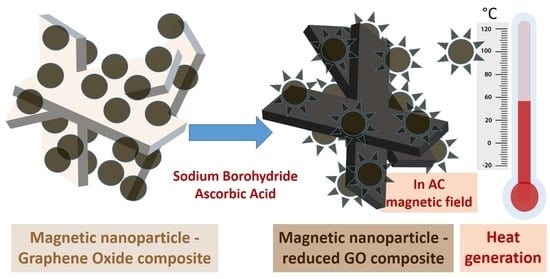
Tunable Magnetic Hyperthermia Properties of Pristine and Mildly Reduced Graphene Oxide/Magnetite Nanocomposite Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Nanocomposites by Heterocoagulation
2.3. Reduction of the Composites
2.4. Experimental Methods
2.4.1. Structure and Morphology
2.4.2. Laser Doppler Electrophoresis Measurements
2.4.3. Dynamic Light Scattering Experiments
2.4.4. Magnetic Hyperthermia Measurements
2.4.5. Determination of Dissolved Iron Concentration
3. Results
3.1. Structural Characterization
3.2. Surface Charge Characteristics
3.3. Heat Generation in Nanocomposite Dispersions
3.3.1. GO/MNP Composites before Reduction
3.3.2. Heat Production of rGO/MNP Composites Obtained by Borohydride Treatment
3.3.3. Heat Production of rGO/MNP Composites Obtained by Ascorbic Acid Treatment
4. Discussion
4.1. Perspectives on Magnetic Hyperthermia of rGO/MNP Composites
4.2. Utility of rGO/MNP Composites Synthesized in This Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Socoliuc, V.; Peddis, D.; Petrenko, V.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic Nanoparticle Systems for Nanomedicine—A Materials Science Perspective. Magnetochemistry 2019, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Knežević, N.Ž.; Jimenez, C.M.; Albino, M.; Vukadinovic, A.; Mrakovic, A.; Illes, E.; Janackovic, D.; Durand, J.-O.; Sangregorio, C.; Peddis, D. Synthesis and Characterization of Core-Shell Magnetic Mesoporous Silica and Organosilica Nanostructures. MRS Adv. 2017, 2, 1037–1045. [Google Scholar] [CrossRef] [Green Version]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Szabó, Á.; Iván, B.; Turcu, R.; Vékás, L.; Zupkó, I.; Jaics, G.; Tombácz, E. Multifunctional PEG-carboxylate copolymer coated superparamagnetic iron oxide nanoparticles for biomedical application. J. Magn. Magn. Mater. 2018, 451, 710–720. [Google Scholar] [CrossRef] [Green Version]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikram, R.; Jan, B.M.; Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Domán, A.; Klébert, S.; Madarász, J.; Sáfrán, G.; Wang, Y.; László, K. Graphene oxide protected copper benzene-1,3,5-tricarboxylate for clean energy gas adsorption. Nanomaterials 2020, 10, 1182. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, B.; He, M.; Huang, X.; Yin, G.; Cui, Y. Preparation of anisotropic aerogels with pristine graphene by heat flow and study of their effects on heat transfer in paraffin. Nanomaterials 2019, 9, 1622. [Google Scholar] [CrossRef] [Green Version]
- Lazarte, J.P.L.; Bautista-Patacsil, L.; Eusebio, R.C.P.; Orbecido, A.H.; Doong, R.A. Sustainable desalination by 3:1 reduced graphene oxide/titanium dioxide nanotubes (rGO/TiONTs) composite via capacitive deionization at different sodium chloride concentrations. Nanomaterials 2019, 9, 1319. [Google Scholar] [CrossRef] [Green Version]
- Paszkiewicz, S.; Pawlikowska, D.; Kurcz, M.; Szymczyk, A.; Irska, I.; Stanik, R.; Gude, M.; Linares, A.; Ezquerra, T.A.; Lipińska, L.; et al. Functional properties of poly(Trimethylene terephthalate)-block-poly(caprolactone) based nanocomposites containing graphene oxide (go) and reduced graphene oxide (rgo). Nanomaterials 2019, 9, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabó, T.; Veres, Á.; Cho, E.; Khim, J.; Varga, N.; Dékány, I. Photocatalyst separation from aqueous dispersion using graphene oxide/TiO2 nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 230–239. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Qi, P.; Liu, X.; Wei, G. Synthesis of three-dimensional graphene-based hybrid materials for water purification: A review. Nanomaterials 2019, 9, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luceño-Sánchez, J.A.; Díez-Pascual, A.M. Grafting of polypyrrole-3-carboxylic acid to the surface of hexamethylene diisocyanate-functionalized graphene oxide. Nanomaterials 2019, 9, 1095. