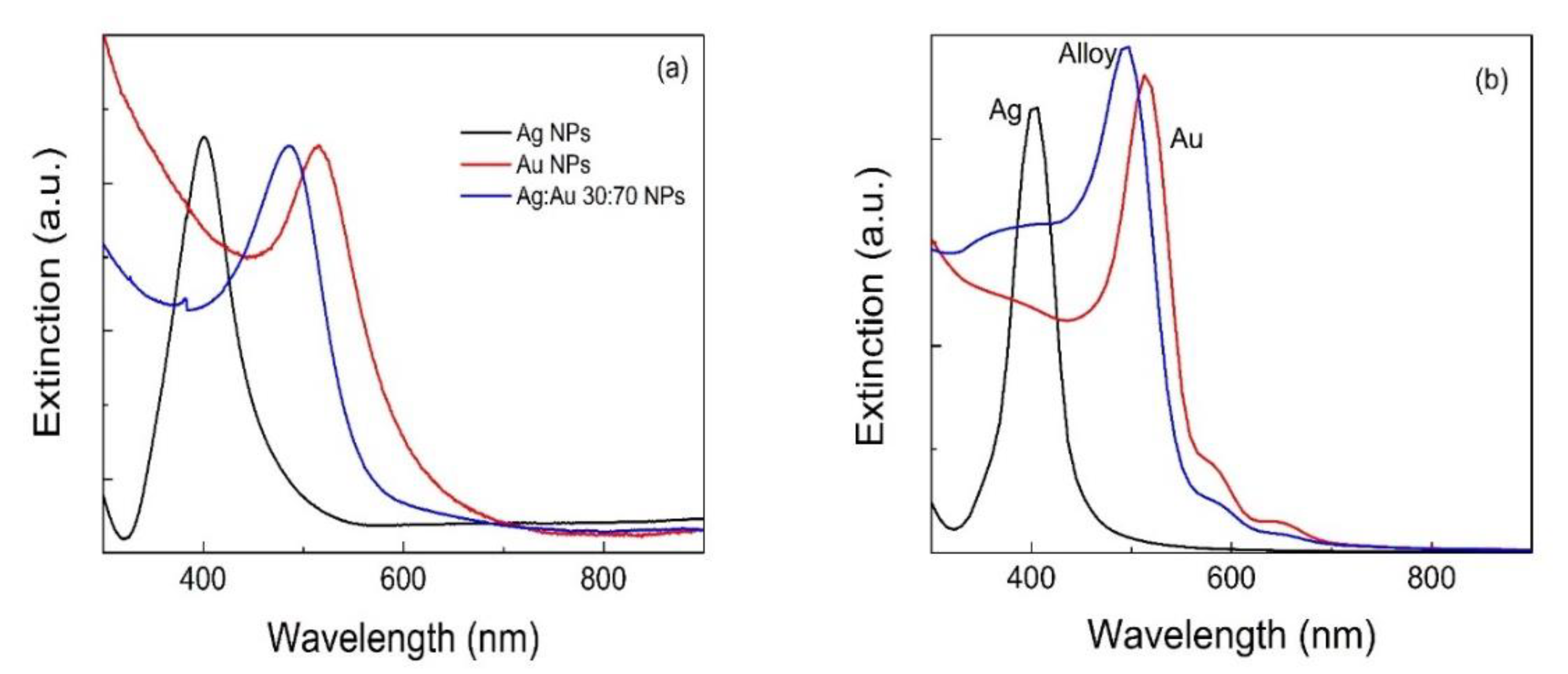
3.1. Plasmonic Properties of Metal Nanoparticles and Plasmon Sensitivity
3.2. Plasmonic Nanocolloids for Biotechnology Applications
3.3. Metal Oxide Nanostructures for UV-SERS Sensing Applications
- The first SERS report is due to Bilmes et al. [201] who observed an increased pyridine Raman activity on an electrochemically roughened Rh substrate. The observed enhancement was very low because visible radiation was employed as the exciting source (far from the wavelength of the Rh SPR absorption peak);
- Lin et al. [202] reported UV-SERS on Rh nanostructured surfaces, and estimated the enhancement factor to be about 102;
- Zettsu et al. [203] published high-quality UV-SERS spectra (under 325 nm excitation) of 4-mercaptopyridine attached to sub-10 nm tripod-shaped stars of Rh;
- Watson et al. [204] reported a comparative study employing Rh tripod geometry by means of SERS, surface enhanced fluorescence (SEF) and photo-induced degradation of p-aminothiophenol (PATP) under UV and visible excitation;
- Ren et al. [205] demonstrated SERS enhancement for pyridine adsorbed on roughened Rh and Ru electrodes with 325 nm excitation;
- Li et al. [206] reported on amorphous rhodium sulfide microbowls, which were successfully designed and synthesized with the guidance of theoretical calculations and characterized by an excellent SERS performance. The amorphous structure favors efficient interfacial charge transfer, and the bowl-like shape is beneficial for photon trapping by multiple light scattering.
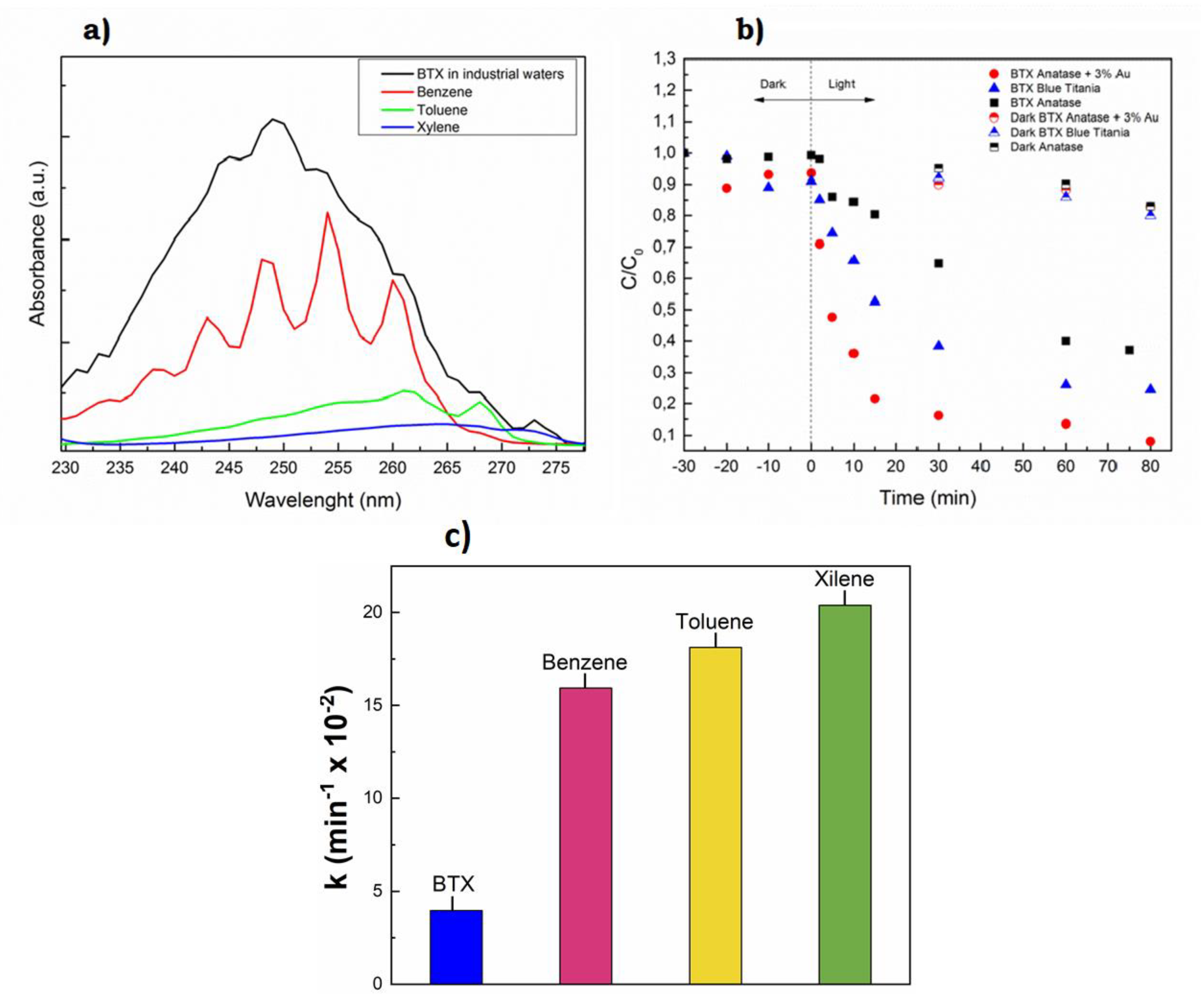
3.4. Semiconductor Nanoparticles for the Degradation of Organic Pollutants
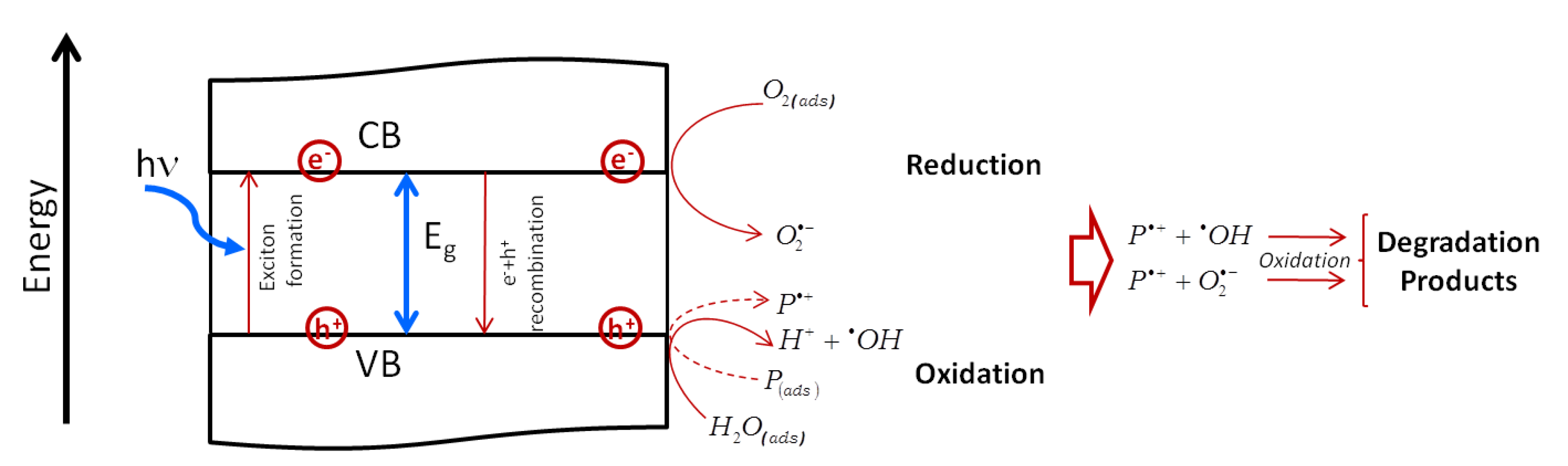
- A decrease in the band gap energy due to the increased structural disorder;
- A strong absorption in the visible range, due to the Surface Resonance Plasmon (SPR) of free electrons (the same effect was observed in Ag-TiO2 catalysts) [260,271,272], favors the injection of photo-excited SPR electrons into the conduction band (CB) of TiO2, thus creating separated electrons-hole pairs and hindering the recombination process [273].
- Metals can act as reservoir, promoting interfacial electron transfer processes from the semiconductor CB toward metal NPs, being the Fermi level of the metal lower than the CB of the semiconductor, leaving holes in the valence band (VB) of the photocatalyst.
- Electrons transferred from Au NPs surface to the oxide can be caught by oxygen atoms giving active O2− species with the increase of the photo-catalytic activity [274].
