Single Particle Approaches to Plasmon-Driven Catalysis
Abstract
1. Introduction
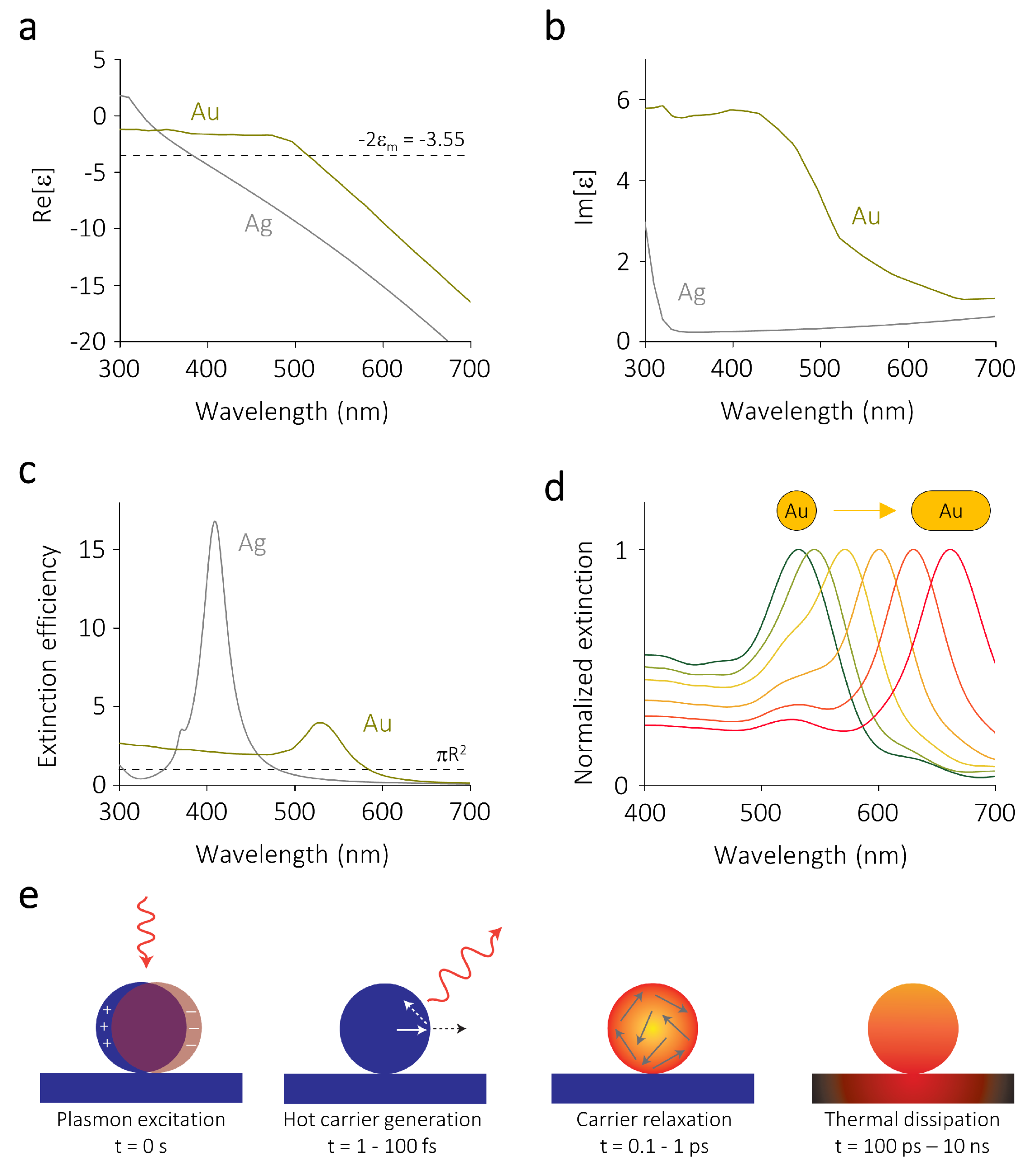
2. Optical Properties of Metallic Nanoparticles
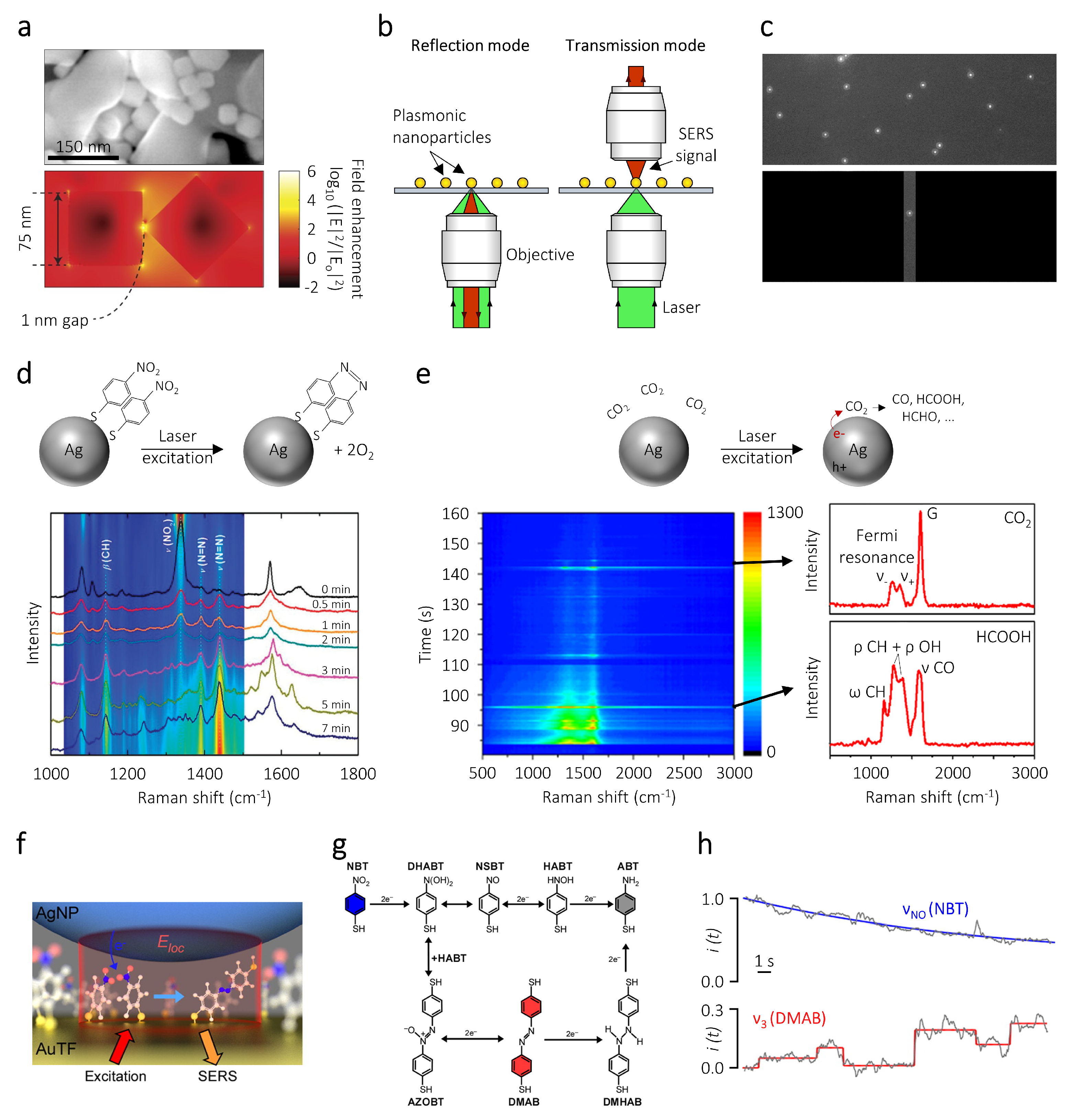
3. Surface-Enhanced Raman Spectroscopy
4. Super-Resolution Fluorescence Microscopy
5. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABT | Aminobenzethiol |
| DHABT | Dihydroxyaminobenzenethiol |
| DMAB | p,p′-dimercaptoazobenzene |
| EF | Enhancement factor |
| HABT | Hydroxylaminobenzenethiol |
| LSPR | Localized surface plasmon resonance |
| NA | Numerical aperature |
| NPoM | Nanoparticle on mirror |
| NSBT | Nitrosobenzenethiol |
| NBT | p-nitrobenzenethiol |
| SERS | Surface-enhanced Raman spectroscopy |
| TEM | Transmission electron microscopy |
| TIRF | Total internal reflection fluorescence |
References
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal Nanostructures for Efficient Conversion of Solar to Chemical Energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced Hot Carrier Science and Technology. Nat. Nanotechnol. 2015, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical Transformations on Plasmonic Metal Nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-plasmon-driven Hot Electron Photochemistry. Chem. Rev. 2017, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic Conversion of Solar to Chemical Energy on Plasmonic Metal Nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Zheng, H.; Xu, H. Plasmon-driven Catalysis on Molecules and Nanomaterials. Acc. Chem. Res. 2019, 52, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Thomann, I.; Pinaud, B.A.; Chen, Z.; Clemens, B.M.; Jaramillo, T.F.; Brongersma, M.L. Plasmon Enhanced Solar-to-fuel Energy Conversion. Nano Lett. 2011, 11, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Thimsen, E.; Le Formal, F.; Gratzel, M.; Warren, S.C. Influence of Plasmonic Au Nanoparticles on the Photoactivity of Fe2o3 Electrodes for Water Splitting. Nano Lett. 2011, 11, 35–43. [Google Scholar] [CrossRef]
- Mubeen, S.; Lee, J.; Singh, N.; Krämer, S.; Stucky, G.D.; Moskovits, M. An Autonomous Photosynthetic Device in Which All Charge Carriers Derive from Surface Plasmons. Nat. Nanotechnol. 2013, 8, 247–251. [Google Scholar] [CrossRef]
- Knight, M.W.; Sobhani, H.; Nordlander, P.; Halas, N.J. Photodetection with Active Optical Antennas. Science 2011, 332, 702–704. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, R.; Liao, L.; Zhou, H.; Bai, J.; Liu, G.; Liu, L.; Huang, Y.; Duan, X. Plasmon Resonance Enhanced Multicolour Photodetection by Graphene. Nat. Commun. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.A.; Luo, L.B. Low-Dimensional Plasmonic Photodetectors: Recent Progress and Future Opportunities. Adv. Opt. Mater. 2018, 6, 1701282. [Google Scholar] [CrossRef]
- Marimuthu, A.; Zhang, J.; Linic, S. Tuning Selectivity in Propylene Epoxidation by Plasmon Mediated Photo-switching of Cu Oxidation State. Science 2013, 339, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zhang, D.; Su, N.Q.; Yang, W.; Everitt, H.O.; Liu, J. Product Selectivity in Plasmonic Photocatalysis for Carbon Dioxide Hydrogenation. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Yu, S.; Wilson, A.J.; Kumari, G.; Zhang, X.; Jain, P.K. Opportunities and Challenges of Solar-energy-driven Carbon Dioxide to Fuel Conversion with Plasmonic Catalysts. ACS Energy Lett. 2017, 2, 2058–2070. [Google Scholar] [CrossRef]
