Human Teeth-Derived Bioceramics for Improved Bone Regeneration
Abstract
:1. Introduction
2. Methods
2.1. Preparation of Nano-Calcium Phosphate Bioceramic
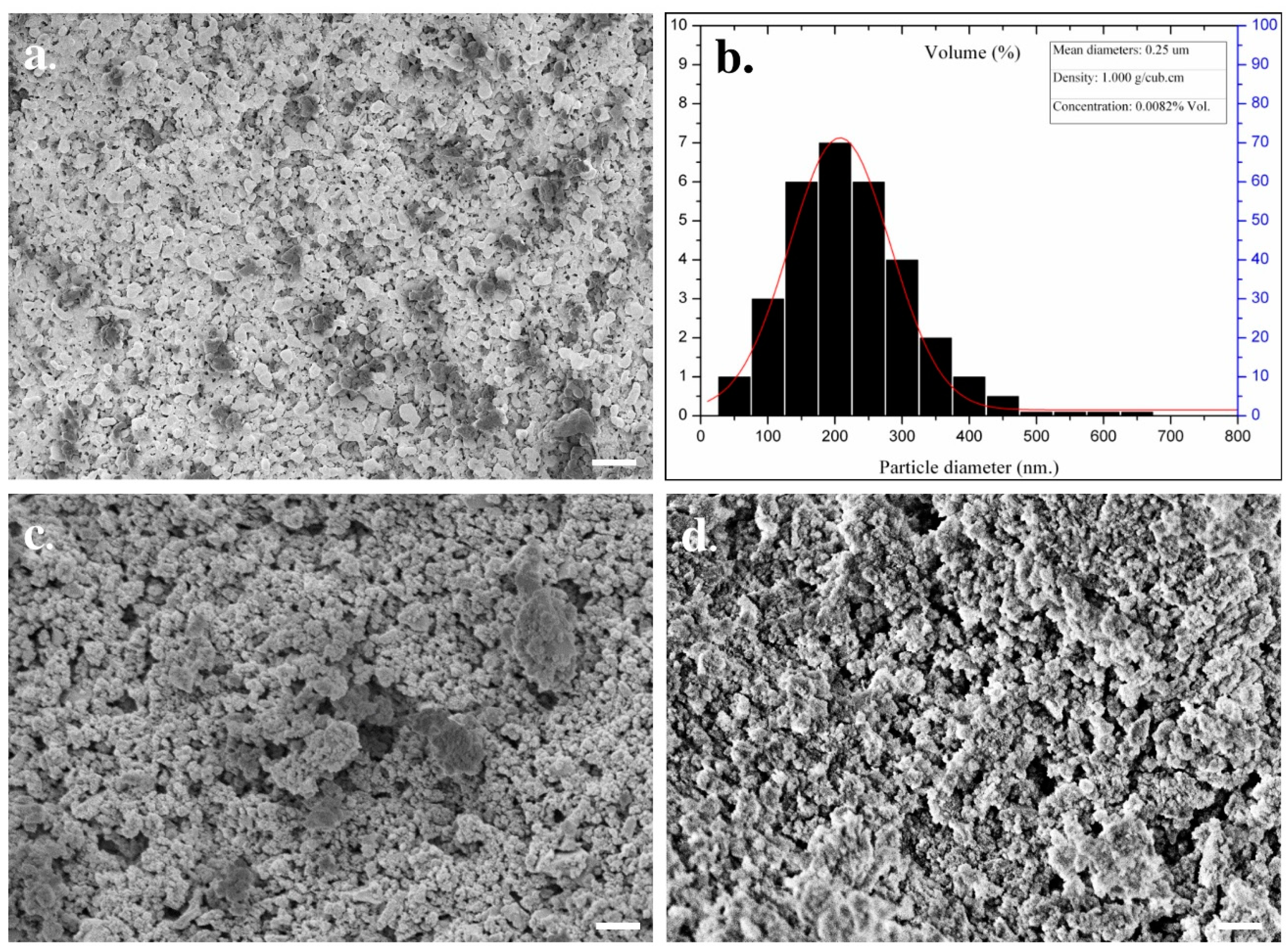
2.2. Phase and Microstructural Characterization of Nano-Calcium Phosphate Bioceramics
2.3. Cell Culture and Maintenance
2.4. Cytotoxicity Evaluation
2.5. Cell Morphology
2.6. Animal Care, In Vivo Bone Formation and Vascularization Study
2.7. Micro-Computed Tomography (µCT) Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethical Statement
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowad, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gupta, A.; Verma, M.; Murugan, P.A.; Sengupta, P.; Matheshwaran, S.; Manna, I.; Balani, K. Site-Specific Antibacterial Efficacy and cyto/Hemo-Compatibility of Zinc Substituted Hydroxyapatite. Ceram. Int. 2019, 45, 12225–12233. [Google Scholar] [CrossRef]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes: A Review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, S36138. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.N.; Tomin, E.; Lane, J.M. Clinical applications of bone graft substitutes. Orthop. Clin. N. Am. 2000, 31, 389–398. [Google Scholar] [CrossRef]
- Verbeeck, L.; Geris, L.; Tylzanowski, P.; Luyten, F.P. Uncoupling of in-Vitro Identity of Embryonic Limb Derived Skeletal Progenitors and Their in-vivo Bone Forming Potential. Sci. Rep. 2019, 9, 5782. [Google Scholar] [CrossRef]
- Cheng, L.; Cai, Z.; Zhao, J.; Wang, F.; Lu, M.; Deng, L.; Cui, W. Black Phosphorus-Based 2D Materials for Bone Therapy. Bioact. Mater. 2020, 5, 1026–1043. [Google Scholar] [CrossRef]
- Patel, D.K.; Jin, B.; Dutta, S.D.; Lim, K.-T. Osteogenic Potential of Human Mesenchymal Stem Cells on Eggshells-Derived Hydroxyapatite Nanoparticles for Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 1953–1960. [Google Scholar] [CrossRef]
- Hornez, J.-C.; Chai, F.; Monchau, F.; Blanchemain, N.; Descamps, M.; Hildebrand, H. Biological and Physico-Chemical Assessment of Hydroxyapatite (HA) With Different Porosity. Biomol. Eng. 2007, 24, 505–509. [Google Scholar] [CrossRef]
- Ferraz, M.P.; Monteiro, F.J.; Manuel, C.M. Hydroxyapatite Nanoparticles: A Review of Preparation Methodologies. J. Appl. Biomater. Biomech. 2010, 2, 74–80. [Google Scholar]
- Bu, S.; Yan, S.; Wang, R.; Xia, P.; Zhang, K.; Li, G.; Yin, J. In Situ Precipitation of Cluster and Acicular Hydroxyapatite onto Porous Poly(γ-Benzyl-l-Glutamate) Microcarriers for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 12468–12477. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, L.; He, M.; Zhang, R.; Tian, Y.; Liu, S.; Gong, T.; Huo, F.; Yang, T.; Zhang, Q.; et al. A Wear-Resistant TiO2 Nanoceramic Coating on Titanium Implants for Visible-Light Photocatalytic Removal of Organic Residues. Acta Biomater. 