4. Conclusions
5. Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ealia, S.A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Neri, G.; Fazio, E.; Mineo, P.G.; Scala, A.; Piperno, A. SERS Sensing Properties of New Graphene/Gold Nanocomposite. Nanomaterials 2019, 9, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzmin, P.G.; Shafeev, G.A.; Bukin, V.V.; Garnov, S.V.; Farcau, C.; Carles, R.; Warot-Fontrose, B.; Guieu, V.; Viau, G. Silicon Nanoparticles Produced by Femtosecond Laser Ablation in Ethanol: Size Control, Structural Characterization, and Optical Properties. J. Phys. Chem. C 2010, 114, 15266–15273. [Google Scholar] [CrossRef]
- Szefler, B. Nanotechnology, from quantum mechanical calculations up to drug delivery. Int. J. Nanomed. 2018, 13, 6143–6176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfsteller, A.; Geyer, N.; Nguyen-Duc, T.-K.; Das Kanungo, P.; Zakharov, N.; Reiche, M.; Erfurth, W.; Blumtritt, H.; Kalem, S.; Werner, P.; et al. Comparison of the top-down and bottom-up approach to fabricate nanowire-based silicon/germanium heterostructures. Thin Solid Films 2010, 518, 2555–2561. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef] [Green Version]
- Damilano, B.; Coulon, P.-M.; Vézian, S.; Brändli, V.; Duboz, J.-Y.; Massies, J.; Shields, P.A. Top-down fabrication of GaN nano-laser arrays by displacement Talbot lithography and selective area sublimation. Appl. Phys. Express 2019, 12, 045007. [Google Scholar] [CrossRef]
- Fazio, E.; Scala, A.; Grimato, S.; Ridolfo, A.; Grassi, G.P.; Neri, F. Laser light triggered smart release of silibinin from a PEGylated–PLGA gold nanocomposite. J. Mater. Chem. B 2015, 3, 9023–9032. [Google Scholar] [CrossRef]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast laser processing of materials: From science to industry. Light. Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef] [Green Version]
- Petridis, C.; Savva, K.; Kymakis, E.; Stratakis, E. Laser generated nanoparticles based photovoltaics. J. Colloid Interface Sci. 2017, 489, 28–37. [Google Scholar] [CrossRef]
- Carter, M.J.; El-Desouky, A.; Andre, M.A.; Bardet, P.; Leblanc, S. Pulsed laser melting of bismuth telluride thermoelectric materials. J. Manuf. Process. 2019, 43, 35–46. [Google Scholar] [CrossRef]
- Zang, X.; Jian, C.; Zhu, T.; Fan, Z.; Wang, W.; Wei, M.; Li, B.; Diaz, M.F.; Ashby, P.; Lu, Z.; et al. Laser-sculptured ultrathin transition metal carbide layers for energy storage and energy harvesting applications. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wang, Y.; Lai, W.; Lin, W.; Lin, Z.; Zhang, Z.; Zou, P.; Xu, Y.; Zhou, S.; Yang, C.; et al. Laser-processed graphene based micro-supercapacitors for ultrathin, rollable, compact and designable energy storage components. Nano Energy 2016, 26, 276–285. [Google Scholar] [CrossRef]
- Picca, R.A.; Di Maria, A.; Riháková, L.; Volpe, A.; Sportelli, M.C.; Lugarà, P.M.; Ancona, A.; Cioffi, N. Laser Ablation Synthesis of Hybrid Copper/Silver Nanocolloids for Prospective Application as Nanoantimicrobial Agents for Food Packaging. MRS Adv. 2016, 1, 3735–3740. [Google Scholar] [CrossRef]
- Soman, P.; Zhang, W.; Umeda, A.; Zhang, Z.J.; Chen, S. Femtosecond laser-assisted optoporation for drug and gene delivery into single mammalian cells. J. Biomed. Nanotechnol. 2011, 7, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Corsaro, C.; Fazio, E. Plasmon-Enhanced Controlled Drug Release from Ag-PMA Capsules. Molecules 2020, 25, 2267–2278. [Google Scholar] [CrossRef]
- Tsuji, T.; Yahata, T.; Yasutomo, M.; Igawa, K.; Tsuji, M.; Ishikawa, Y.; Koshizaki, N. Preparation and investigation of the formation mechanism of submicron-sized spherical particles of gold using laser ablation and laser irradiation in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3099–3107. [Google Scholar] [CrossRef]
- Kucherik, A.O.; Ryabchikov, Y.V.; Kutrovskaya, S.V.; Al-Kattan, A.; Arakelyan, S.M.; Itina, T.E.; Kabashin, A.V. Cavitation-Free Continuous-Wave Laser Ablation from a Solid Target to Synthesize Low-Size-Dispersed Gold Nanoparticles. ChemPhysChem 2017, 18, 1185–1191. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.-Y.; Takeda, A.Y.; Kondow, T.; Sawabe, H. Formation of Gold Nanoparticles by Laser Ablation in Aqueous Solution of Surfactant. J. Phys. Chem. B 2001, 105, 5114–5120. [Google Scholar] [CrossRef]
- Besner, S.; Kabashin, A.V.; Winnik, F.M.; Meunier, M. Ultrafast laser based “green” synthesis of non-toxic nanoparticles in aqueous solutions. Appl. Phys. A 2008, 93, 955–959. [Google Scholar] [CrossRef]
- Pyatenko, A.; Wang, H.; Koshizaki, N.; Tsuji, T. Mechanism of pulse laser interaction with colloidal nanoparticles. Laser Photon Rev. 2013, 7, 596–604. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-Temperature Laser Synthesis in Liquid of Oxide, Metal-Oxide Core-Shells, and Doped Oxide Nanoparticles. Chem. Eur. J. 2020, 26, 9206–9242. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Reddy, D.A.; Kim, Y.; Lee, S.; Ma, R.; Kim, T.K. Synthesis of Ultra-Small Palladium Nanoparticles Deposited on CdS Nanorods by Pulsed Laser Ablation in Liquid: Role of Metal Nanocrystal Size in the Photocatalytic Hydrogen Production. Chem. Eur. J. 2017, 23, 13112–13119. [Google Scholar] [CrossRef]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of Surfactant-Free Colloidal Nanoparticles in Heterogeneous Catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Waag, F.; Li, Y.; Ziefuß, A.R.; Bertin, E.; Kamp, M.; Duppel, V.; Marzun, G.; Kienle, L.; Barcikowski, S.; Gökce, B. Kinetically-controlled laser-synthesis of colloidal high-entropy alloy nanoparticles. RSC Adv. 2019, 9, 18547–18558. [Google Scholar] [CrossRef] [Green Version]
- Lentini, G.; Fazio, E.; Calabrese, F.; De Plano, L.M.; Puliafico, M.; Franco, D.; Nicolò, M.S.; Carnazza, S.; Trusso, S.; Allegra, A.; et al. Phage–AgNPs complex as SERS probe for U937 cell identification. Biosens. Bioelectron. 2015, 74, 398–405. [Google Scholar] [CrossRef]
- Fazio, E.; Santoro, M.; Lentini, G.; Franco, D.; Guglielmino, S.P.P.; Neri, F. Iron oxide nanoparticles prepared by laser ablation: Synthesis, structural properties and antimicrobial activity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 98–103. [Google Scholar] [CrossRef]
- Tommasini, M.; Zanchi, C.; Lucotti, A.; Fazio, E.; Santoro, M.; Spadaro, S.; Neri, F.; Trusso, S.; Ciusani, E.; De Grazia, U.; et al. Laser synthesized nanoparticles for therapeutic drug monitoring. In Advances in the Application of Lasers in Materials Science; Ossi, P.M., Ed.; Springer: Cham, Switzerland, 2018; Volume 274, ISBN 978-3-319-96844-5. [Google Scholar]
- Doñate-Buendía, C.; Frömel, F.; Wilms, M.B.; Streubel, R.; Tenkamp, J.; Hupfeld, T.; Nachev, M.; Gökce, E.; Weisheit, A.; Barcikowski, S.; et al. Oxide dispersion-strengthened alloys generated by laser metal deposition of laser-generated nanoparticle-metal powder composites. Mater. Des. 2018, 154, 360–369. [Google Scholar] [CrossRef]
- Hupfeld, T.; Wegner, A.; Blanke, M.; Doñate-Buendía, C.; Sharov, V.; Nieskens, S.; Piechotta, M.; Giese, M.; Barcikowski, S.; Gökce, B. Plasmonic Seasoning: Giving Color to Desktop Laser 3D Printed Polymers by Highly Dispersed Nanoparticles. Adv. Opt. Mater. 2020, 8, 2000473. [Google Scholar] [CrossRef]
- Fazio, E.; D’Urso, L.; Consiglio, G.; Giuffrida, A.; Compagnini, G.; Puglisi, O.; Patanè, S.; Neri, F.; Forte, G. Nonlinear Scattering and Absorption Effects in Size-Selected Diphenylpolyynes. J. Phys. Chem. C 2014, 118, 28812–28819. [Google Scholar] [CrossRef]
- Flores-Castañeda, M.; Camps, E.; Camacho-López, M.; Muhl, S.; García, E.; Figuero, M. Bismuth nanoparticles synthesized by laser ablation in lubricant oilsfor tribological tests. J. Alloys Compd. 2015, 643, S67–S70. [Google Scholar] [CrossRef]
- Torres-Mendieta, R. Fabrication of High Stable Gold Nanofluid by Pulsed Laser Ablation in Liquids. Adv. Mater. Lett. 2015, 6, 1037–1042. [Google Scholar] [CrossRef]
- Torres-Mendieta, R.; Mondragón, R.; Puerto-Belda, V.; Mendoza-Yero, O.; Lancis, J.; Juliá, J.E.; Mínguez-Vega, G. Characterization of Tin/Ethylene Glycol Solar Nanofluids Synthesized by Femtosecond Laser Radiation. ChemPhysChem 2017, 18, 1055–1060. [Google Scholar] [CrossRef]
- Rao, K.S.; Ganeev, R.A.; Zhang, K.; Fu, Y.; Boltaev, G.S.; Krishnendu, P.S.; Redkin, P.V.; Guo, C. Laser ablation–induced synthesis and nonlinear optical characterization of titanium and cobalt nanoparticles. J. Nanoparticle Res. 2018, 20, 285. [Google Scholar] [CrossRef]
- Fazio, E.; Neri, F.; Patanè, S.; D’Urso, L.; Compagnini, G. Optical limiting effects in linear carbon chains. Carbon 2011, 49, 306–310. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.; Guo, C.; Kulinich, S.; Yang, S.; He, J.; Cai, W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Wagener, P.; Barcikowski, S. Laser fragmentation of organic microparticles into colloidal nanoparticles in a free liquid jet. Appl. Phys. A 2010, 101, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Free Silver Nanoparticles Synthesized by Laser Ablation in Organic Solvents and Their Easy Functionalization. Langmuir 2007, 23, 6766–6770. [Google Scholar] [CrossRef]
