Facile Fabrication of Double-Layered Electrodes for a Self-Powered Energy Conversion and Storage System
Abstract
1. Introduction
2. Experimental Section
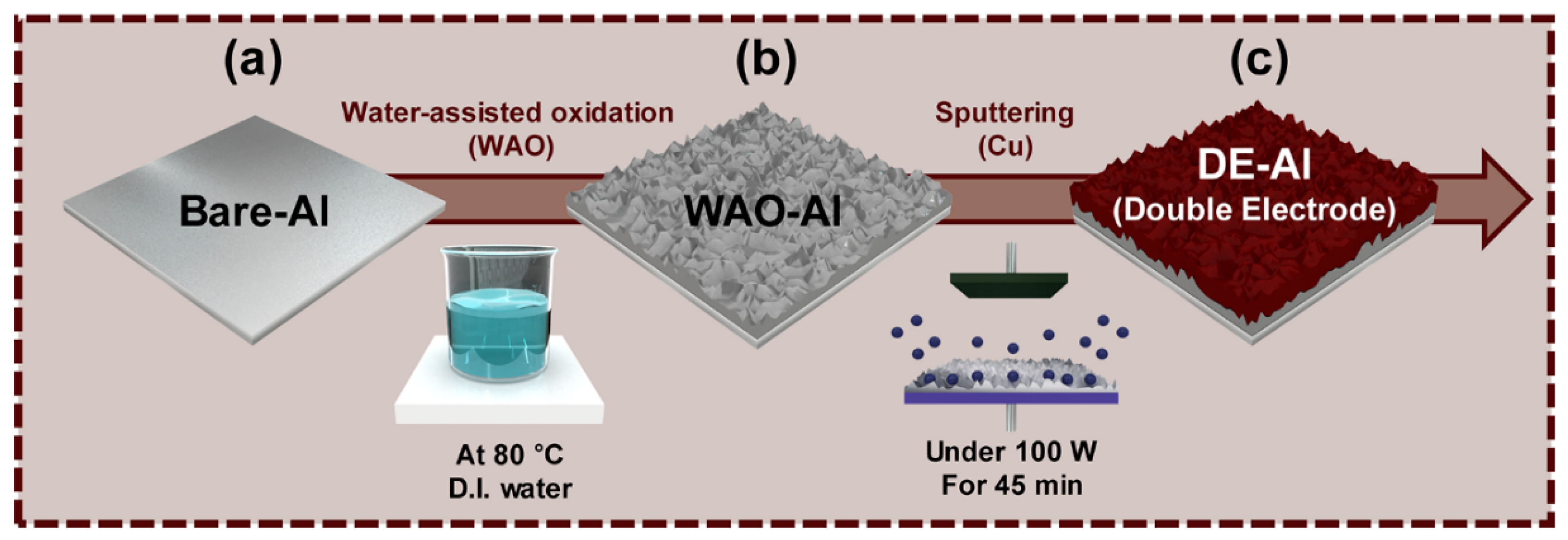
2.1. Synthesis of Double-Layered Electrodes (DEs)
2.2. Preparation of PVA/H3PO4 Electrolyte
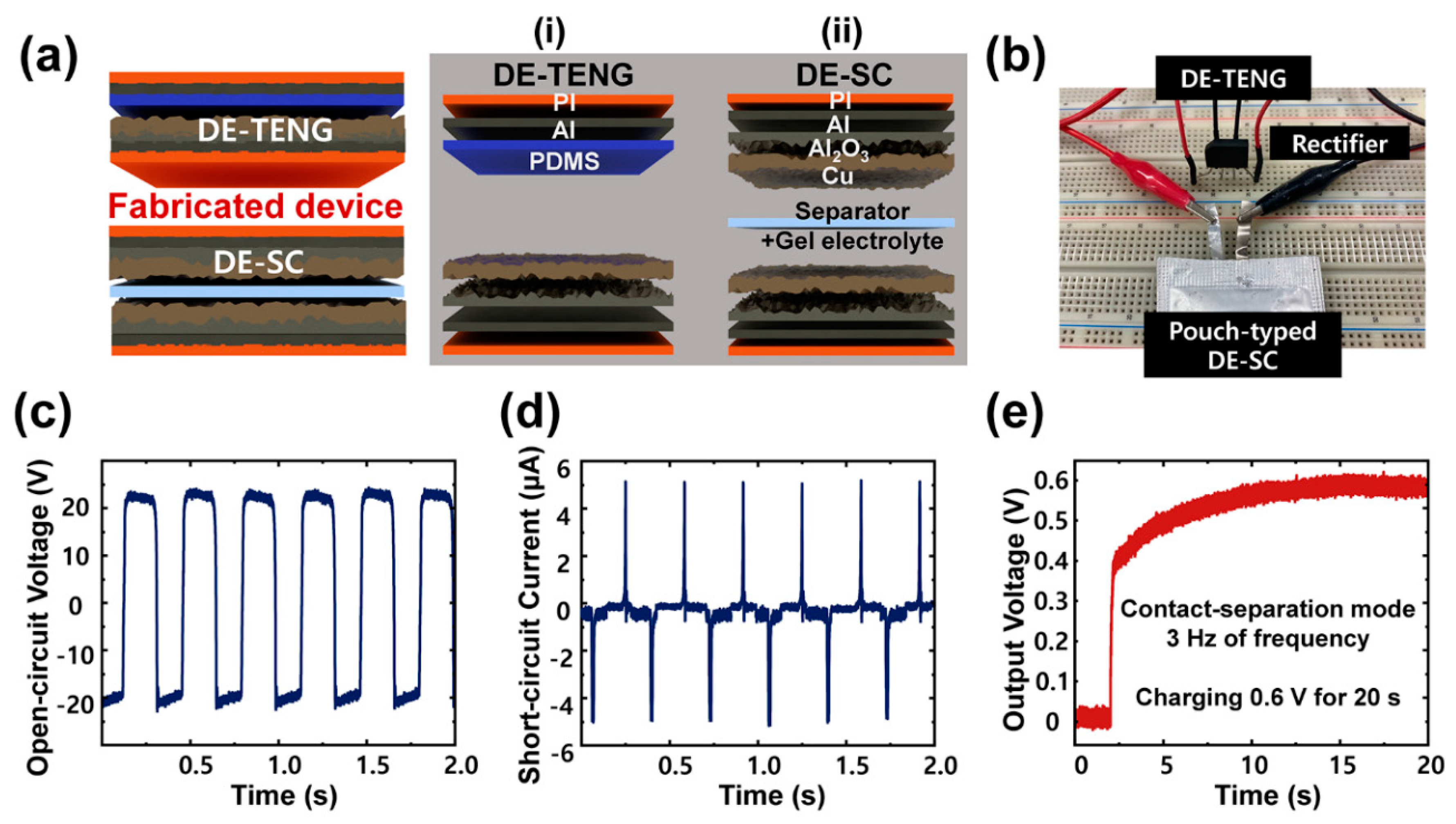
2.3. Preparation of Pouch-Type DE-SC Device
2.4. Fabrication of DE-TENG
2.5. Characterization of Materials
2.6. Electrochemical Characterization
2.7. Coupling of the Energy Conversion and Storage Systems
2.8. Electrochemical Properties
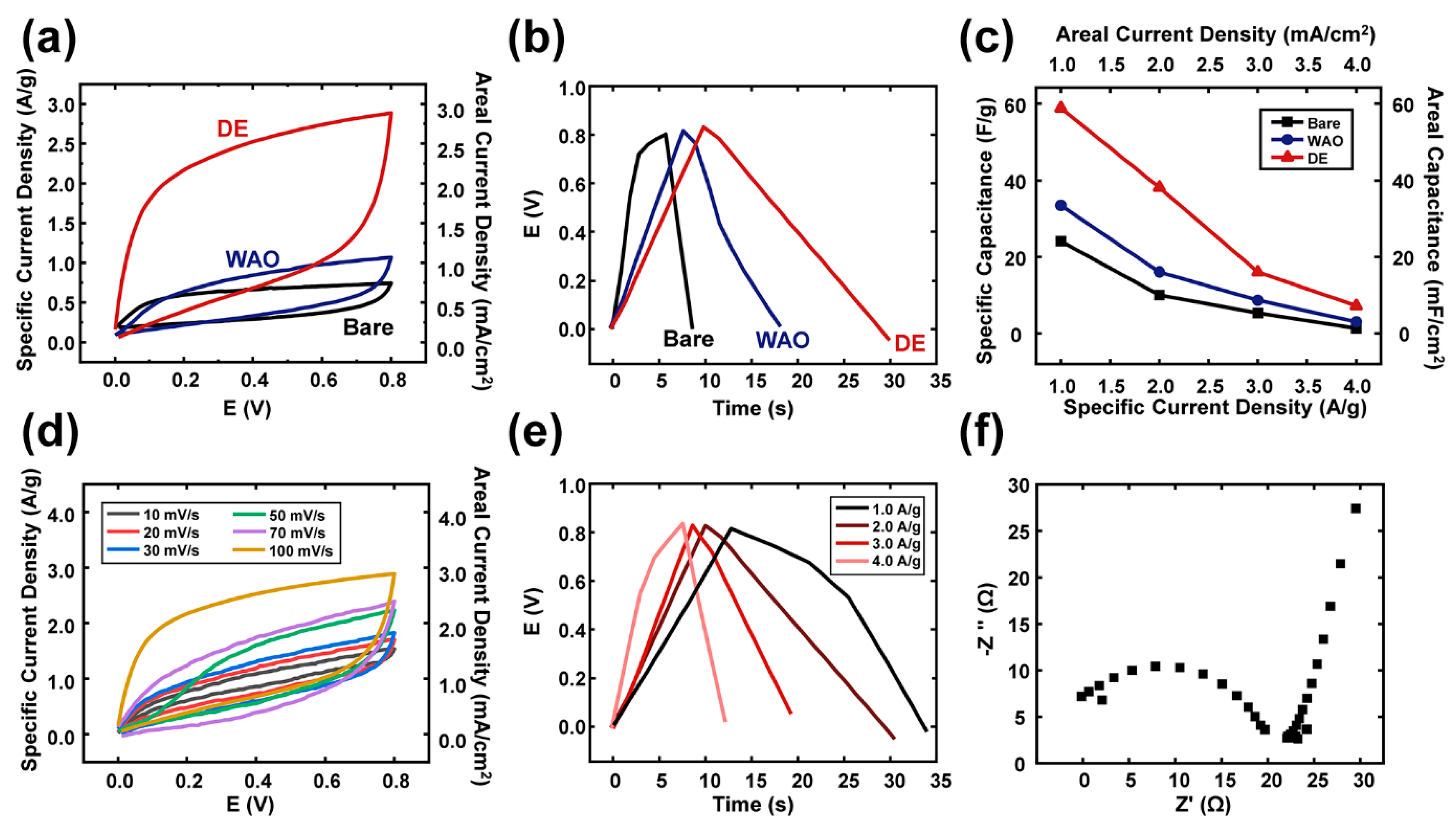
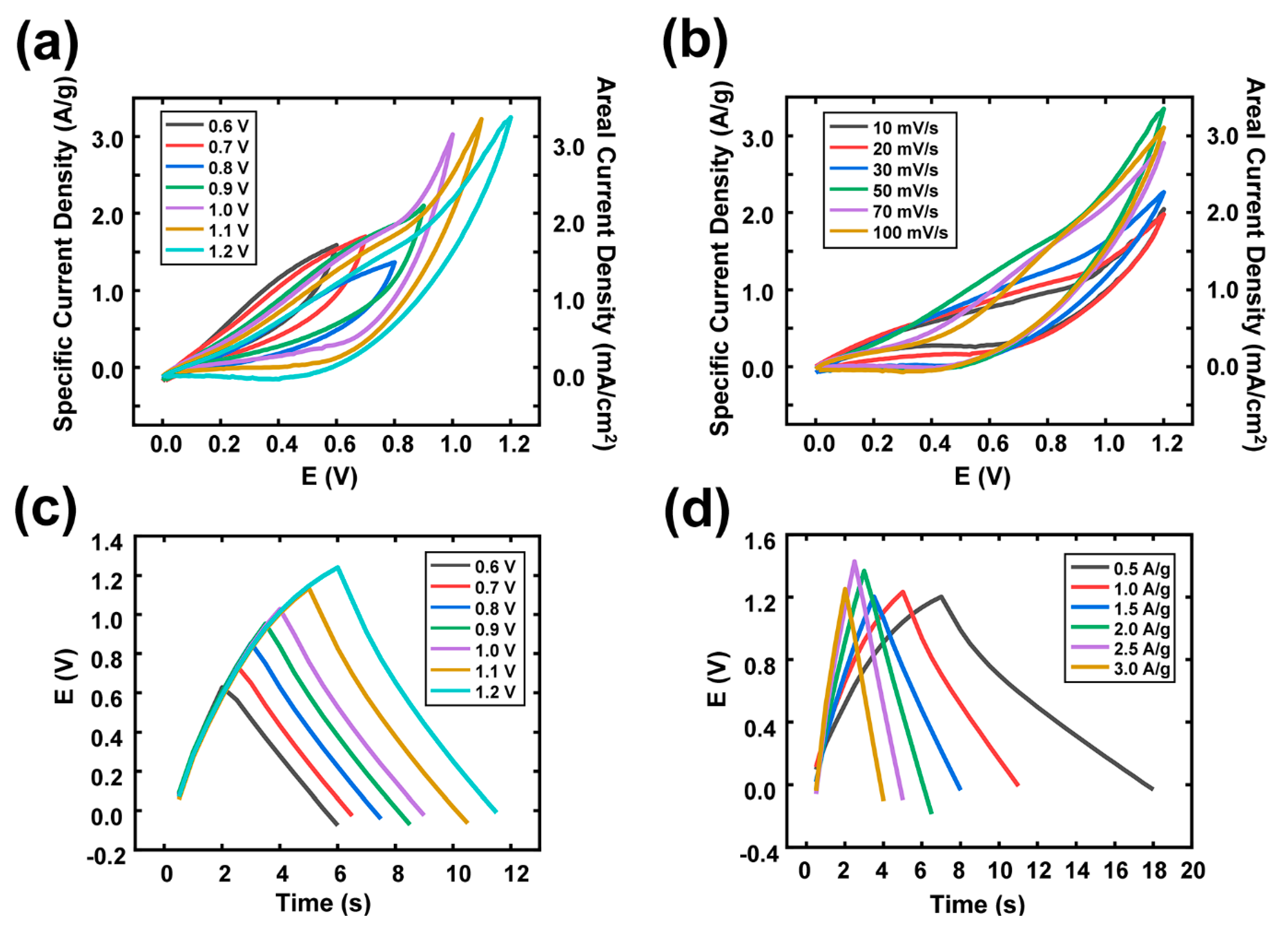
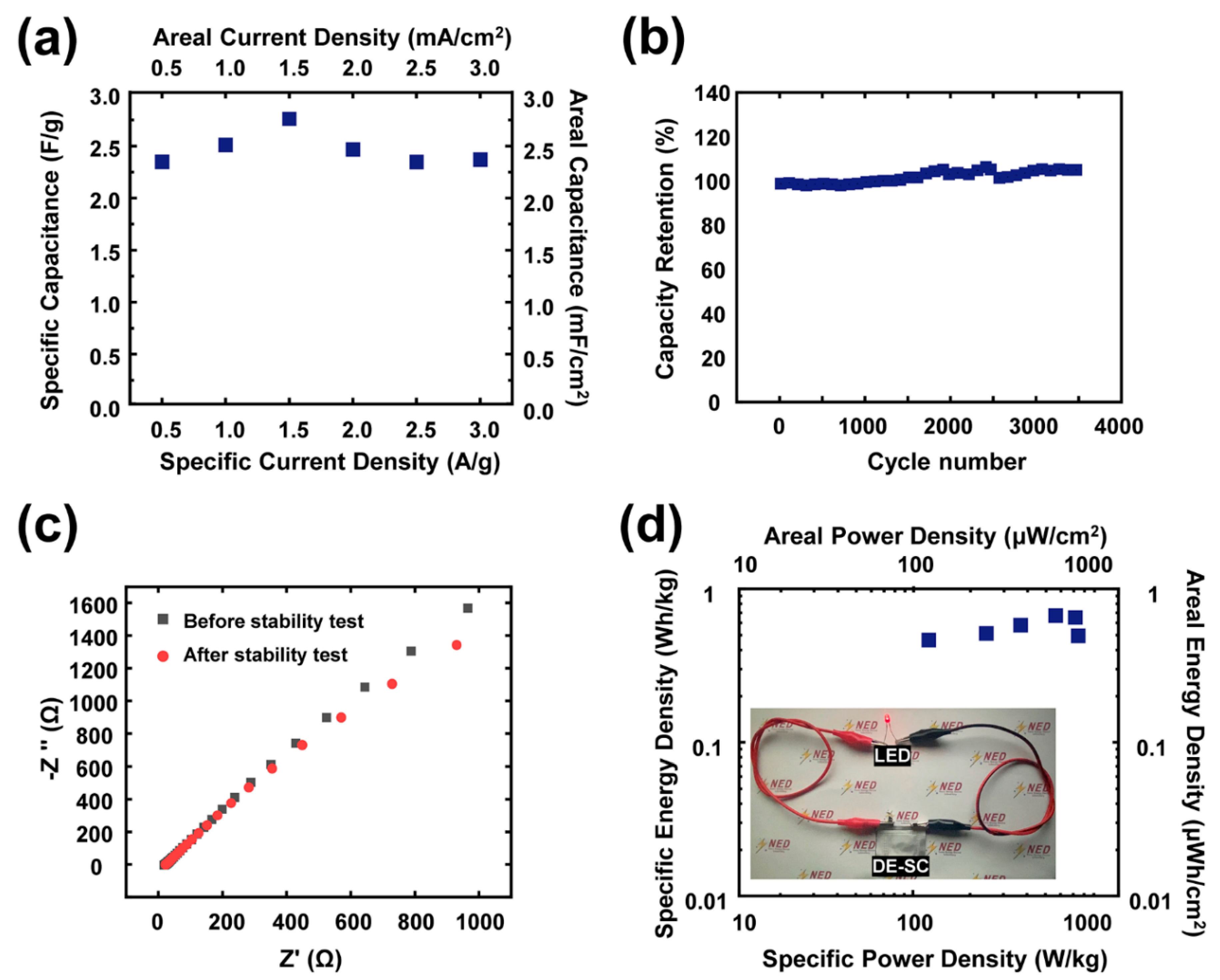
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weinstock, I.B. Recent advances in the US Department of Energy’s energy storage technology research and development programs for hybrid electric and electric vehicles. J. Power Sources 2002, 110, 471–474. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Kousksou, T.; Bruel, P.; Jamil, A.; El Rhafiki, T.; Zeraouli, Y. Energy storage: Applications and challenges. Sol. Energy Mater. Sol. Cells 2014, 120, 59–80. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Shen, P.K. Simultaneous formation of ultrahigh surface area and three-dimensional hierarchical porous graphene-like networks for fast and highly stable supercapacitors. Adv. Mater. 2013, 25, 2474–2480. [Google Scholar] [CrossRef]
- Li, Z.; He, S.; Ji, C.; Mi, H.; Lei, C.; Li, Z.; Pang, H.; Fan, Z.; Yu, C.; Qiu, J. Hierarchical Bimetallic Hydroxides Built by Porous Nanowire-Lapped Bundles with Ultrahigh Areal Capacity for Stable Hybrid Solid-State Supercapac/itors. Adv. Mater. Interfaces 2019, 6. [Google Scholar] [CrossRef]
- Simjee, F.I.; Chou, P.H. Efficient charging of supercapacitors for extended lifetime of wireless sensor nodes. IEEE Trans. Power Electron. 2008, 23, 1526–1536. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Soavi, F. New trends in electrochemical supercapacitors. J. Power Sources 2001, 100, 164–170. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Tivony, R.; Safran, S.; Pincus, P.; Silbert, G.; Klein, J. Charging dynamics of an individual nanopore. Nat. Commun. 2018, 9, 4203. [Google Scholar] [CrossRef] [PubMed]
- Schranger, H.; Barzegar, F.; Abbas, Q. Hybrid electrochemical capacitors in aqueous electrolytes: Challenges and prospects. Curr. Opin. Electrochem. 2020, 21, 167–174. [Google Scholar] [CrossRef]
- Przygocki, P.; Abbas, Q.; Gorska, B.; Béguin, F. High-energy hybrid electrochemical capacitor operating down to −40 °C with aqueous redox electrolyte based on choline salts. J. Power Sources 2019, 427, 283–292. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G. Preparation of highly conductive graphene hydrogels for fabricating supercapacitors with high rate capability. J. Phys. Chem. C 2011, 115, 17206–17212. [Google Scholar] [CrossRef]
- Liu, W.-W.; Yan, X.-B.; Lang, J.-W.; Peng, C.; Xue, Q.-J. Flexible and conductive nanocomposite electrode based on graphene sheets and cotton cloth for supercapacitor. J. Mater. Chem. 2012, 22, 17245. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.-J.; Shao-Horn, Y.; Dincǎ, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Wei, D.; Scherer, M.R.J.; Bower, C.; Andrew, P.; Ryhänen, T.; Steiner, U. A nanostructured electrochromic supercapacitor. Nano Lett. 2012, 12, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Hu, L.; Liu, N.; Wang, H.; Vosgueritchian, M.; Yang, Y.; Cui, Y.; Bao, Z. Enhancing the supercapacitor performance of graphene/mno2nanostructured electrodes by conductive wrapping. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–Metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Ervin, M.H.; Le, L.T.; Lee, W.Y. Inkjet-printed flexible graphene based supercapacitors. ECS Meet. Abstr. 2013, 13, 355–358. [Google Scholar] [CrossRef]
- Li, W.-H.; Ding, K.; Tian, H.-R.; Yao, M.-S.; Nath, B.; Deng, W.-H.; Wang, Y.; Xu, G. Conductive Metal-Organic Framework Nanowire Array Electrodes for High-Performance Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 27. [Google Scholar] [CrossRef]
- Hao, J.; Li, W.; Zhai, J.; Chen, H. Progress in high-strain perovskite piezoelectric ceramics. Mater. Sci. Eng. R Rep. 2019, 135, 1–57. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, S.; Zu, J.; Inman, D. High-Performance Piezoelectric Energy Harvesters and Their Applications. Joule 2018, 2, 642–697. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Pan, H.; Salman, W.; Yuan, Y.; Liu, Y. A portable high-efficiency electromagnetic energy harvesting system using supercapacitors for renewable energy applications in railroads. Energy Convers. Manag. 2016, 118, 287–294. [Google Scholar] [CrossRef]
- Mallineni, S.S.K.; Behlow, H.; Dong, Y.; Bhattacharya, S.; Rao, A.M.; Podila, R. Facile and robust triboelectric nanogenerators assembled using off-the-shelf materials. Nano Energy 2017, 35, 263–270. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, X.; Huo, Z.; Li, X.; Que, M.; Peng, Z.; Wang, H.; Pan, C. A Highly Stretchable Transparent Self-Powered Triboelectric Tactile Sensor with Metallized Nanofibers for Wearable Electronics. Adv. Mater. 2018, 30, e1706738. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-R.; Tian, Z.Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Z.L. Theoretical systems of triboelectric nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, Z.-N. Fundamental theories and basic principles of triboelectric effect: A review. Friction 2019, 7, 2–17. [Google Scholar] [CrossRef]
- Park, S.-J.; Seol, M.-L.; Kim, D.; Jeon, S.-B.; Choi, Y.-K. Triboelectric nanogenerator with nanostructured metal surface using water-assisted oxidation. Nano Energy 2016, 21, 258–264. [Google Scholar] [CrossRef]