- DuChene, J.S.; Tagliabue, G.; Welch, A.J.; Li, X.; Cheng, W.H.; Atwater, H.A. Optical Excitation of a Nanoparticle Cu/p-NiO Photocathode Improves Reaction Selectivity for CO2 Reduction in Aqueous Electrolytes. Nano Lett. 2020, 20, 2348–2358. [Google Scholar] [CrossRef]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot Electrons Do the Impossible: Plasmon-induced Dissociation of H2 on Au. Nano Lett. 2013, 13, 240–247. [Google Scholar] [CrossRef]
- Zhou, L.; Swearer, D.F.; Zhang, C.; Robatjazi, H.; Zhao, H.; Henderson, L.; Dong, L.; Christopher, P.; Carter, E.A.; Nordlander, P.; et al. Quantifying Hot Carrier and Thermal Contributions in Plasmonic Photocatalysis. Science 2018, 362, 69–72. [Google Scholar] [CrossRef]
- Wu, K.; Chen, J.; McBride, J.R.; Lian, T. Efficient Hot-electron Transfer by a Plasmon-induced Interfacial Charge-transfer Transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef]
- Boerigter, C.; Campana, R.; Morabito, M.; Linic, S. Evidence and Implications of Direct Charge Excitation as the Dominant Mechanism in Plasmon-mediated Photocatalysis. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Boerigter, C.; Aslam, U.; Linic, S. Mechanism of Charge Transfer from Plasmonic Nanostructures to Chemically Attached Materials. ACS Nano 2016, 10, 6108–6115. [Google Scholar] [CrossRef] [PubMed]
- Kamarudheen, R.; Castellanos, G.W.; Kamp, L.P.J.; Clercx, H.J.H.; Baldi, A. Quantifying Photothermal and Hot Charge Carrier Effects in Plasmon-driven Nanoparticle Syntheses. ACS Nano 2018, 12, 8447–8455. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sundaresan, V.; Willets, K.A. Hot Carriers Versus Thermal Effects: Resolving the Enhancement Mechanisms for Plasmon-mediated Photoelectrochemical Reactions. J. Phys. Chem. C 2018, 122, 5040–5048. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Reish, M.E.; Zhang, D.; Su, N.Q.; Gutieérrez, Y.; Moreno, F.; Yang, W.; Everitt, H.O.; Liu, J. Plasmon-enhanced Catalysis: Distinguishing Thermal and Nonthermal Effects. Nano Lett. 2018, 18, 1714–1723. [Google Scholar] [CrossRef]
- Aizpurua, J.; Ashfold, M.; Baletto, F.; Baumberg, J.; Christopher, P.; Cortés, E.; de Nijs, B.; Fernandez, Y.D.; Gargiulo, J.; Gawinkowski, S.; et al. Dynamics of Hot Electron Generation in Metallic Nanostructures: General Discussion. Faraday Discuss. 2019, 214, 123–146. [Google Scholar] [CrossRef]
- Jain, P.K. Taking the Heat Off of Plasmonic Chemistry. J. Phys. Chem. C 2019, 123, 24347–24351. [Google Scholar] [CrossRef]
- Sivan, Y.; Baraban, J.; Un, I.W.; Dubi, Y. Comment on “Quantifying Hot Carrier and Thermal Contributions in Plasmonic Photocatalysis”. Science 2019, 364, eaaw9367. [Google Scholar] [CrossRef]
- Kamarudheen, R.; Aalbers, G.J.W.; Hamans, R.F.; Kamp, L.P.J.; Baldi, A. Distinguishing Among All Possible Activation Mechanisms of a Plasmon-Driven Chemical Reaction. ACS Energy Lett. 2020, 5, 2605–2613. [Google Scholar] [CrossRef]
- Xu, W.; Kong, J.S.; Yeh, Y.T.E.; Chen, P. Single-molecule Nanocatalysis Reveals Heterogeneous Reaction Pathways and Catalytic Dynamics. Nat. Mater. 2008, 7, 992–996. [Google Scholar] [CrossRef]
- Chen, T.; Chen, S.; Song, P.; Zhang, Y.; Su, H.; Xu, W.; Zeng, J. Single-molecule Nanocatalysis Reveals Facet-dependent Catalytic Kinetics and Dynamics of Pallidium Nanoparticles. ACS Catal. 2017, 7, 2967–2972. [Google Scholar] [CrossRef]