- Chemin, A.; Lam, J.; Laurens, G.; Trichard, F.; Motto-Ros, V.; LeDoux, G.; Jary, V.; Laguta, V.; Nikl, M.; Dujardin, C.; et al. Doping nanoparticles using pulsed laser ablation in a liquid containing the doping agent. Nanoscale Adv. 2019, 1, 3963–3972. [Google Scholar] [CrossRef] [Green Version]
- Streubel, R.; Barcikowski, S.; Gökce, B. Continuous multigram nanoparticle synthesis by high-power, high-repetition-rate ultrafast laser ablation in liquids. Opt. Lett. 2016, 41, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Manjón, A.; Wagener, P.; Barcikowski, S. Transfer-Matrix Method for Efficient Ablation by Pulsed Laser Ablation and Nanoparticle Generation in Liquids. J. Phys. Chem. C 2011, 115, 5108–5114. [Google Scholar] [CrossRef]
- González-Rubio, G.; Guerrero-Martínez, A.; Liz-Marzán, L.M. Reshaping, Fragmentation, and Assembly of Gold Nanoparticles Assisted by Pulse Lasers. Acc. Chem. Res. 2016, 49, 678–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Rubio, G.; Díaz-Núñez, P.; Rivera, A.; Prada, A.; Tardajos, G.; González-Izquierdo, J.; Bañares, L.; Llombart, P.; MacDowell, L.G.; Palafox, M.A.; et al. Femtosecond laser reshaping yields gold nanorods with ultranarrow surface plasmon resonances. Science 2017, 358, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Doñate-Buendia, C.; Fernández-Alonso, M.; Lancis, J.; Mínguez-Vega, G. Overcoming the barrier of nanoparticle production by femtosecond laser ablation in liquids using simultaneous spatial and temporal focusing. Photon. Res. 2019, 7, 1249–1257. [Google Scholar] [CrossRef]
- Streubel, R.; Bendt, G.; Gökce, B. Pilot-scale synthesis of metal nanoparticles by high-speed pulsed laser ablation in liquids. Nanotechnology 2016, 27, 205602. [Google Scholar] [CrossRef]
- Villa, M.D.A.; Gaudin, J.; Amans, D.; Boudjada, F.; Bozek, J.; Grisenti, R.E.; Lamour, E.; Laurens, G.; Macé, S.; Nicolas, C.; et al. Assessing the Surface Oxidation State of Free-Standing Gold Nanoparticles Produced by Laser Ablation. Langmuir 2019, 35, 11859–11871. [Google Scholar] [CrossRef]
- Lam, J.; Lombard, J.; Dujardin, C.; LeDoux, G.; Merabia, S.; Amans, D. Dynamical study of bubble expansion following laser ablation in liquids. Appl. Phys. Lett. 2016, 108, 074104. [Google Scholar] [CrossRef]
- Itina, T.E. On Nanoparticle Formation by Laser Ablation in Liquids. J. Phys. Chem. C 2010, 115, 5044–5048. [Google Scholar] [CrossRef]
- Kanitz, A.; Kalus, M.-R.; Gurevich, E.L.; Ostendorf, A.; Barcikowski, S.; Amans, D. Review on experimental and theoretical investigations of the early stage, femtoseconds to microseconds processes during laser ablation in liquid-phase for the synthesis of colloidal nanoparticles. Plasma Sources Sci. Technol. 2019, 28, 103001. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Yeom, G.Y.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Schwenke, A.; Wagener, P.; Nolte, S.; Barcikowski, S. Influence of processing time on nanoparticle generation during picosecond-pulsed fundamental and second harmonic laser ablation of metals in tetrahydrofuran. Appl. Phys. A 2011, 104, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-Y.; Streubel, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmidt, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: The origin of the bimodal size distribution. Nanoscale 2018, 10, 6900–6910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casavola, A.R.; Colonna, G.; De Giacomo, A.; De Pascale, O.; Capitelli, M. Experimental and theoretical investigation of laser-induced plasma of a titanium target. Appl. Opt. 2003, 42, 5963–5970. [Google Scholar] [CrossRef]
- Sakka, T.; Iwanaga, S.; Ogata, Y.H.; Matsunawa, A.; Takemoto, T. Laser ablation at solid–liquid interfaces: An approach from optical emission spectra. J. Chem. Phys. 2000, 112, 8645–8653. [Google Scholar] [CrossRef] [Green Version]
- Koubiti, M.; Sheeba, R.S. Spectral Modeling of Hydrogen Radiation Emission in Magnetic Fusion Plasmas. Atoms 2019, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Dell’Aglio, M.; Ros, V.M.; Pelascini, F.; Gornushkin, I.B.; De Giacomo, A. Investigation on the material in the plasma phase by high temporally and spectrally resolved emission imaging during pulsed laser ablation in liquid (PLAL) for NPs production and consequent considerations on NPs formation. Plasma Sources Sci. Technol. 2019, 28, 085017. [Google Scholar] [CrossRef]
- Taccogna, F.; Dell’Aglio, M.; Rutigliano, M.; Valenza, G.; De Giacomo, A. On the growth mechanism of nanoparticles in plasma during pulsed laser ablation in liquids. Plasma Sources Sci. Technol. 2017, 26, 045002. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; De Giacomo, A. Plasma charging effect on the nanoparticles releasing from the cavitation bubble to the solution during nanosecond Pulsed Laser Ablation in Liquid. Appl. Surf. Sci. 2020, 515, 146031. [Google Scholar] [CrossRef]
- Lauterborn, W.; Vogel, A. Shock Wave Emission by Laser Generated Bubbles. Bubble Dynamics and Shock Waves; Springer: Berlin/Heidelberg, Germany, 2013; pp. 67–103. [Google Scholar]
- Brenner, M.P.; Hilgenfeldt, S.; Lohse, D. Single-bubble sonoluminescence. Rev. Mod. Phys. 2002, 74, 425–484. [Google Scholar] [CrossRef] [Green Version]
- Casavola, A.; De Giacomo, A.; Dell’Aglio, M.; Taccogna, F.; Colonna, G.; De Pascale, O.; Longo, S. Experimental investigation and modelling of double pulse laser induced plasma spectroscopy under water. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 975–985. [Google Scholar] [CrossRef]
- Waag, F. Laser Synthesis of Metallic and Oxidic Transition Metal, Multi-Element Nanoparticles for Catalytic Applications. Ph.D. Thesis, University of Duisburg-Essen, Duisburg, Germany, 2019. [Google Scholar]
- Tsuji, T.; Iryo, K.; Ohta, H.; Nishimura, Y. Preparation of Metal Colloids by a Laser Ablation Technique in Solution: Influence of Laser Wavelength on the Efficiencies of Colloid Formation. Jpn. J. Appl. Phys. 2000, 39, L981–L983. [Google Scholar] [CrossRef]
- Šmejkal, P.; Pfleger, J.; Vlčková, B.; Dammer, O. Laser ablation of silver in aqueous ambient: Effect of laser pulse wavelength and energy on efficiency of the process. J. Physics: Conf. Ser. 2007, 59, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Intartaglia, R.; Bagga, K.; Brandi, F. Study on the productivity of silicon nanoparticles by picosecond laser ablation in water: Towards gram per hour yield. Opt. Express 2014, 22, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Reddy, D.A.; Ma, R.; Kim, T.K. The influence of laser wavelength and fluence on palladium nanoparticles produced by pulsed laser ablation in deionized water. Solid State Sci. 2014, 37, 96–102. [Google Scholar] [CrossRef]
- Strutt, H.J. LVIII. On the scattering of light by small particles. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1871, 41, 447–454. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadon, D.G. Ultraviolet–visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Semerok, A.; Chaléard, C.; Detalle, V.; Lacour, J.L.; Mauchien, P.; Meynadier, P.; Nouvellon, C.; Sallé, B.; Palianov, P.; Perdrix, M.; et al. Experimental investigations of laser ablation efficiency of pure metals with femto, pico and nanosecond pulses. Appl. Surf. Sci. 1999, 138, 311–314. [Google Scholar] [CrossRef]
- Stafe, M.; Negutu, C.; Puscas, N.N.; Popescu, I. Pulsed laser ablation of solids. Rom. Rep. Phys. 2010, 62, 758–770. [Google Scholar]
- Chan, P.; Chan, Y.; Ng, H. Reflectivity of metals at high temperatures heated by pulsed laser. Phys. Lett. A 1977, 61, 151–153. [Google Scholar] [CrossRef]
- Beaglehole, D.; Hunderi, O. Study of the interaction of light with rough metal surfaces. I. Experiment. Phys. Rev. B 1970, 2, 309–321. [Google Scholar] [CrossRef]
- Xie, J.; Kar, A. Laser welding of thin sheet steel with surface oxidation. Weld. J. 1999, 78, 343s–348s. [Google Scholar]
- Patel, R.S.; Brewster, M.Q. Effect of Oxidation and Plume Formation on Low Power Nd-Yag Laser Metal Interaction. J. Heat Transf. 1990, 112, 170–177. [Google Scholar] [CrossRef]
- Letzel, A.; Santoro, M.; Frohleiks, J.; Ziefuß, A.R.; Reich, S.; Plech, A.; Fazio, E.; Neri, F.; Barcikowski, S.; Gökce, B. How the re-irradiation of a single ablation spot affects cavitation bubble dynamics and nanoparticles properties in laser ablation in liquids. Appl. Surf. Sci. 2019, 473, 828–837. [Google Scholar] [CrossRef]
- Nedialkov, N.N.; Atanasov, P.A.; Sawczak, M.; Sliwinski, G. Ablation of ceramics with ultraviolet, visible, and infrared nanosecond laser pulses. In Proceedings of the XIV International Symposium on Gas Flow, Chemical Lasers, and High-Power Lasers, Wrocław, Poland, 25–30 August 2002; pp. 703–709. [Google Scholar]
- Sikora, A.; Grojo, D.; Sentis, M. Wavelength scaling of silicon laser ablation in picosecond regime. J. Appl. Phys. 2017, 122, 045702. [Google Scholar] [CrossRef]
- Weber, R.; Hoppius, J.S.; Fiebrandt, M.; Awakowicz, P.; Esen, C.; Ostendorf, A.; Gurevich, E.L. Impact of liquid environment on femtosecond laser ablation. Appl. Phys. A 2017, 123, 674. [Google Scholar]
- Hahn, A. Influences on Nanoparticle Production during Pulsed Laser Ablation. J. Laser Micro/Nanoeng. 2008, 3, 73–77. [Google Scholar] [CrossRef]
