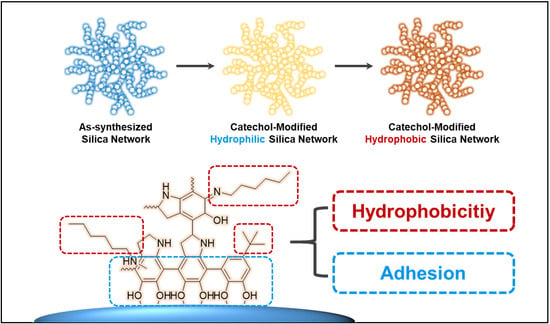
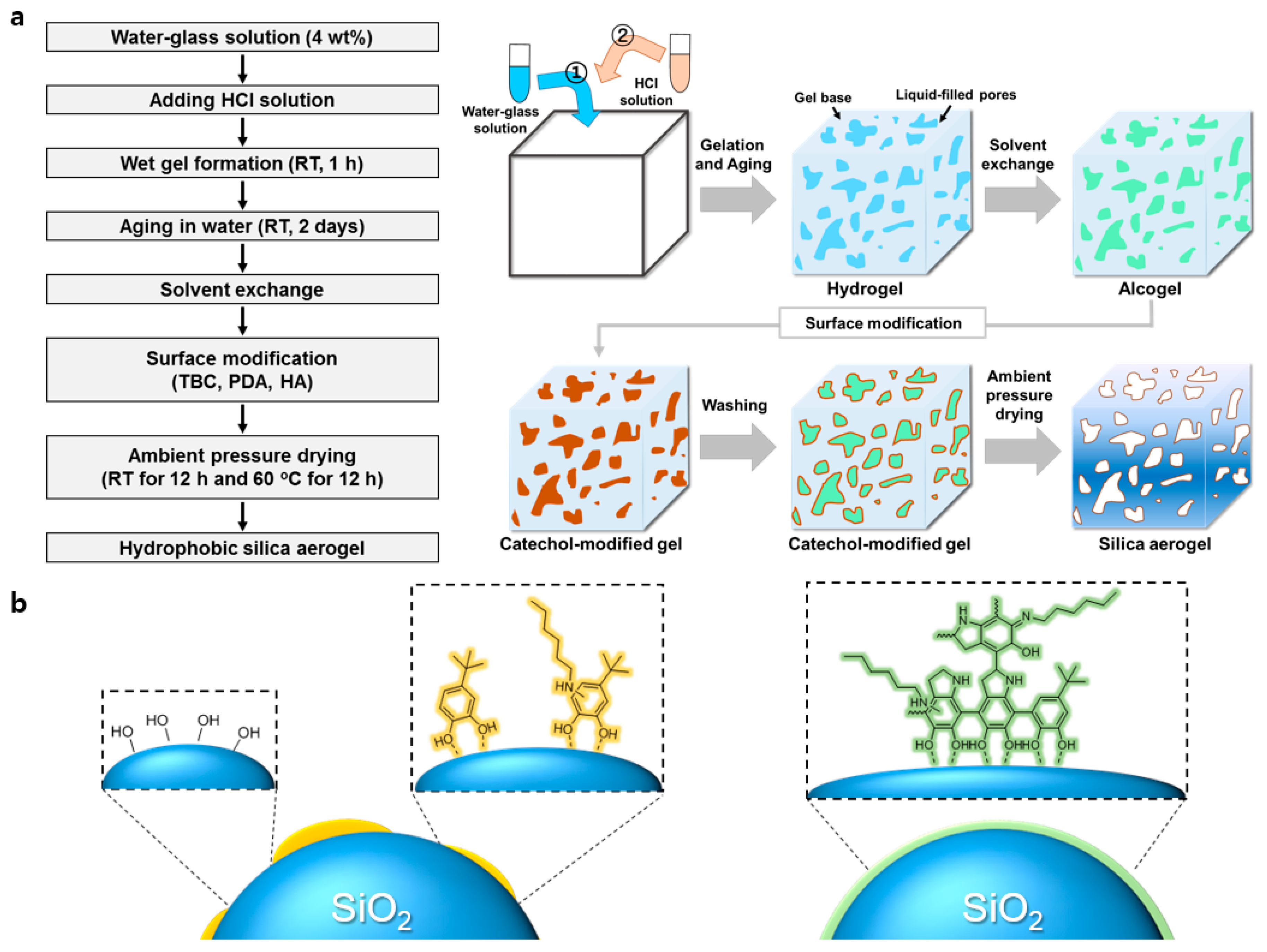
Eco-Friendly Synthesis of Water-Glass-Based Silica Aerogels via Catechol-Based Modifier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Water-Glass Based Silica Wet Gel and Aerogel-Impregnated Glass Wool Sheet
2.3. Preparation of TMCS-Modified Aerogel and Xerogel
2.4. Preparation of Catechol-Modified Aerogel
2.5. Surface Coating of SiO2 Wafer
2.6. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fricke, J.; Emmerling, A. Aerogels. J. Am. Ceram. Soc. 1992, 75, 2027–2036. [Google Scholar] [CrossRef]
- Fricke, J.; Tillotson, T. Aerogels: Production, characterization, and applications. Thin Solid Films 1997, 297, 212–223. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Hrubesh, L.W.; Coronado, P.R.; Satcher, J.H., Jr. Solvent removal from water with hydrophobic aerogels. J. Non-Cryst. Solids 2001, 285, 328–332. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Lee, D.; Cho, J.; Kim, Y.-O.; Lim, S.; Youn, S.; Jung, Y.C.; Kim, S.Y.; Seong, D.G. Super-insulating, flame-retardant, and flexible poly(dimethylsiloxane) composites based on silica aerogel. Compos. Part A Appl. Sci. Manuf. 2019, 123, 108–113. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Salimian, S.; Zadhoush, A. Water-glass based silica aerogel: Unique nanostructured filler for epoxy nanocomposites. J. Porous Mater. 2019, 26, 1755–1765. [Google Scholar] [CrossRef]
- Amiri, T.Y.; Moghaddas, J. Cogeled copper-silica aerogel as a catalyst in hydrogen production from methanol steam reforming. Int. J. Hydrog. Energy 2015, 40, 1472–1480. [Google Scholar] [CrossRef]
- Bangi, U.K.H.; Pandit, S.S.; Bagal, D.B.; Park, H.-H. Preparation of sodium silicate-based aerogels using a two-step sol-gel process and ambient pressure drying. Macromol. Symp. 2019, 387, 1800226. [Google Scholar] [CrossRef]
- De Pooter, S.; Latré, S.; Desplentere, F.; Seveno, D. Optimized synthesis of ambient pressure dried thermal insulating silica aerogel powder from non-ion exchanged water glass. J. Non-Cryst. Solids 2018, 499, 217–226. [Google Scholar] [CrossRef]