- Zhou, X.; Andoy, N.M.; Liu, G.; Choudhary, E.; Han, K.S.; Shen, H.; Chen, P. Quantitative Super-resolution Imaging Uncovers Reactivity Patterns on Single Nanocatalysts. Nat. Nanotechnol. 2012, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Andoy, N.M.; Zhou, X.; Choudhary, E.; Shen, H.; Liu, G.; Chen, P. Single-molecule Catalysis Mapping Quantifies Site-specific Activity and Uncovers Radial Activity Gradient on Single 2D Nanocrystals. J. Am. Chem. Soc. 2013, 135, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.L.; Wagner, J.B.; Helveg, S.; Rostrup-Nielsen, J.R.; Clausen, B.S.; Topsøe, H. Atom-resolved Imaging of Dynamic Shape Changes in Supported Copper Nanocrystals. Science 2002, 295, 2053–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lucas, J.M.; Song, P.; Beberwyck, B.; Fu, Q.; Xu, W.; Alivisatos, A.P. Superresolution Fluorescence Mapping of Single-nanoparticle Catalysts Reveals Spatiotemporal Variations in Surface Reactivity. Proc. Natl. Acad. Sci. USA 2015, 112, 8959–8964. [Google Scholar] [CrossRef]
- Zugic, B.; Wang, L.; Heine, C.; Zakharov, D.N.; Lechner, B.A.; Stach, E.A.; Biener, J.; Salmeron, M.; Madix, R.J.; Friend, C.M. Dynamic Restructuring Drives Catalytic Activity on Nanoporous Gold–silver Alloy Catalysts. Nat. Mater. 2017, 16, 558–564. [Google Scholar] [CrossRef]
- Zhang, X.; Kumari, G.; Heo, J.; Jain, P.K. In Situ Formation of Catalytically Active Graphene in Ethylene Photo-epoxidation. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Buurmans, I.L.C.; Weckhuysen, B.M. Heterogeneities of Individual Catalyst Particles in Space and Time as Monitored by Spectroscopy. Nat. Chem. 2012, 4, 873. [Google Scholar] [CrossRef]
- Novo, C.; Funston, A.M.; Mulvaney, P. Direct Observation of Chemical Reactions on Single Gold Nanocrystals Using Surface Plasmon Spectroscopy. Nat. Nanotechnol. 2008, 3, 598–602. [Google Scholar] [CrossRef]
- Urakawa, A.; Baiker, A. Space-resolved Profiling Relevant in Heterogeneous Catalysis. Top. Catal. 2009, 52, 1312–1322. [Google Scholar] [CrossRef]
- Albinsson, D.; Bartling, S.; Nilsson, S.; Ström, H.; Fritzsche, J.; Langhammer, C. Operando Detection of Single Nanoparticle Activity Dynamics Inside a Model Pore Catalyst Material. Sci. Adv. 2020, 6, eaba7678. [Google Scholar] [CrossRef]
- Beale, A.M.; Jacques, S.D.; Weckhuysen, B.M. Chemical Imaging of Catalytic Solids with Synchrotron Radiation. Chem. Soc. Rev. 2010, 39, 4656–4672. [Google Scholar] [CrossRef] [PubMed]
- Amenabar, I.; Poly, S.; Nuansing, W.; Hubrich, E.H.; Govyadinov, A.A.; Huth, F.; Krutokhvostov, R.; Zhang, L.; Knez, M.; Heberle, J.; et al. Structural Analysis and Mapping of Individual Protein Complexes by Infrared Nanospectroscopy. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berweger, S.; Nguyen, D.M.; Muller, E.A.; Bechtel, H.A.; Perkins, T.T.; Raschke, M.B. Nano-chemical Infrared Imaging of Membrane Proteins in Lipid Bilayers. J. Am. Chem. Soc. 2013, 135, 18292–18295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Dong, Z.; Jiang, S.; Zhang, C.; Chen, L.; Zhang, L.; Liao, Y.; Aizpurua, J.; Luo, Y.E.; et al. Chemical Mapping of a Single Molecule by Plasmon-enhanced Raman Scattering. Nature 2013, 498, 82–86. [Google Scholar] [CrossRef]
- Hartman, T.; Wondergem, C.S.; Kumar, N.; van den Berg, A.; Weckhuysen, B.M. Surface- and Tip-enhanced Raman Spectroscopy in Catalysis. J. Phys. Chem. Lett. 2016, 7, 1570–1584. [Google Scholar] [CrossRef]
- Sambur, J.B.; Chen, P. Approaches to Single-nanoparticle Catalysis. Annu. Rev. Phys. Chem. 2014, 65, 395–422. [Google Scholar] [CrossRef]
- Yu, Y.; Wijesekara, K.D.; Xi, X.; Willets, K.A. Quantifying Wavelength-dependent Plasmonic Hot Carrier Energy Distributions at Metal/Semiconductor Interfaces. ACS Nano 2019, 13, 3629–3637. [Google Scholar] [CrossRef]