- Sattari, R.; Sajti, C.L.; Khan, S.; Barcikowski, S. Scale-up of nanoparticle production during laser ablation of ceramics in liquid media. In Proceedings of the 27th International Congress on Applications of Lasers & Electro-Optics, Temecula, CA, USA, 20–23 October 2008. N204. [Google Scholar]
- Bärsch, N.; Jakobi, J.; Weiler, S.; Barcikowski, S. Pure colloidal metal and ceramic nanoparticles from high-power picosecond laser ablation in water and acetone. Nanotechnology 2009, 20, 445603. [Google Scholar] [CrossRef]
- Al-Mamun, S.A.; Nakajima, R.; Ishigaki, T. Effect of liquid level and laser power on the formation of spherical alumina nanoparticles by nanosecond laser ablation of alumina target. Thin Solid Films 2012, 523, 46–51. [Google Scholar] [CrossRef]
- Cristoforetti, G.; Pitzalis, E.; Spiniello, R.; Ishak, R.; Giammanco, F.; Muniz-Miranda, M.; Caporali, S. Physico–chemical properties of pd nanoparticles produced by pulsed laser ablation in different organic solvents. Appl. Surf. Sci. 2012, 258, 3289–3297. [Google Scholar] [CrossRef]
- Riabinina, D.; Chaker, M.; Margot, J. Dependence of gold nanoparticle production on pulse duration by laser ablation in liquid media. Nanotechnology 2012, 23, 135603. [Google Scholar] [CrossRef] [PubMed]
- Stašić, J.; Živković, L.; Trtica, M. Optimization of silver nanoparticles production by laser ablation in water using a 150-ps laser. J. Nanopart. Res. 2016, 18, 366. [Google Scholar] [CrossRef]
- Kohsakowski, S.; Santagata, A.; Dell’Aglio, M.; De Giacomo, A.; Barcikowski, S.; Wagener, P.; Gökce, B. High productive and continuous nanoparticle fabrication by laser ablation of a wire-target in a liquid jet. Appl. Surf. Sci. 2017, 403, 487–499. [Google Scholar] [CrossRef]
- Wagener, P.; Schwenke, A.; Chichkov, B.N.; Barcikowski, S. Pulsed Laser Ablation of Zinc in Tetrahydrofuran: Bypassing the Cavitation Bubble. J. Phys. Chem. C 2010, 114, 7618–7625. [Google Scholar] [CrossRef]
- Preuss, S.; Demchuk, A.; Stuke, M. Sub-picosecond UV laser ablation of metals. Appl. Phys. A 1995, 61, 33–37. [Google Scholar] [CrossRef]
- Kononenko, T.; Garnov, S.; Klimentov, S.; Konov, V.; Loubnin, E.; Dausinger, F.; Raiber, A.; Taut, C. Laser ablation of metals and ceramics in picosecond–nanosecond pulsewidth in the presence of different ambient atmospheres. Appl. Surf. Sci. 1997, 109–110, 48–51. [Google Scholar] [CrossRef]
- Nolte, S.; Momma, C.; Jacobs, H.; Tünnermann, A.; Chichkov, B.N.; Wellegehausen, B.; Welling, H. Ablation of metals by ultrashort laser pulses. J. Opt. Soc. Am. B 1997, 14, 2716–2722. [Google Scholar] [CrossRef]
- Leitz, K.-H.; Redlingshöfer, B.; Reg, Y.; Otto, A.; Schmidt, M. Metal Ablation with Short and Ultrashort Laser Pulses. Phys. Procedia 2011, 12, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Neuenschwander, B.; Jaeggi, B.; Schmid, M.; Hennig, G. Surface structuring with ultrashort laser pulses: Basics, limitations and needs for high throughput. Phys. Procedia 2014, 56, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Anisimov, S.; Kapeliovich, B.; Perelman, T. Electron emission from metal surfaces exposed to ultrashort laser pulses. Z. Exp. Theor. Phys. 1974, 66, 375–377. [Google Scholar]
- Jiang, L.; Tsai, H.L. A plasma model combined with an improved two-temperature equation for ultrafast laser ablation of dielectrics. J. Appl. Phys. 2008, 104, 093101. [Google Scholar] [CrossRef]
- Byskov-Nielsen, J.; Savolainen, J.M.; Christensen, M.S.; Balling, P. Ultra-short pulse laser ablation of copper, silver and tungsten: Experimental data and two-temperature model simulations. Appl. Phys. A 2011, 103, 447–453. [Google Scholar] [CrossRef]
- Sakka, T.; Masai, S.; Fukami, K.; Ogata, Y.H. Spectral profile of atomic emission lines and effects of pulse duration on laser ablation in liquid. Spectrochim. Acta Part B: At. Spectrosc. 2009, 64, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, S.; Streubel, R.; McDonnell, C.; Huber, H.P.; Barcikowski, S.; Gökce, B. Comparison of the productivity and ablation efficiency of different laser classes for laser ablation of gold in water and air. Appl. Phys. A 2019, 125, 432. [Google Scholar] [CrossRef]
- Schille, J.; Schneider, L.; Lickschat, P.; Loeschner, U.; Ebert, R.; Exner, H. High-pulse repetition frequency ultrashort pulse laser processing of copper. J. Laser Appl. 2015, 27, S28007. [Google Scholar] [CrossRef]
- Jaeggi, B.; Neuenschwander, B.; Schmid, M.; Muralt, M.; Zuercher, J.; Hunziker, U. Influence of the pulse duration in the ps-regime on the ablation efficiency of metals. Phys. Procedia 2011, 12, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Hashida, M.; Semerok, A.; Gobert, O.; Petite, G.; Izawa, Y.; Wagner, J.F. Ablation threshold dependence on pulse duration for copper. Appl. Surf. Sci. 2002, 197–198, 862–867. [Google Scholar] [CrossRef]
- Hanon, M.; Akman, E.; Oztoprak, B.G.; Gunes, M.; Taha, Z.; Hajim, K.; Kacar, E.; Gundogdu, O.; Demir, A. Experimental and theoretical investigation of the drilling of alumina ceramic using Nd:YAG pulsed laser. Opt. Laser Technol. 2012, 44, 913–922. [Google Scholar] [CrossRef]
- Liu, W.; Kosareva, O.; Golubtsov, I.; Iwasaki, A.; Becker, A.; Kandidov, V.; Chin, S. Femtosecond laser pulse filamentation versus optical breakdown in H2O. Appl. Phys. B 2003, 76, 215–229. [Google Scholar] [CrossRef]
- Vogel, A.; Nahen, K.; Theisen, D.; Noack, J. Plasma formation in water by picosecond and nanosecond Nd:YAG laser pulses. I. Optical breakdown at threshold and superthreshold irradiance. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 847–860. [Google Scholar] [CrossRef] [Green Version]
- Noack, J.; Vogel, A. Laser-induced plasma formation in water at nanosecond to femtosecond time scales: Calculation of thresholds, absorption coefficients, and energy density. IEEE J. Quantum Electron. 1999, 35, 1156–1167. [Google Scholar] [CrossRef] [Green Version]
- Hammer, D.X.; Jansen, E.D.; Frenz, M.; Noojin, G.D.; Thomas, R.J.; Noack, J.; Vogel, A.; Rockwell, B.A.; Welch, A.J. Shielding properties of laser-induced breakdown in water for pulse durations from 5 ns to 125 fs. Appl. Opt. 1997, 36, 5630–5640. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Yoshino, K.; Inuishi, Y. Laser-induced breakdown in organic liquids. J. Phys. D: Appl. Phys. 1977, 10, 1975–1983. [Google Scholar] [CrossRef]
- Toyota, K.; Nakashima, S.; Okada, T. Near-infrared laser-induced breakdown of liquid benzene. Chem. Phys. Lett. 2000, 323, 323–328. [Google Scholar] [CrossRef]
- Kovalchuk, T.; Toker, G.; Bulatov, V.; Schechter, I. Laser breakdown in alcohols and water induced by λ=1064nm nanosecond pulses. Chem. Phys. Lett. 2010, 500, 242–250. [Google Scholar] [CrossRef]
- Hammer, D.; Thomas, R.; Noojin, G.; Rockwell, B.; Kennedy, P.; Roach, W. Experimental investigation of ultrashort pulse laser-induced breakdown thresholds in aqueous media. IEEE J. Quantum Electron. 1996, 32, 670–678. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Bakum, I.S. Role of a dissolved gas in the optical breakdown of water. Quantum Electron. 2006, 36, 117–124. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Ninham, B.W.; Babenko, V.A.; Suyazov, N.V.; Sychev, A.A. Role of dissolved gas in optical breakdown of water: Differences between effects due to helium and other gases. J. Phys. Chem. B 2010, 114, 7743–7752. [Google Scholar] [CrossRef]
- Valverde-Alva, M.A.; García-Fernández, T.; Esparza-Alegría, E.; Villagrán-Muniz, M.; Sánchez-Aké, C.; Castañeda-Guzmán, R.; de la Mora, M.B.; Márquez-Herrera, C.E.; Llamazares, J.L.S. Laser ablation efficiency during the production of ag nanoparticles in ethanol at a low pulse repetition rate (1–10 Hz). Laser Phys. Lett. 2016, 13, 106002. [Google Scholar] [CrossRef]
- Barcikowski, S.; Menéndez-Manjón, A.; Chichkov, B.; Brikas, M.; Račiukaitis, G. Generation of nanoparticle colloids by picosecond and femtosecond laser ablations in liquid flow. Appl. Phys. Lett. 2007, 91, 083113. [Google Scholar] [CrossRef]
- Resano-Garcia, A.; Battie, Y.; Koch, A.; En Naciri, A.; Chaoui, N. Influence of the laser light absorption by the colloid on the properties of silver nanoparticles produced by laser ablation in stirred and stationary liquid. J. Appl. Phys. 2015, 117, 113103. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, P.; Liang, Y.; Li, H.B.; Yang, G.W. Promoting the yield of nanoparticles from laser ablation in liquid. Appl. Phys. A 2011, 105, 903–907. [Google Scholar] [CrossRef]
- Kalus, M.R.; Bärsch, N.; Streubel, R.; Gökce, E.; Barcikowski, S.; Gökce, B. How persistent microbubbles shield nanoparticle productivity in laser synthesis of colloids—Quantification of their volume, dwell dynamics, and gas composition. Phys. Chem. Chem. Phys. 2017, 19, 7112–7123. [Google Scholar] [CrossRef]
- Baladi, A.; Mamoory, R.S. Investigation of different liquid media and ablation times on pulsed laser ablation synthesis of aluminum nanoparticles. Appl. Surf. Sci. 2010, 256, 7559–7564. [Google Scholar] [CrossRef]