2019, 97, 597–607. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the Nano Crystal Size on the X-Ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E.; Stifler, C.A.; Sun, C.-Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The Hidden Structure of Human Enamel. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, B.; Samant, A.; Misra, P.K.; Saxena, M. Nanocrystalline Hydroxyapatite: A Potent Material for Adsorption, Biological and Catalytic Studies. Mater. Today Proc. 2019, 9, 689–698. [Google Scholar] [CrossRef]
- Huang, C.; Bhagia, S.; Hao, N.; Meng, X.; Liang, L.; Yong, Q.; Ragauskas, A.J. Biomimetic Composite Scaffold from an in situ Hydroxyapatite Coating on Cellulose Nanocrystals. RSC Adv. 2019, 9, 5786–5793. [Google Scholar] [CrossRef] [Green Version]
- Bayani, M.; Torabi, S.; Shahnaz, A.; Pourali, M. Main Properties of Nanocrystalline Hydroxyapatite as a Bone Graft Material in Treatment of Periodontal Defects. A Review of Literature. Biotechnol. Biotechnol. Equip. 2017, 31, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting PolymersfromCellulose Nanocrystals: Synthesis, Properties, and Applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.C.; Lang, Y. Toxicity of Nanomaterials: Environmental and Healthcare Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Gogoi, S.; Das, B.; Chattopadhyay, D.; Khan, R. Sustainable Nanostructural Materials for Tissue Engineering. In Dynamics of Advanced Sustainable Nanomaterials and Their Related Nanocomposites at the Bio-Nano Interface; Elsevier: Amsterdam, The Netherlands, 2019; pp. 75–100. [Google Scholar]
- Ruksudjarit, A.; Pengpat, K.; Rujijanagul, G.; Tunkasiri, T. Synthesis and Characterization of Nanocrystalline Hydroxyapatite from Natural Bovine Bone. Curr. Appl. Phys. 2008, 8, 270–272. [Google Scholar] [CrossRef]
- Pu’Ad, N.M.; Koshy, P.; Abdullah, H.; Idris, M.; Lee, T. Syntheses of Hydroxyapatite from Natural Sources. Heliyon 2019, 5, e01588. [Google Scholar]
- Lim, K.-T.; Suh, J.D.; Kim, J.; Choung, P.-H.; Chung, J.H. Calcium Phosphate Bioceramics Fabricated from Extracted Human Teeth for Tooth Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey Gehron, P.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Sangsanoh, P.; Waleetorncheepsawat, S.; Suwantong, O.; Wutticharoenmongkol, P.; Weeranantanapan, O.; Chuenjitbuntaworn, B.; Cheepsunthorn, P.; Pavasant, A.P.; Supaphol, P. In Vitro Biocompatibility of Schwann Cells on Surfaces of Biocompatible Polymeric Electrospun Fibrous and Solution-Cast Film Scaffolds. Biomacromolecules 2007, 8, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dutta, S.D.; Hexiu, J.; Ganguly, K.; Lim, K.-T. Bioactive Electrospun Nanocomposite Scaffolds of poly(lactic acid)/Cellulose Nanocrystals for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 162, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Elkayar, A.; El Shazly, Y.; Assaad, M. Properties of Hydroxyapatite from Bovine Teeth. Bone Tissue Regen. Insights 2009, 2, S3728. [Google Scholar] [CrossRef]