- De Cremer, G.; Sels, B.F.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Fluorescence Micro (Spectro) Scopy as a Tool to Study Catalytic Materials in Action. Chem. Soc. Rev. 2010, 39, 4703–4717. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, X.; Andoy, N.M.; Han, K.S.; Choudhary, E.; Zou, N.; Chen, G.; Shen, H. Spatiotemporal Catalytic Dynamics Within Single Nanocatalysts Revealed by Single-molecule Microscopy. Chem. Soc. Rev. 2014, 43, 1107–1117. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Yang, X.; Wang, P.; Qu, Y.; Liang, W.; Sun, M. Plasmon-driven Catalysis in Aqueous Solutions Probed by SERS Spectroscopy. J. Raman Spectrosc. 2016, 47, 877–883. [Google Scholar] [CrossRef]
- Chen, T.; Dong, B.; Chen, K.; Zhao, F.; Cheng, X.; Ma, C.; Lee, S.; Zhang, P.; Kang, S.H.; Ha, J.W.; et al. Optical Super-resolution Imaging of Surface Reactions. Chem. Rev. 2017, 117, 7510–7537. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Devasia, D.; Jain, P.K. Nanoscale Optical Imaging in Chemistry. Chem. Soc. Rev. 2020, 49, 6087–6112. [Google Scholar] [CrossRef] [PubMed]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Yang, H.U.; D’Archangel, J.; Sundheimer, M.L.; Tucker, E.; Boreman, G.D.; Raschke, M.B. Optical Dielectric Function of Silver. Phys. Rev. B 2015, 91, 235137. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Der Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Sousa-Castillo, A.; Comesaña Hermo, M.; Rodriguez-Gonzalez, B.; Pérez-Lorenzo, M.; Wang, Z.; Kong, X.T.; Govorov, A.O.; Correa-Duarte, M.A. Boosting Hot Electron-driven Photocatalysis Through Anisotropic Plasmonic Nanoparticles with Hot Spots in Au–TiO2 Nanoarchitectures. J. Phys. Chem. C 2016, 120, 11690–11699. [Google Scholar] [CrossRef]
- Atta, S.; Pennington, A.M.; Celik, F.E.; Fabris, L. TiO2 on Gold Nanostars Enhances Photocatalytic Water Reduction in the Near-infrared Regime. Chem 2018, 4, 2140–2153. [Google Scholar] [CrossRef]
- Yuan, L.; Lou, M.; Clark, B.D.; Lou, M.; Zhou, L.; Tian, S.; Jacobson, C.R.; Nordlander, P.; Halas, N.J. Morphology-Dependent Reactivity of a Plasmonic Photocatalyst. ACS Nano 2020, 14, 12054–12063. [Google Scholar] [CrossRef]
- Wertz, E.; Isaacoff, B.P.; Flynn, J.D.; Biteen, J.S. Single-molecule Super-resolution Microscopy Reveals How Light Couples to a Plasmonic Nanoantenna on the Nanometer Scale. Nano Lett. 2015, 15, 2662–2670. [Google Scholar] [CrossRef]
- Wertz, E.A.; Isaacoff, B.P.; Biteen, J.S. Wavelength-dependent Super-resolution Images of Dye Molecules Coupled to Plasmonic Nanotriangles. ACS Photonics 2016, 3, 1733–1740. [Google Scholar] [CrossRef]
- Langille, M.R.; Personick, M.L.; Zhang, J.; Mirkin, C.A. Defining Rules for the Shape Evolution of Gold Nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.E.; Burrows, N.D.; Scarabelli, L.; Liz-Marzán, L.M.; Murphy, C.J. Anisotropic Noble Metal Nanocrystal Growth: The Role of Halides. Chem. Mater. 2014, 26, 34–43. [Google Scholar] [CrossRef]
- Bernardi, M.; Mustafa, J.; Neaton, J.B.; Louie, S.G. Theory and Computation of Hot Carriers Generated by Surface Plasmon Polaritons in Noble Metals. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Brown, A.M.; Sundararaman, R.; Narang, P.; Goddard, W.A., III; Atwater, H.A. Nonradiative Plasmon Decay and Hot Carrier Dynamics: Effects of Phonons, Surfaces, and Geometry. ACS Nano 2016, 10, 957–966. [Google Scholar] [CrossRef]
