Impact of Tm3+ and Tb3+ Rare Earth Cations Substitution on the Structure and Magnetic Parameters of Co-Ni Nanospinel Ferrite
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structure
3.2. Surface Morphology
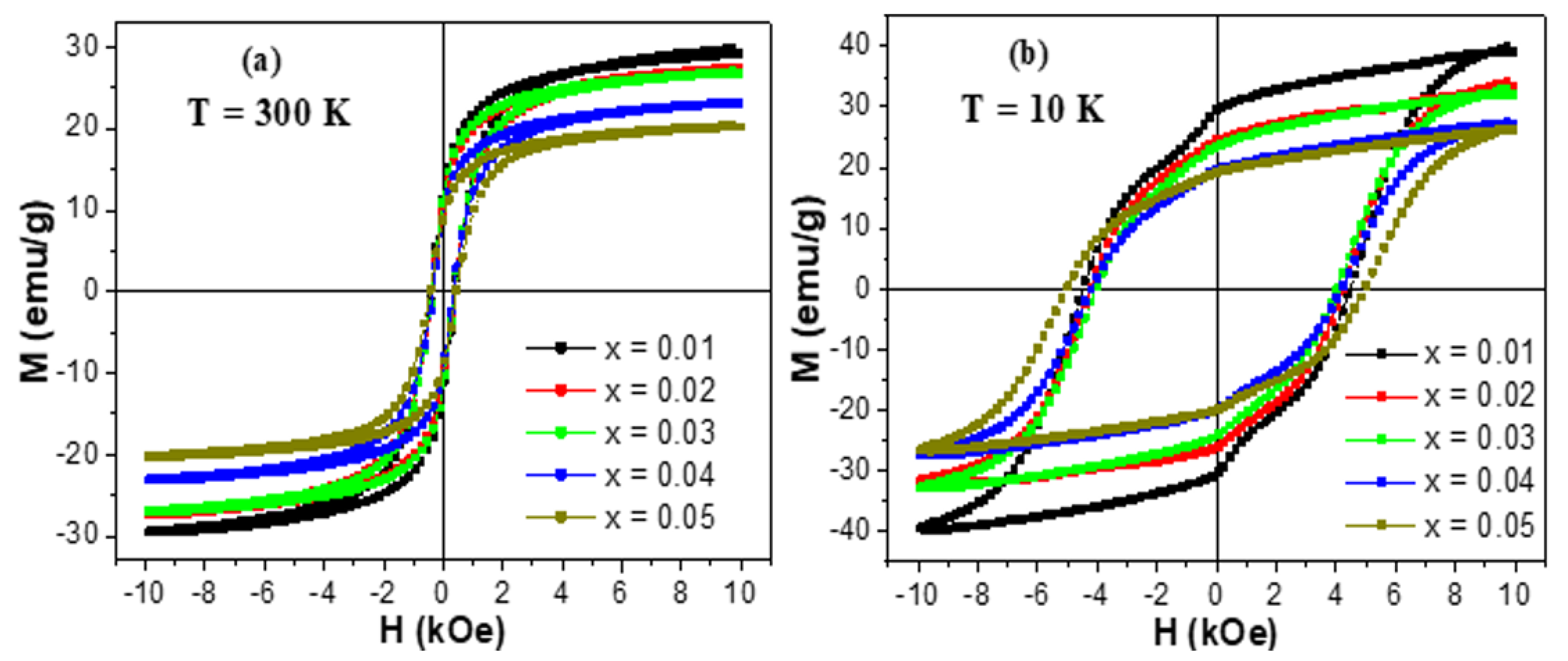
3.3. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, H.-H.; Zhou, D.; Du, C.; Wang, P.-J.; Liu, W.-F.; Pang, L.-X.; Wang, Q.-P.; Su, J.-Z.; Singh, C.; Trukhanov, S. Temperature stable Li2Ti0.75(Mg1/3Nb2/3)0.25O3-based microwave dielectric ceramics with low sintering temperature and ultra-low dielectric loss for dielectric resonator antenna applications. J. Mater. Chem. C 2020, 8, 4690–4700. [Google Scholar] [CrossRef]
- Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe3O4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.M.; Panina, L.V.; Trukhanova, E.L.; Darwish, M.A.; Morchenko, A.T.; Zubar, T.I.; Trukhanov, S.V.; Trukhanov, A.V. Structural, electric and magnetic properties of (BaFe11.9Al0.1O19)1-x-(BaTiO3)x composites. Comp. Part B Eng. 2019, 174, 107054. [Google Scholar] [CrossRef]
- Ketsko, V.A.; Beresnev, E.N.; Kop’eva, M.A.; Rjabkova, L.V.; Baranchicov, A.E.; Stognij, A.I.; Trukhanov, A.V.; Kuznetsov, N.T. Specifics of the pyrohydrolytic and solid-phase syntheses of solid solutions in the (MgGa2O4)x(MgFe2O4)1-x system. Rus. J. Inorg. Chem. 2010, 55, 427–429. [Google Scholar] [CrossRef]
- Nipan, G.D.; Ketsko, V.A.; Stognij, A.I.; Trukhanov, A.V.; Kol’tsova, T.N.; Kop’eva, M.A.; Elesina, L.V.; Kuznetsov, T.N. Properties of Mg(Fe1-XGaX)2O4+δ Solid solutions in stable and metastable state. Inorg. Mater. 2010, 46, 429–433. [Google Scholar] [CrossRef]
- Gang, C.; Zheng, H.; Zhao, H.; Ni, Y.; Pocs, C.A.; Zhang, Y.; Ye, F.; Hoffmann, C.; Wang, X.; Lee, M.; et al. Quantum liquid from strange frustration in the trimer magnet Ba4Ir3O10. NPJ Quantum Mater. 2020, 5, 26. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Silibin, M.V.; Trukhanov, S.V.; Trukhanov, A.V.; Zhaludkevich, A.L.; Latushka, S.I.; Zhaludkevich, D.V.; Khomchenko, V.A.; Alikin, D.O.; Abramov, A.S.; et al. Peculiarities of the crystal structure evolution of BiFeO3-BaTiO3 ceramics across structural phase transitions. Nanomaterials 2020, 10, 801. [Google Scholar] [CrossRef]
- Pisarev, R.V.; Moskvin, A.S.; Kalashnikova, A.M.; Rasing, T. Charge transfer transitions in multiferroic BiFeO3 and related ferrite insulators. Phys. Rev. B 2009, 79, 235128. [Google Scholar] [CrossRef] [Green Version]
- Fodouop, F.K.; Fouokeng, G.C.; Ateuafack, M.E.; Tchoffo, M.; Fai, L.C. Metamagnetoelectric effect in multiferroics A2Cu2Mo3O12 (A=Rb and Cs) quantum spin chain. Phys. B 2020, 598, 412455. [Google Scholar] [CrossRef]
- Da Silveira Lacerda, L.H.; de Lazaro, S.R. Magneto-optical coupling and Kerr effect in PbNiO3, PbCrO3, and PbMnO3 multiferroics: An excited-states approach. J. Magn. Magn. Mater. 2020, 514, 167176. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Kostishin, V.G.; Panina, L.V.; Kazakevich, I.S.; Turchenko, V.A.; Kochervinskiy, V.V. Coexistence of spontaneous polarization and magnetization in substituted M-type hexaferrites BaFe12–xAlxO19 (x ≤ 1.2) at room temperature. JETP Lett. 2016, 103, 100–105. [Google Scholar] [CrossRef]