- Mirzakulova, E.; Khatmullin, R.; Walpita, J.; Corrigan, T.; Vargas-Barbosa, N.M.; Vyas, S.; Oottikkal, S.; Manzer, S.F.; Hadad, C.M.; Glusac, K.D. Electrode-assisted catalytic water oxidation by a flavin derivative. Nat. Chem. 2012, 4, 794–801. [Google Scholar] [CrossRef]
- Wu, M.-S.; Chiang, P.-C.J.; Lin, J.-C. Electrochemical Investigations on Advanced Lithium-Ion Batteries by Three-Electrode Measurements. J. Electrochem. Soc. 2005, 152, A47–A52. [Google Scholar] [CrossRef]
- Hoshikawa, T.; Yamada, M.; Kikuchi, R.; Eguchi, K. Impedance analysis for dye-sensitized solar cells with a three-electrode system. J. Electroanal. Chem. 2005, 577, 339–348. [Google Scholar] [CrossRef]
- Chee, W.; Lim, H.N.; Harrison, I.; Chong, K.; Zainal, Z.; Ng, C.; Huang, N. Performance of Flexible and Binderless Polypyrrole/Graphene Oxide/Zinc Oxide Supercapacitor Electrode in a Symmetrical Two-Electrode Configuration. Electrochim. Acta 2015, 157, 88–94. [Google Scholar] [CrossRef]
- Shieh, J.-Y.; Zhang, S.-H.; Wu, C.-H.; Yu, H.H. A facile method to prepare a high performance solid-state flexible paper-based supercapacitor. Appl. Surf. Sci. 2014, 313, 704–710. [Google Scholar] [CrossRef]