- Khurgin, J.B. Hot Carriers Generated by Plasmons: Where Are They Generated and Where Do They Go from There? Faraday Discuss. 2019, 214, 35–58. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R. Thermo-plasmonics: Using Metallic Nanostructures as Nano-sources of Heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Marimuthu, A.; Linic, S. Singular Characteristics and Unique Chemical Bond Activation Mechanisms of Photocatalytic Reactions on Plasmonic Nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef]
- Li, K.; Hogan, N.J.; Kale, M.J.; Halas, N.J.; Nordlander, P.; Christopher, P. Balancing Near-field Enhancement, Absorption, and Scattering for Effective Antenna–reactor Plasmonic Photocatalysis. Nano Lett. 2017, 17, 3710–3717. [Google Scholar] [CrossRef]
- Seemala, B.; Therrien, A.J.; Lou, M.; Li, K.; Finzel, J.P.; Qi, J.; Nordlander, P.; Christopher, P. Plasmon-mediated Catalytic O2 Dissociation on Ag Nanostructures: Hot Electrons or Near Fields? ACS Energy Lett. 2019, 4, 1803–1809. [Google Scholar] [CrossRef]
- Zhou, L.; Martirez, J.M.P.; Finzel, J.; Zhang, C.; Swearer, D.F.; Tian, S.; Robatjazi, H.; Lou, M.; Dong, L.; Henderson, L.; et al. Light-driven Methane Dry Reforming with Single Atomic Site Antenna-reactor Plasmonic Photocatalysts. Nat. Energy 2020, 5, 61–70. [Google Scholar] [CrossRef]
- Ferry, V.E.; Munday, J.N.; Atwater, H.A. Design Considerations for Plasmonic Photovoltaics. Adv. Mater. 2010, 22, 4794–4808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, Z.; Gu, Y.; Chang, Y.H.; Zhang, L.; Li, L.J.; Zhao, W.; Eda, G.; Zhang, W.; Grinblat, G.; et al. Giant Photoluminescence Enhancement in Tungsten-diselenide–gold Plasmonic Hybrid Structures. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Moskovits, M.; Kneipp, H. Surface-Enhanced Raman Scattering: Physics and Applications; Springer Science & Business Media: Berlin, Germany, 2006; Volume 103. [Google Scholar]
- Zrimsek, A.B.; Chiang, N.; Mattei, M.; Zaleski, S.; McAnally, M.O.; Chapman, C.T.; Henry, A.I.; Schatz, G.C.; Van Duyne, R.P. Single-molecule Chemistry with Surface-and Tip-enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 7583–7613. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic Theories of Surface-enhanced Raman Spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Kang, L.; Xu, P.; Zhang, B.; Tsai, H.; Han, X.; Wang, H.L. Laser Wavelength-and Power-dependent Plasmon-driven Chemical Reactions Monitored Using Single Particle Surface Enhanced Raman Spectroscopy. Chem. Commun. 2013, 49, 3389–3391. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, S.; Radjenovic, P.M.; Tian, Z.Q.; Li, J.F. Core–Shell Nanostructure-Enhanced Raman Spectroscopy for Surface Catalysis. Acc. Chem. Res. 2020, 53, 729–739. [Google Scholar] [CrossRef]
- Cardinal, M.F.; Vander Ende, E.; Hackler, R.A.; McAnally, M.O.; Stair, P.C.; Schatz, G.C.; Van Duyne, R.P. Expanding applications of SERS through versatile nanomaterials engineering. Chem. Soc. Rev. 2017, 46, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Park, W.H.; Park, C.G.; Shin, H.H.; Lee, K.S.; Kim, Z.H. Metal-catalyzed Chemical Reaction of Single Molecules Directly Probed by Vibrational Spectroscopy. J. Am. Chem. Soc. 2016, 138, 4673–4684. [Google Scholar] [CrossRef]