- Turchenko, V.; Trukhanov, A.; Trukhanov, S.; Balasoiu, M.; Lupu, N. Correlation of crystalline and magnetic structures of barium ferrites with dual ferroic properties. J. Magn. Magn. Mater. 2019, 477, 9–16. [Google Scholar] [CrossRef]
- Turchenko, V.; Kostishyn, V.G.; Trukhanov, S.; Damay, F.; Porcher, F.; Balasoiu, M.; Lupu, N.; Bozzo, B.; Fina, I.; Trukhanov, A.; et al. Crystal and magnetic structures, magnetic and ferroelectric properties of strontium ferrite partially substituted with in ions. J. Alloys Compd. 2020, 821, 153412. [Google Scholar] [CrossRef]
- Yakovenko, O.S.; Matzui, L.Y.; Vovchenko, L.L.; Lozitsky, O.V.; Prokopov, O.I.; Lazarenko, O.A.; Zhuravkov, A.V.; Oliynyk, V.V.; Launets, V.L.; Trukhanov, S.V.; et al. Electrophysical properties of epoxy-based composites with graphite nanoplatelets and magnetically aligned magnetite. Mol. Cryst. Liq. Cryst. 2018, 661, 68–80. [Google Scholar] [CrossRef]
- Darwish, M.A.; Trukhanov, A.V.; Senatov, O.S.; Morchenko, A.T.; Saafan, S.A.; Astapovich, K.A.; Trukhanov, S.V.; Trukhanova, E.L.; Pilyushkin, A.A.; Sombra, A.S.B.; et al. Investigation of AC-measurements of epoxy/ferrite composites. Nanomaterials 2020, 10, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trukhanov, A.V.; Astapovich, K.A.; Turchenko, V.A.; Almessiere, M.A.; Slimani, Y.; Baykal, A.; Sombra, A.S.B.; Zhou, D.; Jotania, R.B.; Singh, C.; et al. Influence of the dysprosium ions on structure, magnetic characteristics and origin of the reflection losses in the Ni-Co spinels. J. Alloys Compd. 2020, 841, 155667. [Google Scholar] [CrossRef]
- Gao, F. An Overview of surface-functionalized magnetic nanoparticles: Preparation and application for wastewater treatment. ChemistrySelect 2019, 4, 6805–6811. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernandez, C.d.J.; Sangregorio, C. Exploring the magnetic properties of cobalt-ferrite nanoparticles for the development of a rare-earth-free permanent magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Dong, B.; Li, M.; Xiao, C.; Ding, D.; Gao, G.; Ding, S. Tunable growth of perpendicular cobalt ferrite nanosheets on reduced graphene oxide for energy storage. Nanotechnology 2016, 28, 055401. [Google Scholar] [CrossRef]
- Arbab, A.S.; Jordan, E.K.; Wilson, L.B.; Yocum, G.T.; Lewis, B.K.; Frank, J.A. In Vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum. Gene Ther. 2004, 15, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the application of magnetic nanoparticles for sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar] [CrossRef]
- Mahdi, T.S.; Kadhim, F.J. Effect depositions parameters on the characteristics of Ni0.5Co0.5Fe2O4 nanocomposite films prepared by DC reactive magnetron Co-sputtering technique. Iraqi J. Phys. 2020, 18, 76–88. [Google Scholar] [CrossRef]