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.Z.; Zhao, D.L.; Xu, Y.; Zhang, J.M.; Gao, Y.L.; Zhao, L.Y.; Tang, J.T. Inductive heating property of graphene oxide-Fe3O4 nanoparticles hybrid in an AC magnetic field for localized hyperthermia. Mater. Lett. 2012, 68, 399–401. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Hoan, N.T.V.; Thu, N.T.A.; Van Duc, H.; Cuong, N.D.; Khieu, D.Q.; Vo, V. Fe3O4/Reduced Graphene Oxide Nanocomposite: Synthesis and Its Application for Toxic Metal Ion Removal. J. Chem. 2016, 2016, 2418172. [Google Scholar] [CrossRef] [Green Version]
- Tanwar, S.; Mathur, D. Magnetite-graphene oxide nanocomposites: Facile synthesis and characterization of optical and magnetic property. Mater. Today Proc. 2020, 30, 17–22. [Google Scholar] [CrossRef]
- Scheibe, B.; Mrówczyński, R.; Michalak, N.; Załęski, K.; Matczak, M.; Kempiński, M.; Pietralik, Z.; Lewandowski, M.; Jurga, S.; Stobiecki, F. Anchoring Fe3O4 nanoparticles in a reduced graphene oxide aerogel matrix via polydopamine coating. Beilstein J. Nanotechnol. 2018, 9, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Liu, D.; Lu, W.; Zhang, K. One-pot sonochemical synthesis of magnetite@reduced graphene oxide nanocomposite for high performance Li ion storage. Ultrason. Sonochem. 2018, 45, 167–172. [Google Scholar] [CrossRef]
- Urbas, K.; Aleksandrzak, M.; Jedrzejczak, M.; Jedrzejczak, M.; Rakoczy, R.; Chen, X.; Mijowska, E. Chemical and magnetic functionalization of graphene oxide as a route to enhance its biocompatibility. Nanoscale Res. Lett. 2014, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Kurup Sasikala, A.; Thomas, R.G.; Unnithan, A.R.; Saravanakumar, B.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifunctional Nanocarpets for Cancer Theranostics: Remotely Controlled Graphene Nanoheaters for Thermo-Chemosensitisation and Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 20543. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, X.; Li, J.; Zhu, Y. Comparative Study on the Regeneration of Fe3O4@Graphene Oxide Composites. Front. Chem. 2020, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Karakassides, M.A.; Simopoulos, A.; Petridis, D. Synthesis and characterization of magnetically modified clay composites. Chem. Mater. 2000, 12, 2640–2645. [Google Scholar] [CrossRef]
- Szabo, T.; Bakandritsos, A.; Tzitzios, V.; Papp, S.; Kőrösi, L.; Galbács, G.; Musabekov, K.B.; Bolatova, D.; Petridis, D.; Dékány, I. Magnetic iron oxide/clay composites: Effect of the layer silicate support on the microstructure and phase formation of magnetic nanoparticles. Nanotechnology 2007, 18, 285602. [Google Scholar] [CrossRef]
- Tzitzios, V.; Basina, G.; Bakandritsos, A.; Hadjipanayis, C.G.; Mao, H.; Niarchos, D.; Hadjipanayis, G.C.; Tucek, J.; Zboril, R. Immobilization of magnetic iron oxide nanoparticles on laponite discs—An easy way to biocompatible ferrofluids and ferrogels. J. Mater. Chem. 2010, 20, 5418–5428. [Google Scholar] [CrossRef] [Green Version]
- Seliem, M.K.; Barczak, M.; Anastopoulos, I.; Giannakoudakis, D.A. A novel nanocomposite of activated serpentine mineral decorated with magnetic nanoparticles for rapid and effective adsorption of hazardous cationic dyes: Kinetics and equilibrium studies. Nanomaterials 2020, 10, 684. [Google Scholar] [CrossRef] [Green Version]
- Bourlinos, A.B.; Karakassides, M.A.; Petridis, D. Synthesis and Characterization of Iron-Containing MCM-41 Porous Silica by the Exchange Method of the Template. J. Phys. Chem. B 2000, 104, 4375–4380. [Google Scholar] [CrossRef]