- Giacomo, A.; Dell’Aglio, M.; Santagata, A.; Gaudiuso, R.; De Pascale, O.; Wagener, P.; Messina, G.C.; Compagnini, G.; Barcikowski, S. Cavitation dynamics of laser ablation of bulk and wire-shaped metals in water during nanoparticles production. Phys. Chem. Chem. Phys. 2013, 15, 3083–3092. [Google Scholar] [CrossRef]
- Scaramuzza, S.; Zerbetto, M.; Amendola, V. Synthesis of gold nanoparticles in liquid environment by laser ablation with geometrically confined configurations: Insights to improve size control and productivity. J. Phys. Chem. C 2016, 120, 9453–9463. [Google Scholar] [CrossRef]
- Messina, G.C.; Wagener, P.; Streubel, R.; De Giacomo, A.; Santagata, A.; Compagnini, G.; Barcikowski, S. Pulsed laser ablation of a continuously-fed wire in liquid flow for highyield production of silver nanoparticles. Phys. Chem. Chem. Phys. 2013, 15, 3093–3098. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, P.; Wang, C.X.; Yang, G.W. External field-assisted laser ablation in liquid: An efficient strategy for nanocrystal synthesis and nanostructure assembly. Prog. Mater. Sci. 2017, 87, 140–220. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, P.; Liang, Y.; Li, H.B.; Yang, G.W. High aspect ratio b-MnO2 nanowires and sensor performance for explosive gases. J. Appl. Phys. 2013, 114, 73513. [Google Scholar] [CrossRef]
- Liu, P.; Wang, C.X.; Chen, X.Y.; Yang, G.W. Controllable fabrication and cathodoluminescence performance of high-index facets GeO2 micro- and nanocubes and spindles upon electrical-field-assisted laser ablation in liquid. J. Phys. Chem. C 2008, 112, 13450–13456. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, P.; Yang, G. Fabrication of one-dimensional chain of iron-based bimetallic alloying nanoparticles with unique magnetizations. Cryst. Growth Des. 2014, 14, 5847–5855. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, P.; Li, H.B.; Yang, G.W. ZnMoO4 micro- and nanostructures synthesized by electrochemistry-assisted laser ablation in liquids and their optical properties. Cryst. Growth Des. 2012, 12, 4487–4493. [Google Scholar] [CrossRef]
- Chu, W.G.; Wang, H.F.; Guo, Y.J.; Zhang, L.N.; Han, Z.H.; Li, Q.Q.; Fan, S.S. Catalyst-free growth of quasi-aligned nanorods of single crystal Cu3Mo2O9 and their catalytic properties. Inorg. Chem. 2009, 48, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liang, Y.; Lin, X.; Wang, C.; Yang, G. A general strategy to fabricate simple polyoxometalate nanostructures: Electrochemistry-assisted laser ablation in liquid. ACS Nano 2011, 5, 4748–4755. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, P.; Li, H.B.; Yang, G.W. Synthesis and characterization of copper vanadate nanostructures via electrochemistry assisted laser ablation in liquid and the optical multi-absorptions performance. Cryst. Eng. Comm. 2012, 14, 3291–3296. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, L.F.; Liu, P.; Li, H.B.; Xiao, J.; Ji, X.W.; Yang, G.W. Ag2V4O11 nanostructures for highly ethanol sensitive performance. Cryst. Eng. Comm. 2013, 15, 6131–6135. [Google Scholar] [CrossRef]
- Ismail, R.A.; Fadhil, F.A. Effect of electric field on the properties of bismuth oxide nanoparticles prepared by laser ablation in water. J. Mater. Sci. Mater. Electron. 2014, 25, 1435–1440. [Google Scholar] [CrossRef]
- Sapkota, D.; Li, Y.; Musaev, O.R.; Wrobel, J.M.; Kruger, M.B. Effect of electric fields on tin nanoparticles prepared by laser ablation in water. J. Laser Appl. 2017, 29, 012002. [Google Scholar] [CrossRef]
- Jumaa, T.; Chasib, M.; Hamid, M.K.; Al-Haddad, R. Effect of the electric field on the antibacterial activity of Au nanoparticles on some gram-positive and gramnegative bacteria. Nanosci. Nanotech. Res. 2014, 2, 1–7. [Google Scholar]
- Al-Haddad, R.M.S.; Hamid, M.K.; Jumaa, T. Electric field effect on the synthesis of nanogold particles by PLAL. Int. J. Chem. Nat. Sci. 2015, 3, 269–274. [Google Scholar]
- Moniri, S.; Hantehzadeh, M.R.; Ghoranneviss, M.; Asadabad, M. Study of the optical and structural properties of Pt nanoparticles prepared by laser ablation as a function of the applied electric field. Appl. Phys. A 2017, 123, 684. [Google Scholar] [CrossRef]
- Li, Y.; Musaev, O.R.; Wrobel, J.M.; Kruger, M.B. Laser ablation in liquids of germanium in externally applied electric fields. J. Laser Appl. 2016, 28, 022004. [Google Scholar] [CrossRef]
- Spadaro, S.; Bonsignore, M.; Fazio, E.; Cimino, F.; Speciale, A.; Trombetta, D.; Barreca, F.; Saija, A.; Neri, F. Molybdenum oxide nanocolloids prepared by an external field-assisted laser ablation in water. EPJ Web Conf. 2018, 167, 4009. [Google Scholar] [CrossRef] [Green Version]
- Fazio, E.; Speciale, A.; Spadaro, S.; Bonsignore, M.; Cimino, F.; Cristani, M.; Trombetta, D.; Saija, A.; Neri, F. Evaluation of biological response induced by molybdenum oxide nanocolloids on in vitro cultured NIH/3T3 fibroblast cells by micro-Raman spectroscopy. Colloids Surfaces B: Biointerfaces 2018, 170, 233–241. [Google Scholar] [CrossRef]
- Stockman, M.I. Nanoplasmonics: Past, present, and glimpse into future. Opt. Express 2011, 19, 22029–22106. [Google Scholar] [CrossRef] [Green Version]
- Trugler, A. Optical Properties of Metallic Nanoparticles; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Gramotnev, D.K.; Bozhevolnyi, S.I. Plasmonics beyond the diffraction limit. Nat. Photonics 2010, 4, 83–91. [Google Scholar] [CrossRef]
- Fazio, E.; Saija, R.; Santoro, M.; Abir, S.; Neri, F.; Tommasini, M.; Ossi, P.M. On the Optical Properties of Ag–Au Colloidal Alloys Pulsed Laser Ablated in Liquid: Experiments and Theory. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Lee, I.; Han, S.W.; Kim, K. Production of Au–Ag alloy nanoparticles by laser ablation of bulk alloys. Chem. Commun. 2001, 18, 1782–1783. [Google Scholar] [CrossRef]
- Compagnini, G.; Messina, E.; Puglisi, O.; Nicolosi, V. Laser synthesis of Au/Ag colloidal nano-alloys: Optical properties, structure and composition. Appl. Surf. Sci. 2007, 254, 1007–1011. [Google Scholar] [CrossRef]
- Waxenegger, J.; Trügler, A.; Hohenester, U. Plasmonics simulations with the MNPBEM toolbox: Consideration of substrates and layer structures. Comput. Phys. Commun. 2015, 193, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Farokhnezhad, M.; Esmaeilzadeh, M. Optical and Photothermal Properties of Graphene Coated Au–Ag Hollow Nanoshells: A Modeling for Efficient Photothermal Therapy. J. Phys. Chem. C 2019, 123, 28907–28918. [Google Scholar] [CrossRef]
- Hohenester, U.; Trügler, A. MNPBEM—A Matlab toolbox for the simulation of plasmonic nanoparticles. Comput. Phys. Commun. 2012, 183, 370–381. [Google Scholar] [CrossRef] [Green Version]
- González-Rubio, G.; Kumar, V.; Llombart, P.; Díaz-Núñez, P.; Bladt, E.; Altantzis, T.; Bals, S.; Peña-Rodríguez, O.; Noya, E.G.; MacDowell, L.G.; et al. Disconnecting Symmetry Breaking from Seeded Growth for the Reproducible Synthesis of High Quality Gold Nanorods. ACS Nano 2019, 13, 4424–4435. [Google Scholar] [CrossRef] [Green Version]
- Attia, Y.A.; Flores-Arias, M.T.; Nieto, D.; Vázquez-Vázquez, C.; De La Fuente, G.F.; López-Quintela, M.A. Transformation of Gold Nanorods in Liquid Media Induced by nIR, Visible, and UV Laser Irradiation. J. Phys. Chem. C 2015, 119, 13343–13349. [Google Scholar] [CrossRef]
- Barbosa, S.; Agrawal, A.; Rodríguez-Lorenzo, L.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Kornowski, A.; Weller, H.; Liz-MarzánL, M. Tuning Size and Sensing Properties in Colloidal Gold Nanostars. Langmuir 2010, 26, 14943–14950. [Google Scholar] [CrossRef]
- Hu, G.; Jin, W.; Zhang, W.; Wu, K.; He, J.; Zhang, Y.; Chen, Q.; Zhang, W. Surfactant-assisted shape separation from silver nanoparticles prepared by a seed-mediated method. Colloids Surfaces A: Physicochem. Eng. Asp. 2018, 540, 136–142. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Nguyen, T.D.; Cao, M.T.; Van Viet, P. Fast and simple synthesis of triangular silver nanoparticles under the assistance of light. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 594, 124659. [Google Scholar] [CrossRef]
- Condorelli, M.; Scardaci, V.; D’Urso, L.; Puglisi, O.; Fazio, E.; Compagnini, G. Plasmon sensing and enhancement of laser prepared silver colloidal nanoplates. Appl. Surf. Sci. 2019, 475, 633–638. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: Weinheim, Germany, 1998. [Google Scholar]
- Lee, G.; Bignell, L.; Romeo, T.; Razal, J.; Shepherd, R.; Chen, J.; Minett, A.; Innis, P.; Wallace, G. The citrate-mediated shape evolution of transforming photomorphic silver nanoparticles. Chem. Commun. 2010, 46, 7807–7809. [Google Scholar] [CrossRef] [PubMed]
- Parnklang, T.; Lertvachirapaiboon, C.; Pienpinijtham, P.; Wongravee, K.; Thammacharoen, C.; Ekgasit, S. H2O2-triggered shape transformation of silver nanospheres to nanoprisms with controllable longitudinal LSPR wavelengths. RSC Adv. 2013, 3, 12886–12894. [Google Scholar] [CrossRef]
- Elechiguerra, J.; Reyes-Gasga, J.; Yacaman, M. The role of twinning in shape evolution of anisotropic noble metal nanostructures. J. Mater. Chem. 2006, 16, 3906–3919. [Google Scholar] [CrossRef]
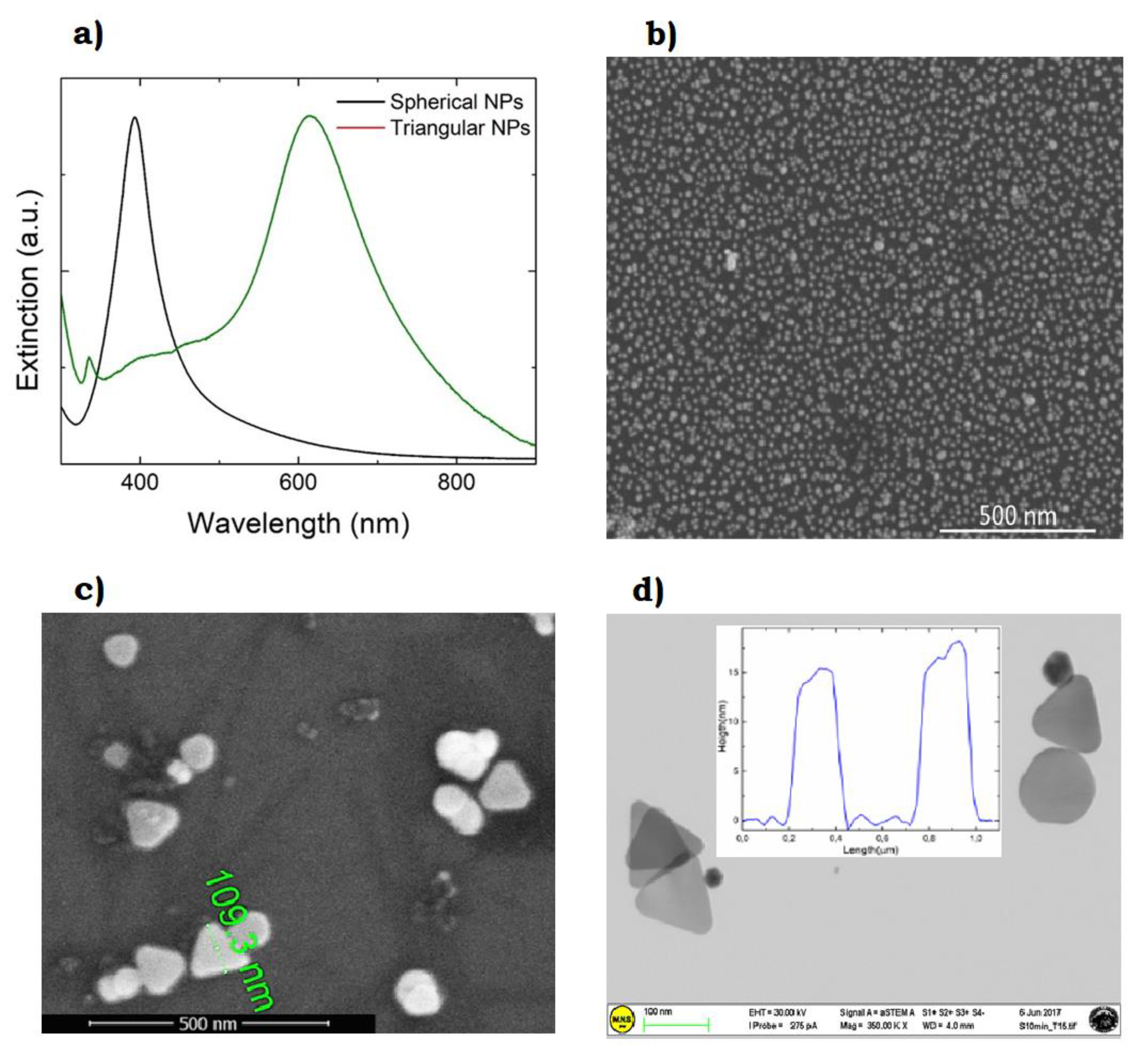
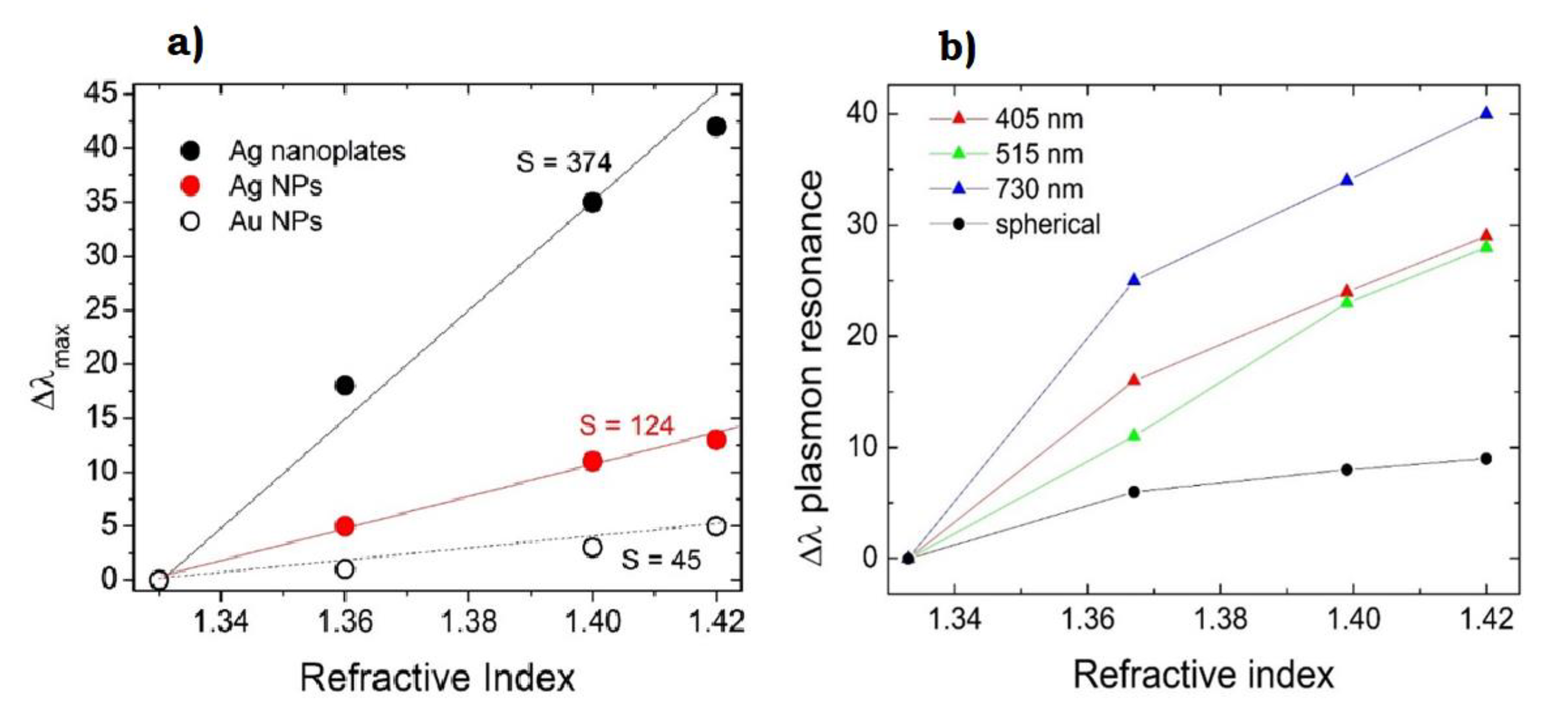