- Huang, Y.; He, S.; Feng, M.; Dai, H.; Pan, Y.; Cheng, X. Organic solvent-saving preparation of water glass based aerogel granules under ambient pressure drying. J. Non-Cryst. Solids 2019, 521, 119507. [Google Scholar] [CrossRef]
- Lee, C.J.; Kim, G.S.; Hyun, S.-H. Synthesis of silica aerogels from waterglass via new modified ambient drying. J. Mater. Sci. 2002, 37, 2237–2241. [Google Scholar] [CrossRef]
- Schwertfeger, F.; Frank, D.; Schmidt, M. Hydrophobic waterglass based aerogels without solvent exchange or supercritical drying. J. Non-Cryst. Solids 1998, 225, 24–29. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Liu, J. Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater. Lett. 2006, 60, 3718–3722. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Jung, H.-H.; Hyun, S.-H.; Ahn, Y.-S. Effective preparation of crack-free silica aerogels via ambient drying. J. Sol-Gel Sci. Technol. 2007, 41, 139–146. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V.; Pajonk, G.M. Hydrophobic and physical properties of the ambient pressure dried silica aerogels with sodium silicate precursor using various surface modification agents. Appl. Surf. Sci. 2007, 253, 6032–6040. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. New tannin-lignin aerogels. Ind. Crop. Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Kadib, A.E.; Bousmina, M. Chitosan Bio-Based Organic-Inorganic Hybrid Aerogel Microspheres. Chem. Eur. J. 2012, 18, 8264–8277. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Yoda, S. Upscaled Preparation of Trimethylsilylated Chitosan Aerogel. Ind. Eng. Chem. Res. 2018, 57, 10421–10430. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Das, S.; Lee, B.H.; Linstadt, R.T.H.; Cunha, K.; Li, Y.; Kaufman, Y.; Levine, Z.A.; Lipshutz, B.H.; Lins, R.D.; Shea, J.-E.; et al. Molecularly Smooth Self-Assembled Monolayer for High-Mobility Organic Field-Effect Transistors. Nano Lett. 2016, 16, 6709–6715. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mahadik, D.B.; Meti, P.; Gong, Y.-D.; Lee, K.-Y.; Park, H.