- de Nijs, B.; Benz, F.; Barrow, S.J.; Sigle, D.O.; Chikkaraddy, R.; Palma, A.; Carnegie, C.; Kamp, M.; Sundararaman, R.; Narang, P.; et al. Plasmonic Tunnel Junctions for Single-molecule Redox Chemistry. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Schmidt, M.K.; Dreismann, A.; Chikkaraddy, R.; Zhang, Y.; Demetriadou, A.; Carnegie, C.; Ohadi, H.; De Nijs, B.; Esteban, R.; et al. Single-molecule Optomechanics in “Picocavities”. Science 2016, 354, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Etchegoin, P.G. Single-molecule Surface-enhanced Raman Spectroscopy. Annu. Rev. Phys. Chem. 2012, 63, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Lee, K.S.; Shin, H.H.; Koo, J.J.; Yeon, G.J.; Kim, Z.H. Single-Molecule Surface-Enhanced Raman Scattering as a Probe of Single-Molecule Surface Reactions: Promises and Current Challenges. Acc. Chem. Res. 2019, 52, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, S.M.; Willets, K.A. Super-resolution Optical Imaging of Single-molecule SERS Hot Spots. Nano Lett. 2010, 10, 3777–3784. [Google Scholar] [CrossRef] [PubMed]
- Chernyshova, I.V.; Somasundaran, P.; Ponnurangam, S. On the Origin of the Elusive First Intermediate of CO2 Electroreduction. Proc. Natl. Acad. Sci. USA 2018, 115, E9261–E9270. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, Y.H.; Morabito, J.V.; Liu, Y.; Koh, C.S.L.; Phang, I.Y.; Pedireddy, S.; Han, X.; Chou, L.Y.; Tsung, C.K.; et al. Driving CO2 to a Quasi-Condensed Phase at the Interface between a Nanoparticle Surface and a Metal–Organic Framework at 1 bar and 298 K. J. Am. Chem. Soc. 2017, 139, 11513–11518. [Google Scholar] [CrossRef]
- Kim, H.; Kosuda, K.M.; Van Duyne, R.P.; Stair, P.C. Resonance Raman and Surface-and Tip-enhanced Raman Spectroscopy Methods to Study Solid Catalysts and Heterogeneous Catalytic Reactions. Chem. Soc. Rev. 2010, 39, 4820–4844. [Google Scholar] [CrossRef]
- Kumari, G.; Zhang, X.; Devasia, D.; Heo, J.; Jain, P.K. Watching Visible Light-driven CO2 Reduction on a Plasmonic Nanoparticle Catalyst. ACS Nano 2018, 12, 8330–8340. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, H.; Alaverdyan, Y.; Dmitriev, A.; Langhammer, C.; Sutherland, D.S.; Zäch, M.; Kasemo, B. Hole–mask Colloidal Lithography. Adv. Mater. 2007, 19, 4297–4302. [Google Scholar] [CrossRef]
- Olson, J.; Dominguez-Medina, S.; Hoggard, A.; Wang, L.Y.; Chang, W.S.; Link, S. Optical Characterization of Single Plasmonic Nanoparticles. Chem. Soc. Rev. 2015, 44, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deckert-Gaudig, T.; Deckert, V. Label-free Monitoring of Plasmonic Catalysis on the Nanoscale. Analyst 2015, 140, 4325–4335. [Google Scholar] [CrossRef]
- Herves, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef]
- Kang, L.; Han, X.; Chu, J.; Xiong, J.; He, X.; Wang, H.L.; Xu, P. In Situ Surface-Enhanced Raman Spectroscopy Study of Plasmon-Driven Catalytic Reactions of 4-Nitrothiophenol under a Controlled Atmosphere. ChemCatChem 2015, 7, 1004–1010. [Google Scholar] [CrossRef]
- Keller, E.L.; Frontiera, R.R. Ultrafast Nanoscale Raman Thermometry Proves Heating Is Not a Primary Mechanism for Plasmon-driven Photocatalysis. ACS Nano 2018, 12, 5848–5855. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, M.; Ueltschi, T.W.; Mosquera, M.A.; Chen, Z.; Schatz, G.C.; Van Duyne, R.P. SERS study of the mechanism of plasmon-driven hot electron transfer between gold nanoparticles and PCBM. J. Phys. Chem. C 2019, 123, 29908–29915. [Google Scholar] [CrossRef]
- Pozzi, E.A.; Zrimsek, A.B.; Lethiec, C.M.; Schatz, G.C.; Hersam, M.C.; Van Duyne, R.P. Evaluating Single-molecule Stokes and Anti-stokes SERS for Nanoscale Thermometry. J. Phys. Chem. C 2015, 119, 21116–21124. [Google Scholar] [CrossRef]
- Airy, G.B. On the Diffraction of an Object-glass with Circular Aperture. Trans. Camb. Philos. Soc. 1835, 5, 283. [Google Scholar]
- Thompson, R.E.; Larson, D.R.; Webb, W.W. Precise Nanometer Localization Analysis for Individual Fluorescent Probes. Biophys. J. 2002, 82, 2775–2783. [Google Scholar] [CrossRef]