- Lim, K.-T.; Patel, D.K.; Choung, H.W.; Seonwoo, H.; Kim, J.; Chung, J.H. Evaluation of Bone Regeneration Potential of Long-Term Soaked Natural Hydroxyapatite. ACS Appl. Bio Mater. 2019, 2, 5535–5543. [Google Scholar] [CrossRef]
- Fragogeorgi, E.; Rouchota, M.; Georgiou, M.; Velez, M.; Bouziotis, P.; Loudos, G. In Vivo Imaging Techniques for Bone Tissue Engineering. J. Tissue Eng. 2019, 10, 2041731419854586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchomel, P.; Barsa, P.; Buchvald, P.; Svobodnik, A.; Vanickova, E. Autologous Versus Allogenic Bone Grafts in Instrumented Anterior Cervical Discectomy and Fusion: A Prospective Study with Respect to Bone Union Pattern. Eur. Spine J. 2004, 13, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous Bone Grafts in Oral implantology—Is It Still a “Gold standard”? A Consecutive Review of 279 Patients with 456 Clinical Procedures. Int. J. Implant. Dent. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Figueiredo, M.J.D.F.M.D.; Fernando, A.; Martins, G.B.; Freitas, J.C.C.; Judas, F. Effect of the Calcination Temperature on the Composition and Microstructure of Hydroxyapatite Derived from Human and Animal Bone. Ceram. Int. 2010, 36, 2383–2393. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, D.; Motelica-Heino, M.; Guégan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niakan, A.; Ramesh, S.; Ganesan, P.; Tan, C.; Purbolaksono, J.; Chandran, H.; Teng, W. Sintering Behaviour of Natural Porous Hydroxyapatite Derived from Bovine Bone. Ceram. Int. 2015, 41, 3024–3029. [Google Scholar] [CrossRef]
- Jinlong, N.; Zhenxi, Z.; Dazong, J. Investigation of Phase Evolution during the Thermochemical Synthesis of Tricalcium Phosphate. J. Mater. Synth. Process. 2001, 9, 235–240. [Google Scholar] [CrossRef]
- De Oliviera Ugarte, J.F.; De Sena, L.A.; de Castro Pérez, C.A.; De Aguiar, P.F.; Rossi, A.M.; Soares, G.A. Influence of Processing Parameters on Structural Characteristics of Porous Calcium Phosphate Samples: A Study Using an Experimental Design Method. Mater. Res. 2005, 8, 71–76. [Google Scholar] [CrossRef]
- Handley-Sidhu, S.; Renshaw, J.C.; Yong, P.; Kerley, R.; Macaskie, L. Nano-Crystalline Hydroxyapatite Bio-Mineral for the Treatment of Strontium from Aqueous Solutions. Biotechnol. Lett. 2010, 33, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-T.; Baik, S.J.; Kim, S.W.; Kim, J.; Seonwoo, H.; Kim, J.-W.; Choung, P.-H.; Choung, Y.-H.; Chung, J.H. Development and Characterization of Fast-Hardening Composite Cements Composed of Natural Ceramics Originated from Horse Bones and Chitosan Solution. Tissue Eng. Regen. Med. 2014, 11, 362–371. [Google Scholar] [CrossRef]