- Harris, V.G. Modern microwave ferrites. IEEE Trans. Magn. 2011, 48, 1075–1104. [Google Scholar] [CrossRef]
- Tong, G.; Liu, Y.; Cui, T.; Li, Y.; Zhao, Y.; Guan, J. Tunable dielectric properties and excellent microwave absorbing properties of elliptical Fe3O4 nanorings. Appl. Phys. Lett. 2016, 108, 072905. [Google Scholar] [CrossRef]
- Datt, G.; Kotabage, C.; Abhyankar, A. Ferromagnetic resonance of NiCoFe2O4 nanoparticles and microwave absorption properties of flexible NiCoFe2O4–carbon black/poly(vinyl alcohol) composites. Phys. Chem. Chem. Phys. 2017, 19, 20699–20712. [Google Scholar] [CrossRef]
- Osaka, T.; Takai, M.; Hayashi, K.; Ohashi, K.; Saito, M.; Yamada, K. A soft magnetic CoNiFe film with high saturation magnetic flux density and low coercivity. Nature 1998, 392, 796–798. [Google Scholar] [CrossRef]
- Mozaffari, M.; Amighian, J.; Darsheshdar, E. Magnetic and structural studies of nickel-substituted cobalt ferrite nanoparticles, synthesized by the sol-gel method. J. Magn. Magn. Mater. 2014, 350, 19–22. [Google Scholar] [CrossRef]
- Almeida, T.P.; Fay, M.W.; Zhu, Y.; Brown, P.D. Hydrothermal synthesis of mixed cobalt-nickel ferrite nanoparticles. J. Phys. Conf. Ser. 2012, 371, 012074. [Google Scholar] [CrossRef]
- Muscas, G.; Yaacoub, N.; Concas, G.; Sayed, F.; Hassan, R.S.; Greneche, J.-M.; Cannas, C.; Musinu, A.; Foglietti, V.; Casciardi, S. Evolution of the magnetic structure with chemical composition in spinel iron oxide nanoparticles. Nanoscale 2015, 7, 13576–13585. [Google Scholar] [CrossRef]
- Datt, G.; Bishwas, M.S.; Raja, M.M.; Abhyankar, A. Observation of magnetic anomalies in one-step solvothermally synthesized nickel–cobalt ferrite nanoparticles. Nanoscale 2016, 8, 5200–5213. [Google Scholar] [CrossRef]
- Bahgat, M.; Paek, M.-K.; Park, C.-H.; Pak, J.-J. Thermal synthesis of nanocrystalline (CoxNi1-x)yFe1-y KOVAR alloy through gaseous reduction of mixed oxides. Mater. Trans. 2008, 49, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Srinivasamurthy, K.; Angadi, V.J.; Kubrin, S.; Matteppanavar, S.; Kumar, P.M.; Rudraswamy, B. Evidence of enhanced ferromagnetic nature and hyperfine interaction studies of Ce-Sm doped Co-Ni ferrite nanoparticles for microphone applications. Ceram. Int. 2018, 44, 18878–18885. [Google Scholar] [CrossRef]
- Asiri, S.; Sertkol, M.; Guner, S.; Gungunes, H.; Batoo, K.; Saleh, T.A.; Sozeri, H.; Almessiere, M.A.; Manikandan, A.; Baykal, A. Hydrothermal synthesis of CoyZnyMn1-2yFe2O4 nanoferrites: Magneto-optical investigation. Ceram. Int. 2018, 44, 5751–5759. [Google Scholar] [CrossRef]
- Auwal, I.; Erdemi, H.; Sözeri, H.; Güngüneş, H.; Baykal, A. Magnetic and dielectric properties of Bi3+ substituted SrFe12O19 hexaferrite. J. Magn. Magn. Mater. 2016, 412, 69–82. [Google Scholar] [CrossRef]