- Compagnini, G.; Condorelli, M.; Fragalà, M.E.; Scardaci, V.; Tinnirello, I.; Puglisi, O.; Neri, F.; Fazio, E. Growth Kinetics and Sensing Features of Colloidal Silver Nanoplates. J. Nanomater. 2019, 2019, 7084731. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzan, L. Colloidal silver nanoplates. State of the art and future challenges. J. Mater. Chem. 2008, 18, 1724–1737. [Google Scholar] [CrossRef]
- Charles, D.E.; Aherne, D.; Gara, M.; Ledwith, D.M.; Gun’ko, Y.K.; Kelly, J.M.; Blau, W.J.; Brennan-Fournet, M.E. Versatile Solution Phase Triangular Silver Nanoplates for Highly Sensitive Plasmon Resonance Sensing. ACS Nano 2010, 4, 55–64. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, L.; Xu, G. Seed-mediated growth of noble metal nanocrystals: Crystal growth and shape control. Nanoscale 2013, 5, 3172–3181. [Google Scholar] [CrossRef]
- Knieke, C.; Berger, A.; Voigt, M.; Taylor, R.N.K.; Röhrl, J.; Peukert, W. Scalable production of graphene sheets by mechanical delamination. Carbon 2010, 48, 3196–3204. [Google Scholar] [CrossRef]
- Taylor, A.B.; Zijlstra, P. Single-Molecule Plasmon Sensing: Current Status and Future Prospects. ACS Sens. 2017, 2, 1103–1122. [Google Scholar] [CrossRef] [Green Version]
- Haes, A.J.; Van Duyne, R.P. A Nanoscale Optical Biosensor: Sensitivity and Selectivity of an Approach Based on the Localized Surface Plasmon Resonance Spectroscopy of Triangular Silver Nanoparticles. J. Am. Chem. Soc. 2002, 124, 10596–10604. [Google Scholar] [CrossRef]
- Yunus, W.M.B.M.; Rahman, A.B.A. Refractive index of solutions at high concentrations. Appl. Opt. 1988, 27, 3341–3343. [Google Scholar] [CrossRef] [PubMed]
- Potara, M.; Gabudean, A.; Astilean, S. Solution-phase, dual LSPR-SERS plasmonic sensors of high sensitivity and stability based on chitosan-coated anisotropic silver nanoparticles. J. Mater. Chem. 2011, 21, 3625–3633. [Google Scholar] [CrossRef]
- Scardaci, V.; Pulvirenti, M.; Condorelli, M.; Compagnini, G. Monochromatic Light Driven Synthesis and Growth of Flat Silver Nanoparticles and their Plasmon Sensitivity. J. Mat. Chem. C 2020, 8, 9734–9741. [Google Scholar] [CrossRef]
- Bertorelle, F.; Pinto, M.; Zappon, R.; Pilot, R.; Litti, L.; Fiameni, S.; Conti, G.; Gobbo, M.; Toffoli, G.; Colombatti, M.; et al. Safe core-satellite magneto-plasmonic nanostructures for efficient targeting and photothermal treatment of tumor cells. Nanoscale 2018, 10, 976–984. [Google Scholar] [CrossRef]
- Biscaglia, F.; Rajendran, S.; Conflitti, P.; Benna, C.; Sommaggio, R.; Litti, L.; Mocellin, S.; Bocchinfuso, G.; Rosato, A.; Palleschi, A.; et al. Enhanced EGFR Targeting Activity of Plasmonic Nanostructures with Engineered GE11 Peptide. Adv. Heal. Mater. 2017, 6, 1700596–1700604. [Google Scholar] [CrossRef]
- Meneghetti, M.; Scarsi, A.; Litti, L.; Marcolongo, G.; Amendola, V.; Gobbo, M.; Di Chio, M.; Boscaini, A.; Fracasso, G.; Colombatti, M. Plasmonic Nanostructures for SERRS Multiplexed Identification of Tumor-Associated Antigens. Small 2012, 8, 3733–3738. [Google Scholar] [CrossRef]
- Calzavara, D.; Ferraro, D.; Litti, L.; Cappozzo, G.; Mistura, G.; Meneghetti, M.; Pierno, M. Single File Flow of Biomimetic Beads for Continuous SERS Recording in a Microfluidic Device. Adv. Condens. Matter Phys. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Litti, L.; Rivato, N.; Fracasso, G.; Bontempi, P.; Nicolato, E.; Marzola, P.; Venzo, A.; Colombatti, M.; Gobbo, M.; Meneghetti, M. A SERRS/MRI multimodal contrast agent based on naked Au nanoparticles functionalized with a Gd(III) loaded PEG polymer for tumor imaging and localized hyperthermia. Nanoscale 2018, 10, 1272–1278. [Google Scholar] [CrossRef]
- Del Tedesco, A.; Piotto, V.; Sponchia, G.; Hossain, K.; Litti, L.; Peddis, D.; Scarso, A.; Meneghetti, M.; Benedetti, A.; Riello, P. Zirconia-Based Magnetoplasmonic Nanocomposites: A New Nanotool for Magnetic-Guided Separations with SERS Identification. ACS Appl. Nano Mater. 2020, 3, 1232–1241. [Google Scholar] [CrossRef]
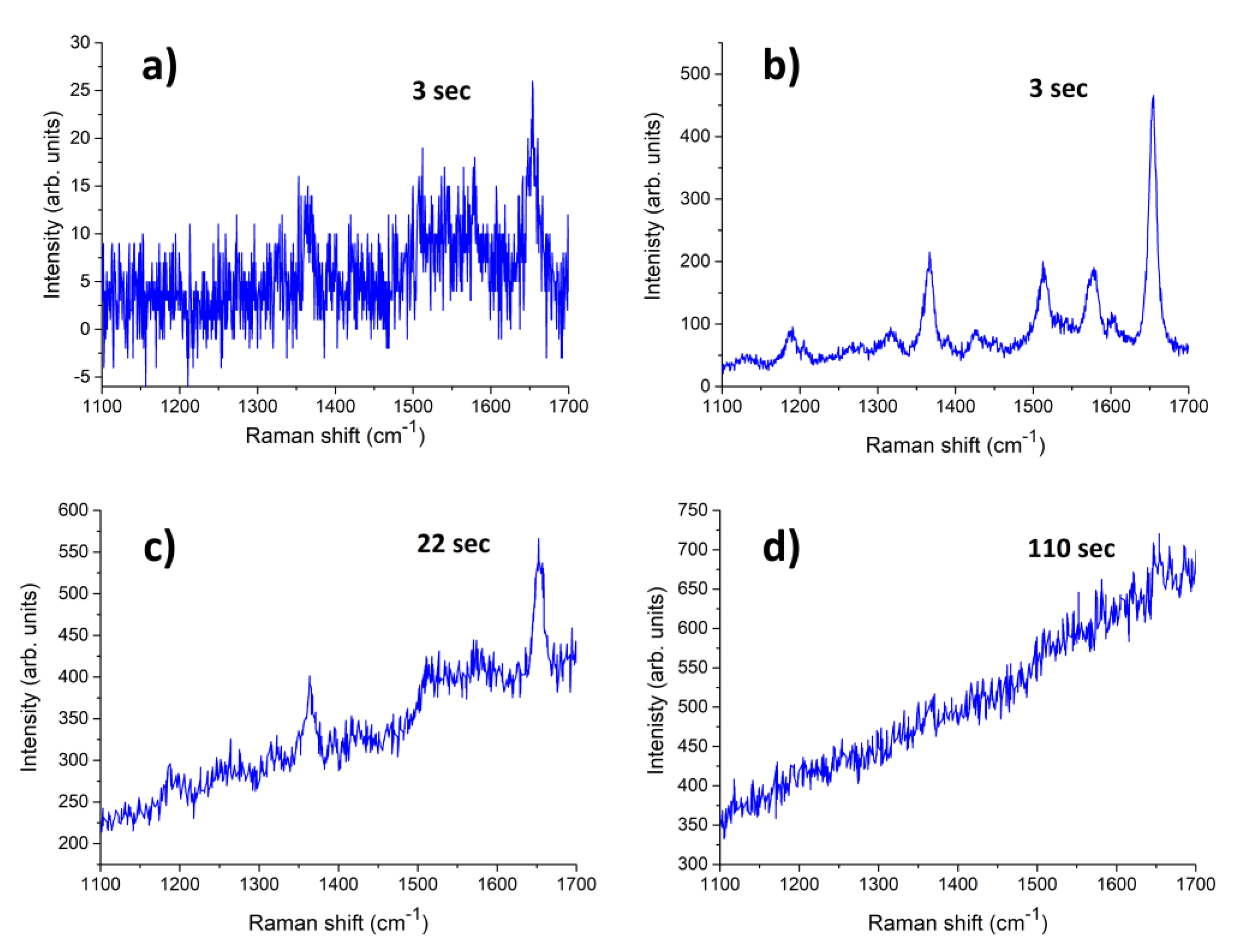
- Litti, L.; Ramundo, A.; Biscaglia, F.; Toffoli, G.; Gobbo, M.; Meneghetti, M. A surface enhanced Raman scattering based colloid nanosensor for developing therapeutic drug monitoring. J. Colloid Interface Sci. 2019, 533, 621–626. [Google Scholar] [CrossRef]
- Litti, L.; Meneghetti, M. Predictions on the SERS enhancement factor of gold nanosphere aggregate samples. Phys. Chem. Chem. Phys. 2019, 21, 15515–15522. [Google Scholar] [CrossRef]
- Fornasaro, S.; Alsamad, F.; Baia, M.; De Carvalho, L.A.E.B.; Beleites, C.; Byrne, H.J.; Chiadò, A.; Chis, M.; Chisanga, M.; Daniel, A.; et al. Surface Enhanced Raman Spectroscopy for Quantitative Analysis: Results of a Large-Scale European Multi-Instrument Interlaboratory Study. Anal. Chem. 2020, 92, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, F.; Quarta, S.; Villano, G.; Turato, C.; Biasiolo, A.; Litti, L.; Ruzzene, M.; Meneghetti, M.; Pontisso, P.; Gobbo, M. PreS1 peptide-functionalized gold nanostructures with SERRS tags for efficient liver cancer cell targetin. Mater. Sci. Eng. C 2019, 103, 109762–109770. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, C.; Di Napoli, B.; Biscaglia, F.; Ripani, G.; Rajendran, S.; Braga, A.; Benna, C.; Mocellin, S.; Gobbo, M.; Meneghetti, M.; et al. Understanding the good and poor cell targeting activity of gold nanostructures functionalized with molecular units for the epidermal growth factor receptor. Nanoscale Adv. 2019, 1, 1970–1979. [Google Scholar] [CrossRef] [Green Version]
- Biscaglia, F.; Ripani, G.; Rajendran, S.; Benna, C.; Mocellin, S.; Bocchinfuso, G.; Meneghetti, M.; Palleschi, A.; Gobbo, M. Gold Nanoparticle Aggregates Functionalized with Cyclic RGD Peptides for Targeting and Imaging of Colorectal Cancer Cells. ACS Appl. Nano Mater. 2019, 2, 6436–6444. [Google Scholar] [CrossRef]
- Prati, S.; Quaranta, M.; Sciutto, G.; Bonacini, I.; Litti, L.; Meneghetti, M.; Mazzeo, R. Use of nano gold obtained by laser ablation for SEIRA analyses of colorants. Herit. Sci. 2014, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chakravadhanula, V.S.K.; Mishra, Y.K.; Kotnur, V.G.; Avasthi, D.K.; Strunskus, T.; Zaporotchenko, V.; Fink, D.; Kienle, L.; Faupel, F. Microstructural and plasmonic modifications in Ag–TiO2 and Au–TiO2 nanocomposites through ion beam irradiation. Beilstein J. Nanotechnol. 2014, 5, 1419–1431. [Google Scholar] [CrossRef] [Green Version]
- Ahmadivand, A.; Sinha, R.; Kaya, S.; Pala, N. Rhodium plasmonics for deep-ultraviolet bio-chemical sensing. Plasmonics 2016, 11, 839–849. [Google Scholar] [CrossRef]
- Yang, Y.; Callahan, J.M.; Kim, T.-H.; Brown, A.S.; Everitt, H.O. Ultraviolet Nanoplasmonics: A Demonstration of Surface-Enhanced Raman Spectroscopy, Fluorescence, and Photodegradation Using Gallium Nanoparticles. Nano Lett. 2013, 13, 2837–2841. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Zhou, L.; Schlather, A.E.; Dong, L.; McClain, M.J.; Swearer, D.F.; Nordlander, P.; Halas, N.J. Al–Pd Nanodisk Heterodimers as Antenna–Reactor Photocatalysts. Nano Lett. 2016, 16, 6677–6682. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nanosci. Technol. 2009, 308–319. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Taguchi, A.; Honda, M.; Watanabe, K.; Saito, Y.; Kawata, S. Indium for Deep-Ultraviolet Surface-Enhanced Resonance Raman Scattering. ACS Photon. 2014, 1, 598–603. [Google Scholar] [CrossRef] [Green Version]
- Dörfer, T.; Schmitt, M.; Popp, J. Deep-UV surface-enhanced Raman scattering. J. Raman Spectrosc. 2007, 38, 1379–1382. [Google Scholar] [CrossRef]
- McMahon, J.M.; Schatz, G.C.; Gray, S.K. Plasmonics in the ultraviolet with the poor metals Al, Ga, In, Sn, Tl, Pb, and Bi. Phys. Chem. Chem. Phys. 2013, 15, 5415–5423. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.W.; King, N.S.; Liu, L.; Everitt, H.O.; Nordlander, P.; Halas, N.J. Aluminum for Plasmonics. ACS Nano 2014, 8, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Ekinci, Y.; Agio, M.; Löffler, J.F. Towards deep-UV surface-enhanced resonance Raman spectroscopy of explosives: Ultrasensitive, real-time and reproducible detection of TNT. Analyst 2015, 140, 5671–5677. [Google Scholar] [CrossRef]
- Gutierrez, Y.; Ortiz, D.; Sanz, J.M.; Saiz, J.M.; Gonzalez, F.; Everitt, H.O.; Moreno, F. How an oxide shell affects the ultraviolet plasmonic behavior of Ga, Mg, and Al nanostructures. Opt. Express 2016, 24, 20621–20631. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Sterl, F.; Strohfeldt, N.; Walter, R.; Griessen, R.; Tittl, A.; Giessen, H. Magnesium as Novel Material for Active Plasmonics in the Visible Wavelength Range. Nano Lett. 2015, 15, 7949–7955. [Google Scholar] [CrossRef] [Green Version]
- Losurdo, M.; Suvorova, A.; Rubanov, S.; Hingerl, K.; Brown, A.S. Thermally stable coexistence of liquid and solid phases in gallium nanoparticles. Nat. Mater. 2016, 15, 995–1002. [Google Scholar] [CrossRef]
- Knight, M.W.; Coenen, T.; Yang, Y.; Brenny, B.J.M.; Losurdo, M.; Brown, A.S.; Everitt, H.O.; Polman, A. Gallium Plasmonics: Deep Subwavelength Spectroscopic Imaging of Single and Interacting Gallium Nanoparticles. ACS Nano 2015, 9, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, A.G.; Cavassin, P.; Machado, T.N.; Woiski, T.D.; Caetano, R.; Schreiner, W.H. Surface-enhanced Raman scattering using bismuth nanoparticles: A study with amino acids. J. Nanopart. Res. 2017, 19, 362. [Google Scholar] [CrossRef]