-H. Dioxybenzene-bridged hydrophobic silica aerogels with enhanced textural and mechanical properties. Microporous Mesoporous Mater. 2020, 294, 109863. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.M.; Dyke, J.C.; Bowman, B.A.; Ko, C.C.; You, W. Investigation of Dopamine Analogues: Synthesis, Mechanistic Understanding, and Structure—Property Relationship. Langmuir 2016, 32, 9873–9882. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.; Lee, J.; Jeong, G.; Kim, H.; Park, J.; Park, H. Bio-Inspired Catecholamine-Derived Surface Modifier for Graphene-Based Organic Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6463–6468. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhang, L.; Wang, Y.X.; Long, Y.H.; Gao, H.; Zhang, X.L.; Zhao, N.; Cai, Y.L.; Xu, J. Mussel-Inspired Chemistry for Robust and Surface-Modifiable Multilayer Films. Langmuir 2011, 27, 13684–13691. [Google Scholar] [CrossRef]
- Yu, H.J.; Liang, X.F.; Wang, J.X.; Wang, M.M.; Yang, S.Y. Preparation and characterization of hydrophobic silica aerogel sphere products by co-precursor method. Solid State Sci. 2015, 48, 155–162. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Cui, S. Fabrication of robust multilayer films by triggering the coupling reaction between phenol and primary amine groups with visible light irradiation. Nanoscale 2011, 3, 3819–3824. [Google Scholar] [CrossRef] [PubMed]
- Gillich, T.; Benetti, E.M.; Rakhmatullina, E.; Konradi, R.; Li, W.; Zhang, A.; Schlüter, A.D.; Textor, M. Self-Assembly of Focal Point Oligo-catechol Ethylene Glycol Dendrons on Titanium Oxide Surfaces: Adsorption Kinetics, Surface Characterization, and Nonfouling Properties. J. Am. Chem. Soc. 2011, 133, 10940–10950. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Oh, D.X.; Heo, J.; Lee, H.-K.; Choy, S.; Hawker, C.J.; Hwang, D.S. Formation, Removal, and Reformation of Surface Coatings on Various Metal Oxide Surfaces Inspired by Mussel Adhesives. ACS Appl. Mater. Interfaces 2015, 7, 24656–24662. [Google Scholar] [CrossRef] [PubMed]