- Baikousi, M.; Bourlinos, A.B.; Douvalis, A.; Bakas, T.; Anagnostopoulos, D.F.; Tuček, J.; Šafářová, K.; Zboril, R.; Karakassides, M.A. Synthesis and Characterization of γ-Fe2O3/Carbon Hybrids and Their Application in Removal of Hexavalent Chromium Ions from Aqueous Solutions. Langmuir 2012, 28, 3918–3930. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Zboril, R.; Petridis, D. A simple route towards magnetically modified zeolites. Microporous Mesoporous Mater. 2003, 58, 155–162. [Google Scholar] [CrossRef]
- Rahmani-Sani, A.; Singh, P.; Raizada, P.; Lima, E.C.; Anastopoulos, I.; Giannakoudakis, D.A.; Sivamani, S.; Dontsova, T.A.; Hosseini-Bandegharaei, A. Use of chicken feather and eggshell to synthesize a novel magnetized activated carbon for sorption of heavy metal ions. Bioresour. Technol. 2020, 297, 122452. [Google Scholar] [CrossRef]
- Minati, L.; Speranza, G.; Micheli, V.; Serra, M.D.; Clamer, M. Graphene oxide nanocomposite magnetic microbeads for the remediation of positively charged aromatic compounds. Dalt. Trans. 2020, 49, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.; Nánai, L.; Nesztor, D.; Barna, B.; Malina, O.; Tombácz, E. A Simple and Scalable Method for the Preparation of Magnetite/Graphene Oxide Nanocomposites under Mild Conditions. Adv. Mater. Sci. Eng. 2018, 2018, 1390651. [Google Scholar] [CrossRef] [Green Version]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabó, T.; Szeri, A.; Dékány, I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Karim, M.R.; Hayami, S. Chemical, Thermal, and Light-Driven Reduction of Graphene Oxide: Approach to Obtain Graphene and its Functional Hybrids. In Graphene Materials-Advanced Applications; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Azizighannad, S.; Mitra, S. Stepwise reduction of Graphene Oxide (GO) and its effects on chemical and colloidal properties. Sci. Rep. 2018, 8, 10083. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide vial-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Habte, A.T.; Ayele, D.W.; Hu, M. Synthesis and Characterization of Reduced Graphene Oxide (rGO) Started from Graphene Oxide (GO) Using the Tour Method with Different Parameters. Adv. Mater. Sci. Eng. 2019, 2019, 5058163. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Park, W.K.; Hwang, T.-M.; Yoon, D.H.; Yang, W.S.; Kang, J.-W. Comparative evaluation of magnetite-graphene oxide and magnetite-reduced graphene oxide composite for As(III) and As(V) removal. J. Hazard. Mater. 2016, 304, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E.; Tóth, I.Y.; Kovács, K.; Illés, E.; Szekeres, M.; Barna, B.; Csicsor, A.; Szabó, T. Striking analogies and dissimilarities between graphene oxides and humic acids: pH-dependent charging and colloidal stability. J. Mol. Liq. 2020, 306, 112948. [Google Scholar] [CrossRef]
- Szekeres, M.; Tóth, I.Y.; Illés, E.; Jedlovszky-Hajdu, A.; Zupkó, I.; Farkas, K.; Oszlánczi, G.; Tiszlavicz, L.; Tombácz, E. Chemical and colloidal stability of carboxylated core-shell magnetite nanoparticles designed for biomedical applications. Int. J. Mol. Sci. 2013, 14, 4550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tombácz, E.; Illés, E.; Majzik, A.; Hajdú, A.; Rideg, N.; Szekeres, M. Ageing in the inorganic nanoworld: Example of magnetite nanoparticles in aqueous medium. Croat. Chem. Acta 2007, 80, 503–515. [Google Scholar]
- Mykhaylyk, O.; Antequera, Y.S.; Vlaskou, D.; Plank, C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat. Protoc. 2007, 2, 2391–2411. [Google Scholar] [CrossRef]
- Szabó, T.; Tombácz, E.; Illés, E.; Dékány, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon N. Y. 2006, 44, 537–545. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef]