- Maity, D.; Ding, J.; Xue, J.-M. Synthesis of magnetite nanoparticles by thermal decomposition: Time, temperature, surfactant and solvent effects. Func. Mater. Lett. 2008, 1, 189–193. [Google Scholar] [CrossRef]
- Biehl, P.; Von der Lühe, M.; Dutz, S.; Schacher, F.H. Synthesis, characterization, and applications of magnetic nanoparticles featuring polyzwitterionic coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Nkurikiyimfura, I.; Pan, Z. Sonochemical synthesis of magnetic nanoparticles. Chem. Eng. Comm. 2015, 202, 616–621. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Nkurikiyimfura, I.; Wang, Y.; Pan, Z. Effect of chain-like magnetite nanoparticle aggregates on thermal conductivity of magnetic nanofluid in magnetic field. Exp. Therm. Fluid Sci. 2013, 44, 607–612. [Google Scholar] [CrossRef]
- Marchegiani, G.; Imperatori, P.; Mari, A.; Pilloni, L.; Chiolerio, A.; Allia, P.; Tiberto, P.; Suber, L. Sonochemical synthesis of versatile hydrophilic magnetite nanoparticles. Ultrason. Sonochem. 2012, 19, 877–882. [Google Scholar] [CrossRef]
- De Biasi, R.S.; de Souza Lopes, R.D. Magnetocrystalline anisotropy of NiCoFe2O4 nanoparticles. Ceram. Int. 2016, 42, 9315–9318. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, A.; Zhao, X.; Suo, N.; Yu, L.; Zuo, Z. Structural and magnetic properties of La3+ ion doped Ni-Cu-Co nano ferrites prepared by sol-gel auto-combustion method. J. Sol Gel Sci. Technol. 2019, 90, 599–610. [Google Scholar] [CrossRef]
- Hossain, M.; Khan, M.; Nahar, A.; Ali, M.; Matin, M.; Hoque, S.; Hakim, M.; Jamil, A. Tailoring the properties of Ni-Zn-Co ferrites by Gd3+ substitution. J. Magn. Magn. Mater. 2020, 497, 165978. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Trukhanov, A.V.; Khan, F.A.; Slimani, Y.; Tashkandi, N.; Turchenko, V.A.; Zubar, T.I.; Tishkevich, D.I.; Trukhanov, S.V.; Panina, L.V.; et al. Correlation between microstructure parameters and anti-cancer activity of the [Mn0.5Zn0.5](EuxNdxFe2-2x)O4 nanoferrites produced by modified sol-gel and ultrasonic methods. Ceram. Int. 2020, 46, 7346–7354. [Google Scholar] [CrossRef]
- Sadaqat, A.; Almessiere, M.; Slimani, Y.; Guner, S.; Sertkol, M.; Albetran, H.; Baykal, A.; Shirsath, S.E.; Ozcelik, B.; Ercan, I. Structural, optical and magnetic properties of Tb3+ substituted Co nanoferrites prepared via sonochemical approach. Ceram. Int. 2019, 45, 22538–22546. [Google Scholar] [CrossRef]
- Almessiere, M.; Slimani, Y.; Guner, S.; Sertkol, M.; Korkmaz, A.D.; Shirsath, S.E.; Baykal, A. Sonochemical synthesis and physical properties of Co0.3Ni0.5Mn0.2EuxFe2−xO4 nano-spinel ferrites. Ultrason. Sonochem. 2019, 58, 104654. [Google Scholar] [CrossRef]
- Almessiere, M.; Slimani, Y.; Kurtan, U.; Guner, S.; Sertkol, M.; Shirsath, S.E.; Akhtar, S.; Baykal, A.; Ercan, I. Structural, magnetic, optical properties and cation distribution of nanosized Co0.7Zn0.3TmxFe2−xO4 (0.0≤x≤0.04) spinel ferrites synthesized by ultrasonic irradiation. Ultrason. Sonochem. 2019, 58, 104638. [Google Scholar] [CrossRef]