- Yah, W.O.; Xu, H.; Soejima, H.; Ma, W.; Lvov, Y.; Takahara, A. Biomimetic Dopamine Derivative for Selective Polymer Modification of Halloysite Nanotube Lumen. J. Am. Chem. Soc. 2012, 134, 12134–12137. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.R.; Fan, H.L.; Zha, D.A.; Wang, L.; Jin, Z.X. Characterizations of the Formation of Polydopamine-Coated Halloysite Nanotubes in Various pH Environments. Langmuir 2016, 32, 10377–10386. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, M.; Mishra, R.; Wiener, J.; Militky, J.; Kotresh, T.M.; Vaclavik, M. Novel techniques to analyse thermal performance of aerogel-treated blankets under extreme temperatures. J. Text. Inst. 2015, 106, 736–747. [Google Scholar] [CrossRef]
- Venkataraman, M.; Mishra, R.; Militky, J. Comparative Analysis of High Performance Thermal Insulation Materials. J. Text. Eng. Fash. Technol. 2017, 2, 401–409. [Google Scholar] [CrossRef] [Green Version]

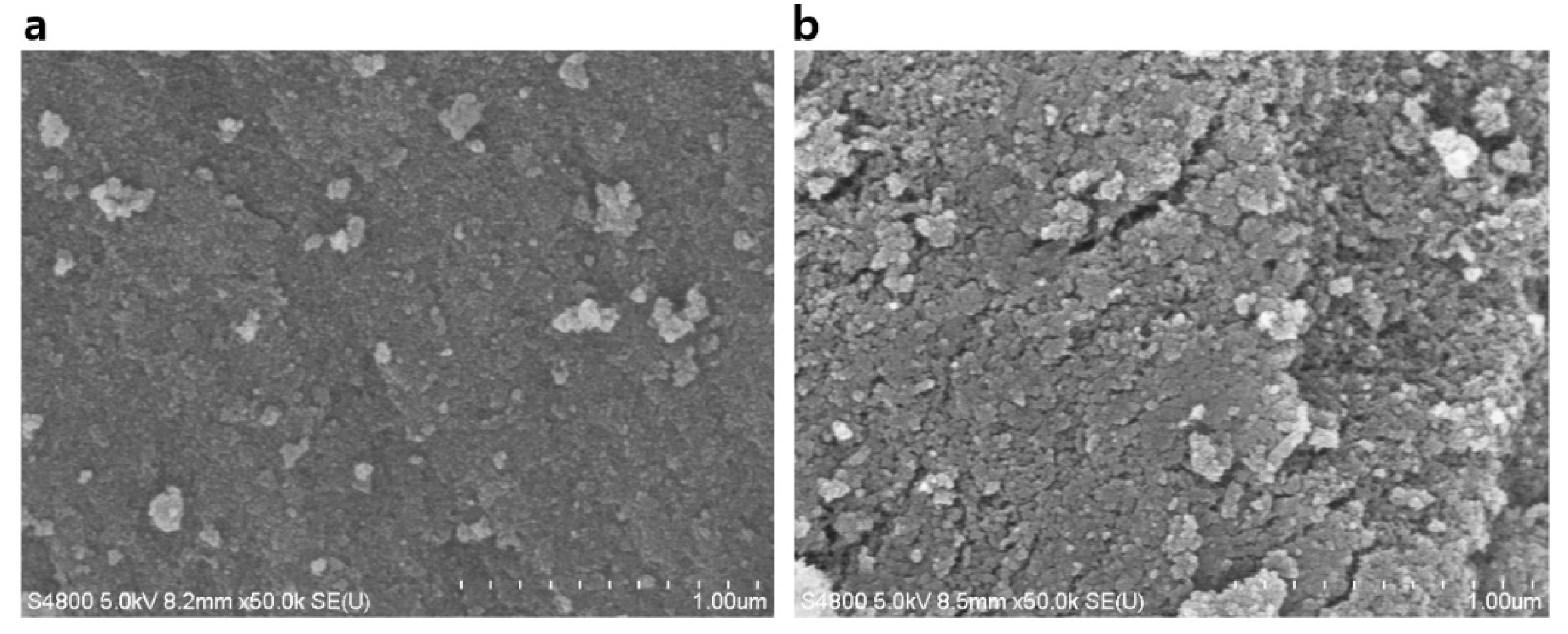


| Physical Property | Xerogel | TBC | TBC + HA | PDA + TBC | PDA + TBC + HA | TMCS |
|---|---|---|---|---|---|---|
| BET surface area (m2/g) | 98 | 308 | 340 | 375 | 377 | 473 |
| Pore volume (cm3/g) | 0.08 | 1.77 | 2.13 | 2.14 | 1.93 | 4.15 |
| Average pore diameter (nm) | 3.23 | 22.90 | 25.02 | 22.82 | 20.52 | 35.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, K.; Kim, H.; Lee, D.J.; Park, J. Eco-Friendly Synthesis of Water-Glass-Based Silica Aerogels via Catechol-Based Modifier. Nanomaterials 2020, 10, 2406. https://doi.org/10.3390/nano10122406
Kim H, Kim K, Kim H, Lee DJ, Park J. Eco-Friendly Synthesis of Water-Glass-Based Silica Aerogels via Catechol-Based Modifier. Nanomaterials. 2020; 10(12):2406. https://doi.org/10.3390/nano10122406
Chicago/Turabian StyleKim, Hyeonjung, Kangyong Kim, Hyunhong Kim, Doo Jin Lee, and Jongnam Park. 2020. "Eco-Friendly Synthesis of Water-Glass-Based Silica Aerogels via Catechol-Based Modifier" Nanomaterials 10, no. 12: 2406. https://doi.org/10.3390/nano10122406
APA StyleKim, H., Kim, K., Kim, H., Lee, D. J., & Park, J. (2020). Eco-Friendly Synthesis of Water-Glass-Based Silica Aerogels via Catechol-Based Modifier. Nanomaterials, 10(12), 2406. https://doi.org/10.3390/nano10122406