- Mortensen, K.I.; Churchman, L.S.; Spudich, J.A.; Flyvbjerg, H. Optimized Localization Analysis for Single-molecule Tracking and Super-resolution Microscopy. Nat. Methods 2010, 7, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.T.; Girirajan, T.P.K.; Mason, M.D. Ultra-high Resolution Imaging by Fluorescence Photoactivation Localization Microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit Imaging by Stochastic Optical Reconstruction Microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef]
- Johlin, E.; Solari, J.; Mann, S.A.; Wang, J.; Shimizu, T.S.; Garnett, E.C. Super-resolution Imaging of Light–matter Interactions near Single Semiconductor Nanowires. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Mack, D.L.; Cortés, E.; Giannini, V.; Török, P.; Roschuk, T.; Maier, S.A. Decoupling Absorption and Emission Processes in Super-resolution Localization of Emitters in a Plasmonic Hotspot. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef][Green Version]
- Hamans, R.F.; Parente, M.; Castellanos, G.W.; Ramezani, M.; Gómez Rivas, J.; Baldi, A. Super-resolution Mapping of Enhanced Emission by Collective Plasmonic Resonances. ACS Nano 2019, 13, 4514–4521. [Google Scholar] [CrossRef]
- Ristanović, Z.; Kubarev, A.V.; Hofkens, J.; Roeffaers, M.B.; Weckhuysen, B.M. Single Molecule Nanospectroscopy Visualizes Proton-transfer Processes Within a Zeolite Crystal. J. Am. Chem. Soc. 2016, 138, 13586–13596. [Google Scholar] [CrossRef]
- Roeffaers, M.B.J.; Sels, B.F.; Uji-i, H.; De Schryver, F.C.; Jacobs, P.A.; De Vos, D.E.; Hofkens, J. Spatially Resolved Observation of Crystal-face-dependent Catalysis by Single Turnover Counting. Nature 2006, 439, 572–575. [Google Scholar] [CrossRef]
- Martin-Fernandez, M.L.; Tynan, C.J.; Webb, S.E.D. A ‘Pocket Guide’ to Total Internal Reflection Fluorescence. J. Microsc. 2013, 252, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Ruberu, T.P.A.; Han, R.; Dong, B.; Vela, J.; Fang, N. Super-resolution Mapping of Photogenerated Electron and Hole Separation in Single Metal–semiconductor Nanocatalysts. J. Am. Chem. Soc. 2014, 136, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-dependent Catalytic Activity and Dynamics of Gold Nanoparticles at the Single-molecule Level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Liao, H.; Hafner, J.H. Monitoring Gold Nanorod Synthesis by Localized Surface Plasmon Resonance. J. Phys. Chem. B 2006, 110, 22323–22327. [Google Scholar] [CrossRef]
- Zou, N.; Zhou, X.; Chen, G.; Andoy, N.M.; Jung, W.; Liu, G.; Chen, P. Cooperative Communication within and between Single Nanocatalysts. Nat. Chem. 2018, 10, 607–614. [Google Scholar] [CrossRef]
- Zou, N.; Chen, G.; Mao, X.; Shen, H.; Choudhary, E.; Zhou, X.; Chen, P. Imaging Catalytic Hotspots on Single Plasmonic Nanostructures via Correlated Super-resolution and Electron Microscopy. ACS Nano 2018, 12, 5570–5579. [Google Scholar] [CrossRef]
- Li, W.; Miao, J.; Peng, T.; Lv, H.; Wang, J.G.; Li, K.; Zhu, Y.; Li, D. Single-molecular Catalysis Identifying Activation Energy of the Intermediate Product and Rate-limiting Step in Plasmonic Photocatalysis. Nano Lett. 2020, 20, 2507–2513. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Xu, W. Single-molecule Nanocatalysis Reveals Catalytic Activation Energy of Single Nanocatalysts. J. Am. Chem. Soc. 2016, 138, 12414–12421. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Xu, W. Revealing the Thermodynamics of Individual Catalytic Steps Based on Temperature-dependent Single-particle Nanocatalysis. Phys. Chem. Chem. Phys. 2019, 21, 21806–21813. [Google Scholar] [CrossRef]