- Tanbir, K.; Ghosh, M.P.; Singh, R.K.; Kar, M.; Mukherjee, S. Effect of doping different rare earth ions on microstructural, optical, and magnetic properties of nickel-cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2020, 31, 435–443. [Google Scholar] [CrossRef]
- Kadam, A.; Mande, V.K.; Kadam, S.; Kadam, R.; Shirsath, S.E.; Borade, R.B. Influence of gadolinium (Gd3+) ion substitution on structural, magnetic and electrical properties of cobalt ferrites. J. Alloys Compd. 2020, 840, 155669. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Mane, M.; Yasukawa, Y.; Liu, X.; Morisako, A. Chemical tuning of structure formation and combustion process in CoDy0.1Fe1.9O4 nanoparticles: Influence@pH. J. Nanoparticle Res. 2013, 15, 1976. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Mane, M.L.; Yasukawa, Y.; Liu, X.; Morisako, A. Self-ignited high temperature synthesis and enhanced super-exchange interactions of Ho3+–Mn2+–Fe3+–O2− ferromagnetic nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Aparicio, M.; Jitianu, A. Handbook of Sol-Gel Science and Technology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Abdallah, H.; Msomi, J.; Moyo, T.; Dolo, J.; Lančok, A. Mössbauer and magnetic studies of Mn0.1Sr0.2Co0.7Fe2O4 nanoferrite. Hyperfine Interact. 2011, 203, 99–104. [Google Scholar] [CrossRef]
- Bloch, F. Zur Theorie des Ferromagnetismus. Zeitschrift für Physik 1930, 61, 206–219. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Algarou, N.A.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Tishkevich, D.I.; Vinnik, D.A.; Vakhitov, M.G.; Klygach, D.S.; Silibin, M.V.; et al. Peculiarities of the microwave properties of hard-soft functional composites SrTb0.01Tm0.01Fe11.98O19-AFe2O4 (A = Co, Ni, Zn, Cu and Mn). RSC Adv. 2020, 10, 32638–32651. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Gungunes, H.; Manikandan, A.; Baykal, A. Investigation of the effects of Tm3+ on the structural, microstructural, optical, and magnetic properties of Sr hexaferrites. Res. Phys. 2019, 13, 102166. [Google Scholar] [CrossRef]
- Tung, L.; Kolesnichenko, V.; Caruntu, D.; Chou, N.; O’Connor, C.; Spinu, L. Magnetic properties of ultrafine cobalt ferrite particles. J. Appl. Phys. 2003, 93, 7486–7488. [Google Scholar] [CrossRef]
- Franco, A., Jr.; e Silva, F. High temperature magnetic properties of cobalt ferrite nanoparticles. Appl. Phys. Lett. 2010, 96, 172505. [Google Scholar] [CrossRef]
- Coey, J. Noncollinear spin structures. Can. J. Phys. 1987, 65, 1210–1232. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.; Nawaz, M.; Baykal, A.; Akhtar, S.; Ercan, I.; Belenli, I. Effect of bimetallic (Ca, Mg) substitution on magneto-optical properties of NiFe2O4 nanoparticles. Ceram. Int. 2019, 45, 6021–6029. [Google Scholar] [CrossRef]
- Nakagomi, F.; Da Silva, S.; Garg, V.; Oliveira, A.; Morais, P.; Franco, A., Jr.; Lima, E. The influence of cobalt population on the structural properties of CoxFe3−xO4. J. Appl. Phys. 2007, 101, 09M514. [Google Scholar] [CrossRef]