- Xie, S.; Liu, X.Y.; Xia, Y. Shape-controlled syntheses of rhodium nanocrystals for the enhancement of their catalytic properties. Nano Res. 2015, 8, 82–96. [Google Scholar] [CrossRef]
- Bilmes, S.A. SERS of pyridine adsorbed on rhodium electrodes. Chem. Phys. Lett. 1990, 171, 141–146. [Google Scholar] [CrossRef]
- Lin, X.-F.; Ren, B.; Yang, Z.-L.; Liu, G.-K.; Tian, Z.-Q. Surface-enhanced Raman spectroscopy with ultraviolet excitation. J. Raman Spectrosc. 2005, 36, 606–612. [Google Scholar] [CrossRef]
- Zettsu, N.; McLellan, J.M.; Wiley, B.; Yin, Y.; Li, Z.-Y.; Xia, Y. Synthesis, Stability, and Surface Plasmonic Properties of Rhodium Multipods, and Their Use as Substrates for Surface-Enhanced Raman Scattering. Angew. Chem. Int. Ed. 2006, 45, 1288–1292. [Google Scholar] [CrossRef]
- Watson, A.M.; Zhang, X.; De La Osa, R.A.; Sanz, J.M.; González, F.; Moreno, F.; Finkelstein, G.; Liu, J.; Everitt, H.O. Rhodium Nanoparticles for Ultraviolet Plasmonics. Nano Lett. 2015, 15, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.; Lin, X.F.; Yang, Z.L.; Liu, G.K.; Aroca, R.F.; Mao, B.W.; Tian, Z.Q. Surface-enhanced Raman scattering in the ultraviolet spectral region: UV-SERS on rhodium and ruthenium electrodes. J. Am. Chem. Soc. 2003, 125, 9598–9599. [Google Scholar] [CrossRef]
- Li, A.; Lin, J.; Huang, Z.; Wang, X.; Guo, L. Surface-Enhanced Raman Spectroscopy on Amorphous Semiconducting Rhodium Sulfide Microbowl Substrates. iScience 2018, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Cui, L.; Ren, B.; Tian, Z.Q. Electrochemical preparation of platinum nanothorn assemblies with high surface enhanced Raman scattering activity. Chem. Commun. 2006, 39, 4090–4092. [Google Scholar] [CrossRef]
- Humphrey, S.M.; Grass, M.E.; Habas, S.E.; Niesz, K.; Somorjai, G.A.; Don Tilley, T. Rhodium nanoparticles from cluster seeds: Control of size and shape by precursor addition rate. Nano Lett. 2007, 7, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Soni, R.K. Rhodium nanocubes and nanotripods for highly sensitive ultraviolet surface-enhanced Raman spectroscopy. Analyst 2018, 143, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Passoni, M.; Dellasega, D.; Grosso, G.; Conti, C.; Ubaldi, M.; Bottani, C.E. Nanostructured rhodium films produced by pulsed laser deposition for nuclear fusion applications. J. Nucl. Mater. 2010, 404, 1–5. [Google Scholar] [CrossRef]
- Uccello, A.; Dellasega, D.; Perissinotto, S.; Lecis, N.; Passoni, M. Nanostructured rhodium films for advanced mirrors produced by Pulsed Laser Deposition. J. Nucl. Mater. 2013, 432, 261–265. [Google Scholar] [CrossRef]
- Fazio, E.; Leonardi, S.; Santoro, M.; Donato, N.; Neri, G.; Neri, F. Synthesis, characterization and hydrogen sensing properties of nanosized colloidal rhodium oxides prepared by Pulsed Laser Ablation in water. Sens. Actuators B: Chem. 2018, 262, 79–85. [Google Scholar] [CrossRef]
- Santoro, M.; Fazio, E.; Trusso, S.; Ossi, P.M.; Tommasini, M.; Neri, F. Rhodium nanoparticles synthesized by nanosecond and picosecond Pulsed Laser Ablation in Liquid. GISR2016. In Proceedings of the Italian Meeting on Raman Spectroscopy and Non Linear Optic Effects, Padova, Italy, 14–16 September 2016. [Google Scholar]
- Santoro, M.; Bertoncini, A.; Fazio, E.; Tommasini, M.; Neri, F.; Ossi, P.M.; Trusso, S. Nanostructured Rhodium Thin Films Deposited by Pulsed Laser Deposition for SERS Detection. CNS2018—4°. In Proceedings of the Congresso Nazionale Sensori, Catania, Italy, 21–23 February 2018. [Google Scholar]
- Borodko, Y.; Sook Lee, H.; Joo, S.H.; Zhang, Y.; Somorjai, G. Spectroscopic Study of the Thermal Degradation of PVP-Capped Rh and Pt Nanoparticles in H2 and O2 Environments. J. Phys. Chem. C 2010, 114, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Abe, T.; Kawamura, M.; Sasaki, M. Preparation of RhO2 Thin Films by Reactive Sputtering and Their Characterizations. Jpn. J. Appl. Phys. 2001, 40, 2399. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of Pharmaceuticals in Sewage Treatment Plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef]
- Dawson, A.; Kamat, P.V. Semiconductor-metal nanocomposites. Photoinduced fusion and photocatalysis of gold-capped TiO2 (TiOa/Gold) nanoparticles. J. Phys. Chem. B. 2001, 105, 960–966. [Google Scholar] [CrossRef]
- Raj Pant, H.; Pant, B.; Joo Kim, H.; Amarjargal, A.; Hee Park, C.; Tijing, L.D.; Kyo Kim, E.; Sang Kim, C. A green and facile one-pot synthesis of Ag-ZnO/RGO nanocomposite with effective photocatalytic activity for removal of organic pollutants. Ceram. Int. 2013, 39, 5083–5091. [Google Scholar] [CrossRef]
- Safajou, H.; Khojasteh, H.; Salavati-Niasari, M.; Mortazavi-Derazkola, S. Enhanced photocatalytic degradation of dyes over graphene/Pd/TiO2 nanocomposites: TiO2 nanowires versus TiO2 nanoparticles. J. Colloid Interface Sci. 2017, 498, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Zhang, Q.; Hu, Y.; Ge, J.; Lu, Z.; He, L.; Chen, Z.; Yin, Y. Magnetically Recoverable Core-Shell Nanocomposites with Enhanced Photocatalytic Activity. Chem. A Eur. J. 2010, 16, 6243–6250. [Google Scholar] [CrossRef] [PubMed]
- Mitsudome, T.; Mikami, Y.; Matoba, M.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Design of a Silver-Cerium Dioxide Core-Shell Nanocomposite Catalyst for Chemoselective Reduction Reactions. Angew. Chem. Int. Ed. 2012, 51, 136–139. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energ. Mater. Sol. Cells. 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Fenoll, J.; Hellín, P.; Martínez, C.M.; Flores, P.; Navarro, S. Semiconductor-sensitized photodegradation of s-triazine and chloroacetanilide herbicides in leaching water using TiO2 and ZnO as catalyst under natural sunlight. J. Photochem. Photobiol. A Chem. 2012, 238, 81–87. [Google Scholar] [CrossRef]
- Yamaki, T.; Umebayashi, T.; Sumita, T.; Yamamoto, S.; Maekawa, M.; Kawasuso, A.; Itoh, H. Fluorine-doping in titanium dioxide by ion implantation technique. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. Atoms 2003, 206, 254–258. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, J.P. Effects of dopant states on photoactivity in carbon-doped TiO2. J. Physics: Condens. Matter 2005, 17, L209–L213. [Google Scholar] [CrossRef]
- Diwald, O.; Thompson, T.L.; Zubkov, T.; Goralski, E.G.; Walck, S.D.; Yates, J.T. Photochemical activity of nitrogen-doped rutile TiO2(110) in visible light. J. Phys. Chem. B 2004, 108, 6004–6008. [Google Scholar] [CrossRef]
- Eibner, A. Action of Light on Pigments. Chem. Ztg. 1911, 35, 753–775. [Google Scholar]
- Keidel, E. The Fading of Aniline Dyes in the Presence of Titanium White. Farben Ztg. 1929, 34, 1242–1243. [Google Scholar]
- Kandavelu, V.; Kastien, H.; Thampi, K.R. Photocatalytic degradation of isothiazolin-3-ones in water and emulsion paints containing nanocrystalline TiO2 and ZnO catalysts. Appl. Catal. B Environ. 2004, 48, 101–111. [Google Scholar] [CrossRef]
- Fenoll, J.; Ruiz, E.; Hellín, P.; Flores, P.; Navarro, S. Heterogeneous photocatalytic oxidation of cyprodinil and fludioxonil in leaching water under solar irradiation. Chemosphere 2011, 85, 1262–1268. [Google Scholar] [CrossRef]
- Rao, G.G.; Dhar, N.R. Photosennsitized oxidation of ammonia and ammonium salt and problem of nitrification in soils. Soil Sci. 1931, 31, 379–384. [Google Scholar] [CrossRef]
- Osugi, M.; Aoki, S. On the photoxidation of ammonium compounds in solution and soil. Jpn. J. Soil Manure. 1936, 10, 11–24. [Google Scholar]
- Souza, R.P.; Freitas, T.K.; Domingues, F.S.; Pezoti, O.; Ambrosio, E.; Ferrari-Lima, A.M.; Garcia, J.C. Photocatalytic activity of TiO2, ZnO and Nb2O5 applied to degradation of textile wastewater. J. Photochem. Photobiol. A Chem. 2016, 329, 9–17. [Google Scholar] [CrossRef]
- Frenkel, J. On the Absorption of Light and the Trapping of Electrons and Positive Holes in Crystalline Dielectrics. Phys. Zs. Sowjetunion. 1936, 9, 158–186. [Google Scholar]
- Goodeve, C.F. The absorption spectra and photo-sensitising activity of white pigments. Trans. Faraday Soc. 1937, 33, 340–347. [Google Scholar] [CrossRef]
- Goodeve, C.F.; Kitchener, J.A. The mechanism of photosensitisation by solids. Trans. Faraday Soc. 1938, 34, 902–908. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Heterogeneous Photocatalytic Oxidation of Cyanide Ion in Aqueous Solutions at TiO2 Powder. J. Am. Chem. Soc. 1977, 99, 303–304. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Cavanagh, J.E.; Weinberg, H.S.; Avram, G.; Sangalah, R.; Dean, M.; Glaze, W.H.; Collette, T.W.; Richardson, S.D.; Thruston, A.D. Ozonation Byproducts: Identification of Bromohydrins from the Ozonation of Natural Waters with Enhanced Bromide Levels. Environ. Sci. Technol. 1992, 26, 1658–1662. [Google Scholar] [CrossRef]