- Thiele, H. Graphit und Graphitsäure. Z. Anorg. Allg. Chem. 1930, 190, 145–160. [Google Scholar] [CrossRef]
- Kotov, N.A.; Dékány, I.; Fendler, J.H. Ultrathin graphite oxide-polyelectrolyte composites prepared by self-assembly: Transition between conductive and non-conductive states. Adv. Mater. 1996, 8, 637–641. [Google Scholar] [CrossRef]
- Gutiérrez, L.; De La Cueva, L.; Moros, M.; Mazarío, E.; De Bernardo, S.; De La Fuente, J.M.; Morales, M.D.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Di Fan, D.; Ding, J. Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, J.G.; Cabrera, D.; Carrey, J.; Valdivielso, T.; Salas, G.; Teran, F.J. Effects of inter- and intra-aggregate magnetic dipolar interactions on the magnetic heating efficiency of iron oxide nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 10954–10963. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.O.; Baldi, G.; Doumett, S.; Garcia-Hevia, L.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; et al. Multifunctional graphene-based magnetic nanocarriers for combined hyperthermia and dual stimuli-responsive drug delivery. Mater. Sci. Eng. C 2018, 93, 206–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, T.; Akaike, J.; Kawashita, M.; Lim, H.N. In vitro apatite mineralization and heat generation of magnetite-reduced graphene oxide nanocomposites for hyperthermia treatment. Mater. Sci. Eng. C 2019, 99, 68–72. [Google Scholar] [CrossRef]
- Sugumaran, P.J.; Liu, X.L.; Herng, T.S.; Peng, E.; Ding, J. GO-Functionalized Large Magnetic Iron Oxide Nanoparticles with Enhanced Colloidal Stability and Hyperthermia Performance. ACS Appl. Mater. Interfaces 2019, 11, 22703–22713. [Google Scholar] [CrossRef]
- Gupta, J.; Prakash, A.; Jaiswal, M.K.; Agarrwal, A.; Bahadur, D. Superparamagnetic iron oxide-reduced graphene oxide nanohybrid-a vehicle for targeted drug delivery and hyperthermia treatment of cancer. J. Magn. Magn. Mater. 2018, 448, 332–338. [Google Scholar] [CrossRef]







| NaBH4 | Acidic (pH ~ 3.4–3.7) ~25 °C, 4 days | Alkaline (pH ~ 9.2–9.5) ~95 °C, 30 min | |
|---|---|---|---|
| GO/MNP 1/0 GO:1 g/L, MNP: 0 g/L | 50 mM | LAA 5 mM | LAA: 5 mM, NH3: 5 µL/mL |
| GO/MNP 1/5 GO: 1 g/L, MNP: 5 g/L | 50 mM | LAA 5 mM | LAA: 5 mM, NH3: 5 µL/mL |
| GO/MNP 1/10 GO: 0.5 g/L, MNP: 5 g/L | 50 mM | LAA 5 mM | LAA: 5 mM, NH3: 5 µL/mL |
| Without Reduction | After Reduction with | |||
|---|---|---|---|---|
| NaBH4 | LAA, pH ~ 3.5 | LAA, pH ~ 9.3 | ||
| GO | 3.6 | 7.1 | 2.6 | 1.9 |
| 1/5 GO/MNP | 9.2 | 12.7 | 7.6 | 20.3 |
| 1/10 GO/MNP | 8.8 | 16.2 | 11.8 | 11.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illés, E.; Tombácz, E.; Hegedűs, Z.; Szabó, T. Tunable Magnetic Hyperthermia Properties of Pristine and Mildly Reduced Graphene Oxide/Magnetite Nanocomposite Dispersions. Nanomaterials 2020, 10, 2426. https://doi.org/10.3390/nano10122426
Illés E, Tombácz E, Hegedűs Z, Szabó T. Tunable Magnetic Hyperthermia Properties of Pristine and Mildly Reduced Graphene Oxide/Magnetite Nanocomposite Dispersions. Nanomaterials. 2020; 10(12):2426. https://doi.org/10.3390/nano10122426
Chicago/Turabian StyleIllés, Erzsébet, Etelka Tombácz, Zsófia Hegedűs, and Tamás Szabó. 2020. "Tunable Magnetic Hyperthermia Properties of Pristine and Mildly Reduced Graphene Oxide/Magnetite Nanocomposite Dispersions" Nanomaterials 10, no. 12: 2426. https://doi.org/10.3390/nano10122426
APA StyleIllés, E., Tombácz, E., Hegedűs, Z., & Szabó, T. (2020). Tunable Magnetic Hyperthermia Properties of Pristine and Mildly Reduced Graphene Oxide/Magnetite Nanocomposite Dispersions. Nanomaterials, 10(12), 2426. https://doi.org/10.3390/nano10122426