- Ikeda, T.; Kasai, M.; Tatsukawa, E.; Kamitakahara, M.; Shibata, Y.; Yokoi, T.; Nemoto, T.K.; Ioku, K. A Bone Substitute with High Affinity for Vitamin D-binding Protein―Relationship with Niche of Osteoclasts. J. Cell. Mol. Med. 2014, 18, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Meejoo, S.; Maneeprakorn, W.; Winotai, P. Phase and Thermal Stability of Nanocrystalline Hydroxyapatite Prepared via Microwave Heating. Thermochim. Acta 2006, 447, 115–120. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, A.; Sun, Z. Synthesis of Nano-Hydroxyapatite (nHA) from Waste Mussel Shells Using a Rapid Microwave Method. Mater. Chem. Phys. 2015, 149, 607–616. [Google Scholar] [CrossRef]
- Patel, D.K.; Rana, D.; Aswal, V.K.; Srivastava, S.; Roy, P.; Maiti, P. Influence of Graphene on Self-Assembly of Polyurethane and Evaluation of Its Biomedical Properties. Polymer 2015, 65, 183–192. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Seo, Y.-R.; Park, C.-W.; Lee, S.-H.; Kim, J.-W.; Kim, J.; Seonwoo, H.; Lim, K.-T. In Vitro Biocompatibility of Electrospun Poly(ε-Caprolactone)/Cellulose Nanocrystals-Nanofibers for Tissue Engineering. J. Nanomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Water on Graphene: Hydrophobicity and Dipole Moment Using Density Functional Theory. Phys. Rev. B 2009, 79, 235440. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size Effect of Hydroxyapatite Nanoparticles on Proliferation and Apoptosis of Osteoblast-Like Cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, Y.W.; Fu, X.W.; Best, S.M.; Brooks, R.A.; Rushton, N.; Bonfield, W. Development of Nano-Sized Hydroxyapatite Reinforced Composites for Tissue Engineering Scaffolds. J. Mater. Sci. Mater. Electron. 2007, 18, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Lee, Y.-K.; Huh, K.M.; Kim, S.; Park, K. Safety and Efficacy of Nano/Micro Materials; Springer: Berlin/Heidelberg, Germany, 2008; pp. 63–88. [Google Scholar]
- Daculsi, G.; LeGeros, R.Z.; Deudon, C. Scanning and Transmission Electron Microscopy, and Electron Probe Analysis of the Interface Between Implants and Host Bone. Osseo-Coalescence Versus Osseo-Integration. Scanning Microsc. 1990, 4, 309–314. [Google Scholar]
- Okumura, M.; Ohgushi, H.; Dohi, Y.; Katuda, T.; Tamai, S.; Koerten, H.K.; Tabata, S. Osteoblastic Phenotype Expression on the Surface of Hydroxyapatite Ceramics. J. Biomed. Mater. Res. 1997, 37, 122–129. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, K.; Zhao, R.; Ye, X.; Chen, X.; Xiao, Z.; Yang, X.; Zhu, X.; Zhang, K.; Fan, Y.; et al. Bone Regeneration with micro/Nano Hybrid-Structured Biphasic Calcium Phosphate Bioceramics at Segmental Bone Defect and the Induced Immunoregulation of MSCs. Biomaterials 2017, 147, 133–144. [Google Scholar] [CrossRef]