- Saffari, F.; Kameli, P.; Rahimi, M.; Ahmadvand, H.; Salamati, H. Effects of Co-substitution on the structural and magnetic properties of NiCoxFe2−xO4 ferrite nanoparticles. Ceram. Int. 2015, 41, 7352–7358. [Google Scholar] [CrossRef]
- Algarou, N.A.; Slimani, Y.; Almessiere, M.A.; Sadaqat, A.; Trukhanov, A.V.; Gondal, M.A.; Hakeem, A.S.; Trukhanov, S.V.; Vakhitov, M.G.; Klygach, D.S.; et al. Functional Sr0.5Ba0.5Sm0.02Fe11.98O4/x(Ni0.8Zn0.2Fe2O4) hard-soft ferrite nanocomposites: Structure, magnetic and microwave properties. Nanomaterials 2020, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Chermahini, M.D.; Baghbaderani, H.A.; Shahraki, M.M.; Kazazi, M. Low temperature sintering of magnetic Ni0.5Co0.5Fe2O4 ceramics prepared from mechanochemically synthesized nanopowders. Ceram. Int. 2019, 45, 5491–5495. [Google Scholar] [CrossRef]
- Kumar, Y.; Shirage, P.M. Highest coercivity and considerable saturation magnetization of CoFe2O4 nanoparticles with tunable band gap prepared by thermal decomposition approach. J. Mater. Sci. 2017, 52, 4840–4851. [Google Scholar] [CrossRef]
- Almessiere, M.; Slimani, Y.; Güner, S.; Baykal, A.; Ercan, I. Effect of dysprosium substitution on magnetic and structural properties of NiFe2O4 nanoparticles. J. Rare Earths 2019, 37, 871–878. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.; Güner, S.; Tashkandi, N.; Baykal, A.; Sarac, M.; Nawaz, M.; Ercan, I. Calcination effect on the magneto-optical properties of vanadium substituted NiFe2O4 nanoferrites. J. Mater. Sci. Mater. Electron. 2019, 30, 9143–9154. [Google Scholar] [CrossRef]
- Slimani, Y.; Unal, B.; Almessiere, M.; Korkmaz, A.D.; Shirsath, S.E.; Yasin, G.; Trukhanov, A.; Baykal, A. Investigation of structural and physical properties of Eu3+ ions substituted Ni0.4Cu0.2Zn0.4Fe2O4 spinel ferrite nanoparticles prepared via sonochemical approach. Res. Phys. 2020, 17, 103061. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.; Bertino, M. Temperature dependent coercivity and magnetization of nickel ferrite nanoparticles. J. Magn. Magn. Mater. 2010, 322, 2199–2202. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.; Güner, S.; Kurtan, U.; Shirsath, S.E.; Baykal, A.; Ercan, I. Magnetic and microstructural features of Dy3+ substituted NiFe2O4 nanoparticles derived by sol-gel approach. J. Sol Gel Sci. Technol. 2020, 95, 202–210. [Google Scholar] [CrossRef]
- Iglesias, O.; Labarta, A.; Batlle, X. Exchange bias phenomenology and models of core/shell nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 2761–2780. [Google Scholar] [CrossRef] [Green Version]
- Slimani, Y.; Almessiere, M.; Korkmaz, A.D.; Guner, S.; Güngüneş, H.; Sertkol, M.; Manikandan, A.; Yildiz, A.; Akhtar, S.; Shirsath, S.E. Ni0.4Cu0.2Zn0.4TbxFe2-xO4 nanospinel ferrites: Ultrasonic synthesis and physical properties. Ultrason. Sonochem. 2019, 59, 104757. [Google Scholar] [CrossRef] [PubMed]