- Mao, X.; Liu, C.; Hesari, M.; Zou, N.; Chen, P. Super-resolution Imaging of Non-fluorescent Reactions via Competition. Nat. Chem. 2019, 11, 687–694. [Google Scholar] [CrossRef]
- Chen, G.; Zou, N.; Chen, B.; Sambur, J.B.; Choudhary, E.; Chen, P. Bimetallic Effect of Single Nanocatalysts Visualized by Super-resolution Catalysis Imaging. ACS Cent. Sci. 2017, 3, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Akhlaghi, Y.; Kompany-Zareh, M.; Rinnan, Å. Nucleic Acid Based Fluorescent Nanothermometers. ACS Nano 2014, 8, 10372–10382. [Google Scholar] [CrossRef] [PubMed]
- Carattino, A.; Caldarola, M.; Orrit, M. Gold Nanoparticles as Absolute Nanothermometers. Nano Lett. 2018, 18, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Kamarudheen, R.; Kumari, G.; Baldi, A. Plasmon-driven Synthesis of Individual Metal@Semiconductor Core@Shell Nanoparticles. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baffou, G. Thermoplasmonics: Heating Metal Nanoparticles Using Light; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Barella, M.; Violi, I.L.; Gargiulo, J.; Martinez, L.P.; Goschin, F.; Guglielmotti, V.; Pallarola, D.; Schlücker, S.; Pilo-Pais, M.; Acuna, G.P.; et al. In Situ Photothermal Response of Single Gold Nanoparticles through Hyperspectral Imaging Anti-Stokes Thermometry. ACS Nano 2020. [Google Scholar] [CrossRef] [PubMed]
- Baffou, G.; Bordacchini, I.; Baldi, A.; Quidant, R. Simple Experimental Procedures to Distinguish Photothermal from Hot-carrier Processes in Plasmonics. Light. Sci. Appl. 2020, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Félidj, N.; Aubard, J.; Lévi, G.; Krenn, J.R.; Salerno, M.; Schider, G.; Lamprecht, B.; Leitner, A.; Aussenegg, F. Controlling the Optical Response of Regular Arrays of Gold Particles for Surface-enhanced Raman Scattering. Phys. Rev. B 2002, 65, 075419. [Google Scholar] [CrossRef]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured Plasmonic Sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.; Brolo, A.G. A Review on the Fabrication of Substrates for Surface Enhanced Raman Spectroscopy and Their Applications in Analytical Chemistry. Anal. Chim. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef]
- Cortés, E.; Xie, W.; Cambiasso, J.; Jermyn, A.S.; Sundararaman, R.; Narang, P.; Schlücker, S.; Maier, S.A. Plasmonic Hot Electron Transport Drives Nano-localized Chemistry. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Jermyn, A.S.; Tagliabue, G.; Atwater, H.A.; Goddard, W.A., III; Narang, P.; Sundararaman, R. Transport of Hot Carriers in Plasmonic Nanostructures. Phys. Rev. Mater. 2019, 3, 075201. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; García de Abajo, F.J. Nanoscale Control of Optical Heating in Complex Plasmonic Systems. ACS Nano 2010, 4, 709–716. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamans, R.F.; Kamarudheen, R.; Baldi, A. Single Particle Approaches to Plasmon-Driven Catalysis. Nanomaterials 2020, 10, 2377. https://doi.org/10.3390/nano10122377
Hamans RF, Kamarudheen R, Baldi A. Single Particle Approaches to Plasmon-Driven Catalysis. Nanomaterials. 2020; 10(12):2377. https://doi.org/10.3390/nano10122377
Chicago/Turabian StyleHamans, Ruben F., Rifat Kamarudheen, and Andrea Baldi. 2020. "Single Particle Approaches to Plasmon-Driven Catalysis" Nanomaterials 10, no. 12: 2377. https://doi.org/10.3390/nano10122377
APA StyleHamans, R. F., Kamarudheen, R., & Baldi, A. (2020). Single Particle Approaches to Plasmon-Driven Catalysis. Nanomaterials, 10(12), 2377. https://doi.org/10.3390/nano10122377