| Sample | Element (wt%) | Atomic (%) | ||||
|---|---|---|---|---|---|---|
| Al | O | Cu | Al | O | Cu | |
| Bare-Al | 98.8 | 1.2 | 98.0 | 2.0 | ||
| WAO-Al | 80.6 | 19.4 | 71.1 | 28.9 | ||
| DE-Al | 31.7 | 5.8 | 62.5 | 46.6 | 14.4 | 39.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Jayababu, N.; Kim, D. Facile Fabrication of Double-Layered Electrodes for a Self-Powered Energy Conversion and Storage System. Nanomaterials 2020, 10, 2380. https://doi.org/10.3390/nano10122380
Jo S, Jayababu N, Kim D. Facile Fabrication of Double-Layered Electrodes for a Self-Powered Energy Conversion and Storage System. Nanomaterials. 2020; 10(12):2380. https://doi.org/10.3390/nano10122380
Chicago/Turabian StyleJo, Seungju, Nagabandi Jayababu, and Daewon Kim. 2020. "Facile Fabrication of Double-Layered Electrodes for a Self-Powered Energy Conversion and Storage System" Nanomaterials 10, no. 12: 2380. https://doi.org/10.3390/nano10122380
APA StyleJo, S., Jayababu, N., & Kim, D. (2020). Facile Fabrication of Double-Layered Electrodes for a Self-Powered Energy Conversion and Storage System. Nanomaterials, 10(12), 2380. https://doi.org/10.3390/nano10122380