- Tegus, O.; Brück, E.; Buschow, K.; De Boer, F. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature 2002, 415, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.S.; Moyo, T. Temperature dependence of coercivity and magnetization of Sr1/3Mn1/3Co1/3Fe2O4 ferrite nanoparticles. J. Supercond. Nov. Magn. 2016, 29, 361–366. [Google Scholar] [CrossRef]
- Chauhan, C.C.; Kagdi, A.R.; Jotania, R.B.; Upadhyay, A.; Sandhu, C.S.; Shirsath, S.E.; Meena, S.S. Structural, magnetic and dielectric properties of Co-Zr substituted M-type calcium hexagonal ferrite nanoparticles in the presence of α-Fe2O3 phase. Ceram. Int. 2018, 44, 17812–17823. [Google Scholar] [CrossRef]
- Almessiere, M.; Slimani, Y.; Korkmaz, A.; Taskhandi, N.; Sertkol, M.; Baykal, A.; Shirsath, S.E.; Ercan, İ.; Ozcelik, B. Sonochemical synthesis of Eu3+ substituted CoFe2O4 nanoparticles and their structural, optical and magnetic properties. Ultrason. Sonochem. 2019, 58, 104621. [Google Scholar] [CrossRef] [PubMed]






| x | a (Å) | V (Å)3 | DXRD (nm) ±0.05 | χ2 (chi2) | RBragg | Cations Distribution | |
|---|---|---|---|---|---|---|---|
| A-Site | B-Site | ||||||
| 0.00 | 8.357 (3) | 583.73 | 23.9 | 1.21 | 12.4 | Co0.1Ni0.1Fe0.8 | Co0.4Ni0.4Fe1.2 |
| 0.01 | 8.336 (4) | 579.34 | 13.32 | 1.85 | 1.74 | Co0.05Ni0.15Fe0.8 | Co0.45Ni0.35Tm0.01Tb0.01Fe1.18 |
| 0.02 | 8.320 (3) | 575.99 | 11.24 | 1.35 | 4.12 | Co0.05Ni0.15Fe0.8 | Co0.45Ni0.35Tm0.02Tb0.02Fe1.16 |
| 0.03 | 8.316 (5) | 575.21 | 13.88 | 1.05 | 0.89 | Co0.05Ni0.15Fe0.8 | Co0.45Ni0.35Tm0.03Tb0.03Fe1.14 |
| 0.04 | 8.316 (1) | 575.12 | 12.48 | 1.08 | 2.20 | Co0.05Ni0.15Fe0.8 | Co0.45Ni0.35Tm0.04Tb0.04Fe1.12 |
| 0.05 | 8.310 (6) | 573.97 | 13.93 | 1.04 | 3.48 | Co0.05Ni0.15Fe0.8 | Co0.45Ni0.35Tm0.05Tb0.05Fe1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almessiere, M.A.; Slimani, Y.; Auwal, İ.A.; Shirsath, S.E.; Manikandan, A.; Baykal, A.; Özçelik, B.; Ercan, İ.; Trukhanov, S.V.; Vinnik, D.A.; et al. Impact of Tm3+ and Tb3+ Rare Earth Cations Substitution on the Structure and Magnetic Parameters of Co-Ni Nanospinel Ferrite. Nanomaterials 2020, 10, 2384. https://doi.org/10.3390/nano10122384
Almessiere MA, Slimani Y, Auwal İA, Shirsath SE, Manikandan A, Baykal A, Özçelik B, Ercan İ, Trukhanov SV, Vinnik DA, et al. Impact of Tm3+ and Tb3+ Rare Earth Cations Substitution on the Structure and Magnetic Parameters of Co-Ni Nanospinel Ferrite. Nanomaterials. 2020; 10(12):2384. https://doi.org/10.3390/nano10122384
Chicago/Turabian StyleAlmessiere, Munirah A., Yassine Slimani, İsmail A. Auwal, Sagar E. Shirsath, Ayyar Manikandan, Abdulhadi Baykal, Bekir Özçelik, İsmail Ercan, Sergei V. Trukhanov, Denis A. Vinnik, and et al. 2020. "Impact of Tm3+ and Tb3+ Rare Earth Cations Substitution on the Structure and Magnetic Parameters of Co-Ni Nanospinel Ferrite" Nanomaterials 10, no. 12: 2384. https://doi.org/10.3390/nano10122384