- He, Y.; Liu, A.; Ke, X.; Sun, M.; He, Y.; Yang, X.; Fu, J.; Zhang, L.; Yang, G.; Liu, Y.; et al. 3D Robocasting Magnesium-Doped wollastonite/TCP Bioceramic Scaffolds with Improved Bone Regeneration Capacity in Critical Sized Calvarial Defects. J. Mater. Chem. B 2017, 5, 2941–2951. [Google Scholar]
- Mansour, A.; Abu-Nada, L.; Al-Waeli, H.; Mezour, M.A.; Abdallah, M.; Kinsella, J.M.; Kort-Mascort, J.; Henderson, J.E.; Luna, J.L.R.-G.; Tran, S.D.; et al. Bone Extracts Immunomodulate and Enhance the Regenerative Performance of Dicalcium Phosphates Bioceramics. Acta Biomater. 2019, 89, 343–358. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Lopezalvarez, M.R.; Serra, J.; González, P.; Landín, M. Current Stage of Marine Ceramic Grafts for 3D Bone Tissue Regeneration. Mar. Drugs 2019, 17, 471. [Google Scholar] [CrossRef] [Green Version]
- Widholz, B.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Westhauser, F. Pooling of Patient-Derived Mesenchymal Stromal Cells Reduces Inter-Individual Confounder-Associated Variation Without Negative Impact on Cell Viability, Proliferation and Osteogenic Differentiation. Cells 2019, 8, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Product | Ca5(PO4)3(OH) | Bioceramics |
|---|---|---|
| Ca | 39.9 | 36.66 |
| P | 18.5 | 16.40 |
| Ca/P Ratio | 1.61 | 1.67 |
| Na | - | 0.79 |
| Mg | - | 0.60 |
| Al | - | 0.04 |
| Si | - | 0.07 |
| Cu | - | 0.01 |
| Cl | - | 0.20 |
| K | - | 0.03 |
| Zn | - | 0.14 |
| Sr | - | 0.03 |
| Y | - | 0.04 |
| Zr | - | 0.60 |
| Ag | - | 0.02 |
| Fe | - | 0.01 |
| S | - | 0.03 |
| Composition (%) | ||
|---|---|---|
| Element | Fresh Bioceramics | SBF-Soaked Bioceramics (12 Months) |
| Calcium | 35.70 | 40.50 |
| Phosphorus | 16.70 | 16.66 |
| Oxygen | 47.60 | 39.91 |
| Sodium | - | 1.23 |
| Chlorine | - | 1.09 |
| Magnesium | - | 0.61 |
| Total | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.-T.; Patel, D.K.; Dutta, S.D.; Choung, H.-W.; Jin, H.; Bhattacharjee, A.; Chung, J.H. Human Teeth-Derived Bioceramics for Improved Bone Regeneration. Nanomaterials 2020, 10, 2396. https://doi.org/10.3390/nano10122396
Lim K-T, Patel DK, Dutta SD, Choung H-W, Jin H, Bhattacharjee A, Chung JH. Human Teeth-Derived Bioceramics for Improved Bone Regeneration. Nanomaterials. 2020; 10(12):2396. https://doi.org/10.3390/nano10122396
Chicago/Turabian StyleLim, Ki-Taek, Dinesh K. Patel, Sayan Deb Dutta, Han-Wool Choung, Hexiu Jin, Arjak Bhattacharjee, and Jong Hoon Chung. 2020. "Human Teeth-Derived Bioceramics for Improved Bone Regeneration" Nanomaterials 10, no. 12: 2396. https://doi.org/10.3390/nano10122396