- Kormann, C.; Hoffmann, M.R.; Bahnemann, D.W. Photolysis of Chloroform and Other Organic Molecules in Aqueous TiO2 Suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Spadaro, J.T.; Isabelle, L.; Renganathan, V. Hydroxyl Radical Mediated Degradation of Azo Dyes: Evidence for Benzene Generation. Environ. Sci. Technol. 1994, 28, 1389–1393. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Can the photocatalyst TiO2 be incorporated into a wastewater treatment method? Background and prospects. Catal. Today 2020, 340, 334–346. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Waldner, G.; Pourmodjib, M.; Bauer, R.; Neumann-Spallart, M. Photoelectrocatalytic degradation of 4-chlorophenol and oxalic acid on titanium dioxide electrodes. Chemosphere 2003, 50, 989–998. [Google Scholar]
- Sonawane, R.S.; Kale, B.B.; Dongare, M.K. Preparation and photo-catalytic activity of Fe-TiO2 thin films prepared by sol-gel dip coating. Mater. Chem. Phys. 2004, 85, 52–57. [Google Scholar] [CrossRef]
- Al-Qaradawi, S.; Salman, S.R. Photocatalytic degradation of methyl orange as a model compound. J. Photochem. Photobiol. A Chem. 2002, 148, 161–168. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Scirè, S.; Palmisano, L. Effect of the addition of different doping agents on visible light activity of porous TiO2 photocatalysts. Mol. Catal. 2018, 455, 108–120. [Google Scholar] [CrossRef]
- Amadelli, R.; Samiolo, L.; Borsa, M.; Bellardita, M.; Palmisano, L. N-TiO2 Photocatalysts highly active under visible irradiation for NOX abatement and 2-propanol oxidation. Catal. Today 2013, 206, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Filice, S.; Compagnini, G.; Fiorenza, R.; Scirè, S.; D’Urso, L.; Fragalà, M.E.; Russo, P.; Fazio, E.; Scalese, S. Laser processing of TiO2 colloids for an enhanced photocatalytic water splitting activity. J. Colloid Interface Sci. 2017, 489, 131–137. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Gulino, A.; Spitaleri, L.; Privitera, V.; Impellizzeri, G. Molecularly imprinted N-doped TiO2 photocatalysts for the selective degradation of o-phenylphenol fungicide from water. Mater. Sci. Semicond. Process 2020, 112, 105019. [Google Scholar] [CrossRef]
- D’Urso, L.; Spadaro, S.; Bonsignore, M.; Santangelo, S.; Compagnini, G.; Neri, F.; Fazio, E. Zinc oxide nanocolloids prepared by picosecond pulsed laser ablation in water at different temperatures. EPJ Web Conf. 2018, 167, 04008. [Google Scholar] [CrossRef] [Green Version]
- Rather, R.A.; Singh, S.; Pal, B. Visible and direct sunlight induced H2 production from water by plasmonic Ag-TiO2 nanorods hybrid interface. Sol. Energ. Mater. Sol. Cells 2017, 160, 463–469. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sakamoto, H.; Sugano, Y.; Ichikawa, S.; Hirai, T. Pt-Cu bimetallic alloy nanoparticles supported on anatase TiO2: Highly active catalysts for aerobic oxidation driven by visible light. ACS Nano 2013, 7, 9287–9297. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, X.; Yang, H.; Zhou, Y.; Cui, H.; Liu, H. High yield production of reduced TiO2 with enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 360, 738–743. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Vijayan, B.; Dimitrijevic, N.M.; Rajh, T.; Gray, K. Effect of calcination temperature on the photocatalytic reduction and oxidation processes of hydrothermally synthesized titania nanotubes. J. Phys. Chem. C 2010, 114, 12994–13002. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Li, J.; Yin, J. TiO2-coated active carbon composites with increased photocatalytic activity prepared by a properly controlled sol–gel method. Mater. Lett. 2005, 59, 2659–2663. [Google Scholar] [CrossRef]
- Henrich, V.E.; Kurtz, R.L. Surface electronic structure of TiO2: Atomic geometry, ligand coordination, and the effect of adsorbed hydrogen. Phys. Rev. B 1981, 23, 6280–6287. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-Doped Black Titania with Very High Solar Absorption and Excellent Photocatalysis Enhanced by Localized Surface Plasmon Resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Z.; Zhou, Q.; Meng, B.; Meng, X.; Qiu, J. Highly efficient low-temperature plasma-assisted modification of TiO2 nanosheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem. A Eur. J. 2014, 20, 14763–14770. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Liu, K.; Wang, C.; Liu, L.; Li, B.; Zhang, Z.; Shen, D. Laser-Modified Black Titanium Oxide Nanospheres and Their Photocatalytic Activities under Visible Light. ACS Appl. Mater. Interfaces 2015, 7, 16070–16077. [Google Scholar] [CrossRef]
- Fiorenza, R.; Condorelli, M.; D’Urso, L.; Compagnini, G.; Bellardita, M.; Palmisano, L.; Scirè, S. Catalytic and Photothermo-catalytic Applications of TiO-CoOx Composites. J. Photocatal. 2020, 1, 3–15. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Sau, T.K.; Murphy, C.J. Shape-dependent plasmon-resonant gold nanoparticles. Small 2006, 2, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Juárez, R.; Marino, T.; Molinari, R.; García, H. Influence of Excitation Wavelength (UV or Visible Light) on the Photocatalytic Activity of Titania Containing Gold Nanoparticles for the Generation of Hydrogen or Oxygen from Water. J. Am. Chem. Soc. 2011, 133, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Manna, J.; Vinod, T.P.; Flomin, K.; Jelinek, R. Photocatalytic hybrid Au/ZnO nanoparticles assembled through a one-pot method. J. Colloid Interface Sci. 2015, 460, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Aramendía, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Synthesis, characterization and photocatalytic activity of different metal-doped titania systems. Appl. Catal. A Gen. 2006, 306, 120–127. [Google Scholar] [CrossRef]
- Araña, J.; Doña-Rodríguez, J.; González-Díaz, O.; Rendón, E.T.; Melián, J.H.; Colón, G.; Navío, J.; Pena, J.P. Gas-phase ethanol photocatalytic degradation study with TiO2 doped with Fe, Pd and Cu. J. Mol. Catal. A Chem. 2004, 215, 153–160. [Google Scholar] [CrossRef]
- Zhao, B.; Mele, G.; Pio, I.; Li, J.; Palmisano, L.; Vasapollo, G. Degradation of 4-nitrophenol (4-NP) using Fe–TiO2 as a heterogeneous photo-Fenton catalyst. J. Hazard. Mater. 2010, 176, 569–574. [Google Scholar] [CrossRef]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, D.Y.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrogen Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Wu, N.L.; Lee, M.S. Enhanced TiO2 photocatalysis by Cu in hydrogen production from aqueous methanol solution. Int. J. Hydrogen Energy 2004, 29, 1601–1605. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Murcia, J.; Rojas, H.; Navío, J.; Hidalgo, M. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Appl. Catal. B Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Negrea, P.; Sidea, F.; Negrea, A.; Lupa, L.; Ciopec, M.; Muntean, C. Stuies regarding the Benzene, Toluene and o-Xylene Removal from Waste Water. Politeh. Univ. 2008, 53, 1–2. [Google Scholar]
- Parker, J.C.; Siegel, R.W. Calibration of the Raman spectrum to the oxygen stoichiometry of nanophase TiO2. Appl. Phys. Lett. 1990, 57, 943–945. [Google Scholar] [CrossRef]
- Stucchi, M.; Galli, F.; Bianchi, C.L.; Pirola, C.; Boffito, D.C.; Biasioli, F.; Capucci, V. Simultaneous photodegradation of VOC mixture by TiO2 powders. Chemosphere 2018, 193, 198–206. [Google Scholar] [CrossRef]





| Rate Constant k of Degradation of TiO2 (h−1) in Different Time with Different Solar Intensities W h/m2 | Effect of Concentration on the Degradation Rate (h−1) during April 2001 | ||||||
|---|---|---|---|---|---|---|---|
| Month | 1st Rate Constant | Solar Intensity | [MO] April 2001 | k (465 nm) | R2 | pH before Reaction | pH after Reaction |
| July 1999 | 0.6195 | 4480 | 2 × 10−3 M | 0.0412 | 0.94 | 6.4 | 6.1 |
| November 1999 | 0.367 | 2535 | 4 × 10−4 M | 0.1329 | 0.96 | 6.4 | 6.0 |
| December 1999 (cloudy) | 0.2455 | 2177 | 8 × 10−5 M | 0.2010 | 0.98 | 6.5 | 6.1 |
| January 2000 | 0.3314 | 3060 | 4 × 10−5 M | 0.6393 | 0.92 | 6.5 | 6.4 |
| February 2000 | 0.6489 | 3419 | 1 × 10−5 M | 0.2912 | 0.90 | 5.9 | 6.2 |
| March 2000 | 0.7953 | 4255 | |||||
| May 2000 | 0.5445 | 4814 | |||||
| June 2000 | 0.4645 | 3969 | |||||
| October 2000 | 0.3491 | 3970 | |||||
| pH | 9.1 |
|---|---|
| Benzene | 90.3% |
| Toluene | 6.1% |
| m-xylene | 0.85% |
| o-xylene | 0.46% |
| p-xylene | 0.32% |
| Etylenbenzene | 1.32% |
| Aromatics | 1060 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. https://doi.org/10.3390/nano10112317
Fazio E, Gökce B, De Giacomo A, Meneghetti M, Compagnini G, Tommasini M, Waag F, Lucotti A, Zanchi CG, Ossi PM, et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials. 2020; 10(11):2317. https://doi.org/10.3390/nano10112317
Chicago/Turabian StyleFazio, Enza, Bilal Gökce, Alessandro De Giacomo, Moreno Meneghetti, Giuseppe Compagnini, Matteo Tommasini, Friedrich Waag, Andrea Lucotti, Chiara Giuseppina Zanchi, Paolo Maria Ossi, and et al. 2020. "Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications" Nanomaterials 10, no. 11: 2317. https://doi.org/10.3390/nano10112317
APA StyleFazio, E., Gökce, B., De Giacomo, A., Meneghetti, M., Compagnini, G., Tommasini, M., Waag, F., Lucotti, A., Zanchi, C. G., Ossi, P. M., Dell’Aglio, M., D’Urso, L., Condorelli, M., Scardaci, V., Biscaglia, F., Litti, L., Gobbo, M., Gallo, G., Santoro, M., ... Neri, F. (2020). Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials, 10(11), 2317. https://doi.org/10.3390/nano10112317