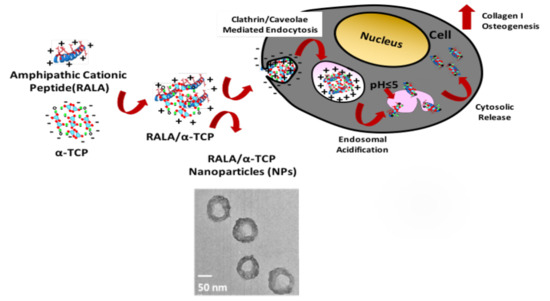
Improving the Intercellular Uptake and Osteogenic Potency of Calcium Phosphate via Nanocomplexation with the RALA Peptide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. RALA Peptide
2.1.2. Synthesis of Alpha-Tri Calcium Phosphate
2.1.3. Plasmid
2.2. Methods
2.2.1. X-ray Diffraction
2.2.2. Fourier Transform Infra-Red Spectroscopy
2.2.3. Scanning Electron Microscopy
2.2.4. Particle Size and Zeta Potential Analysis
2.2.5. Cell Culture
2.2.6. Nanoparticle Transfection
2.2.7. Transmission Electron Microscopy
2.2.8. Cell Viability: MTS Cell Proliferation Assay
2.2.9. Cell Toxicity: Lactate Dehydrogenase Assay
2.2.10. Immunocytochemistry
2.2.11. Mineralization: Alizarin Red Stain Assay
2.2.12. Alizarin Red CPC Extraction
2.2.13. Statistical Analysis
3. Results
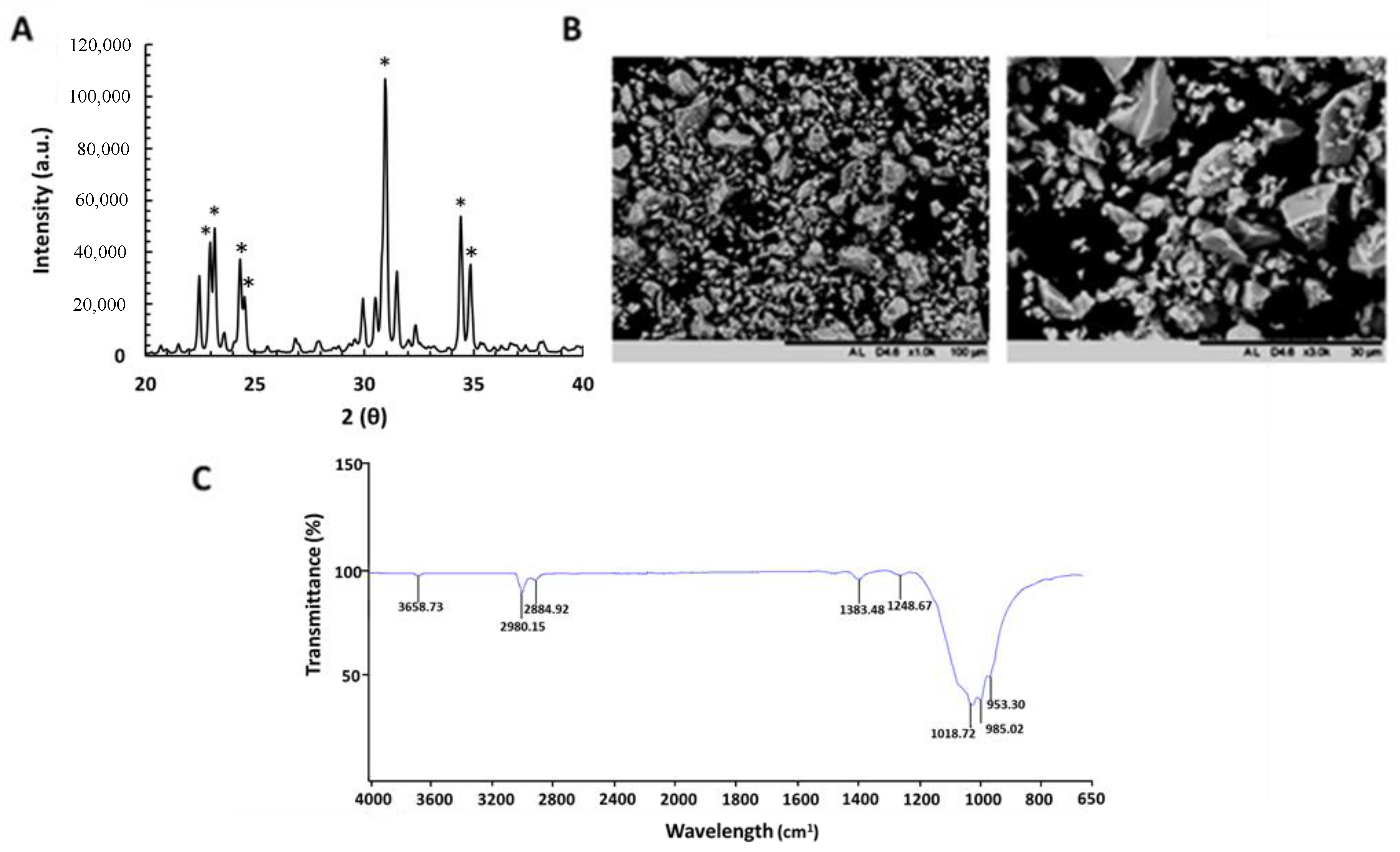
3.1. Physiochemical Properties of α-TCP Particles
3.2. Characterization of RALA/α-TCP Nanoparticles
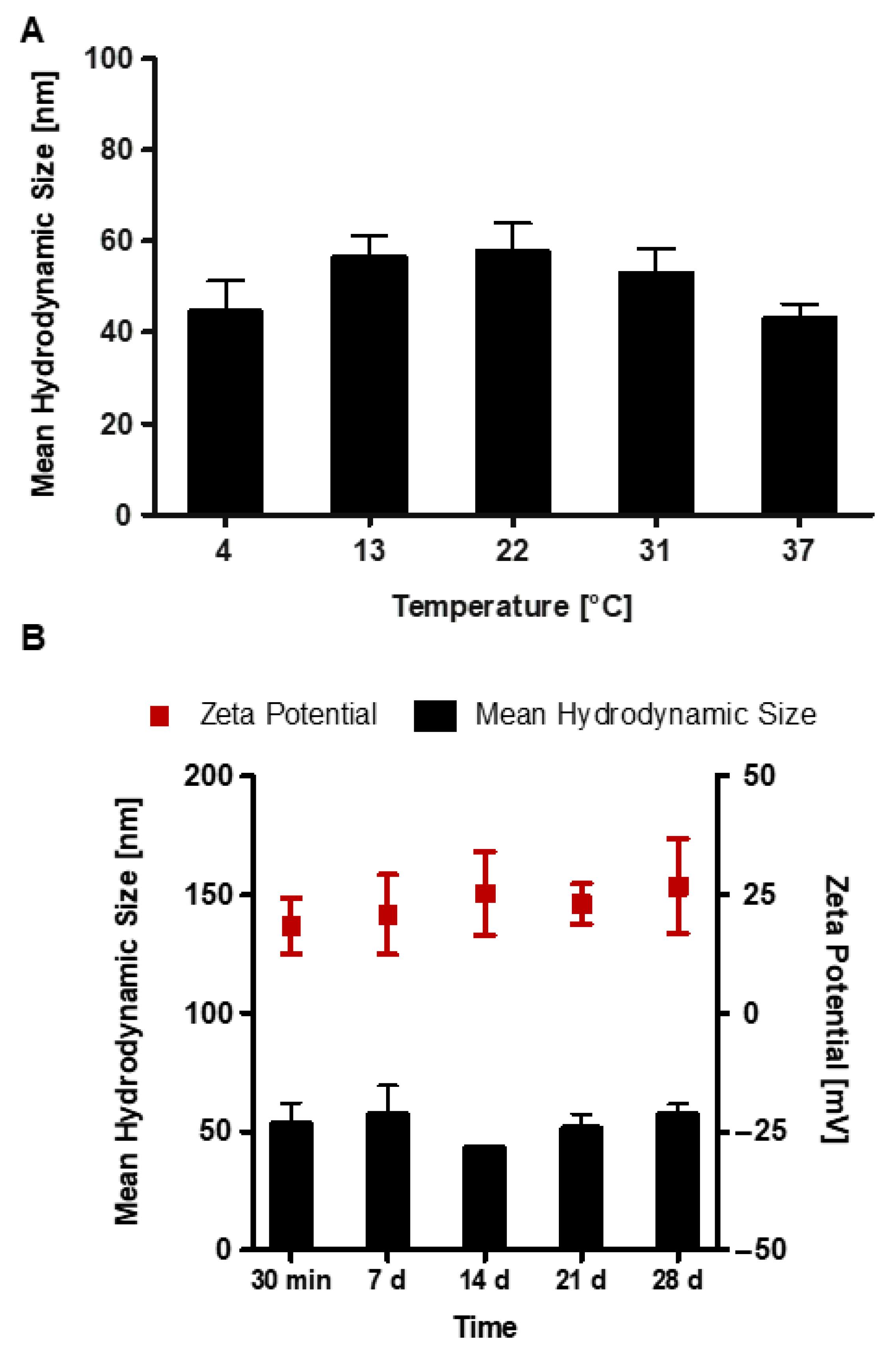
3.3. Stability of RALA/α-TCP Nanoparticles
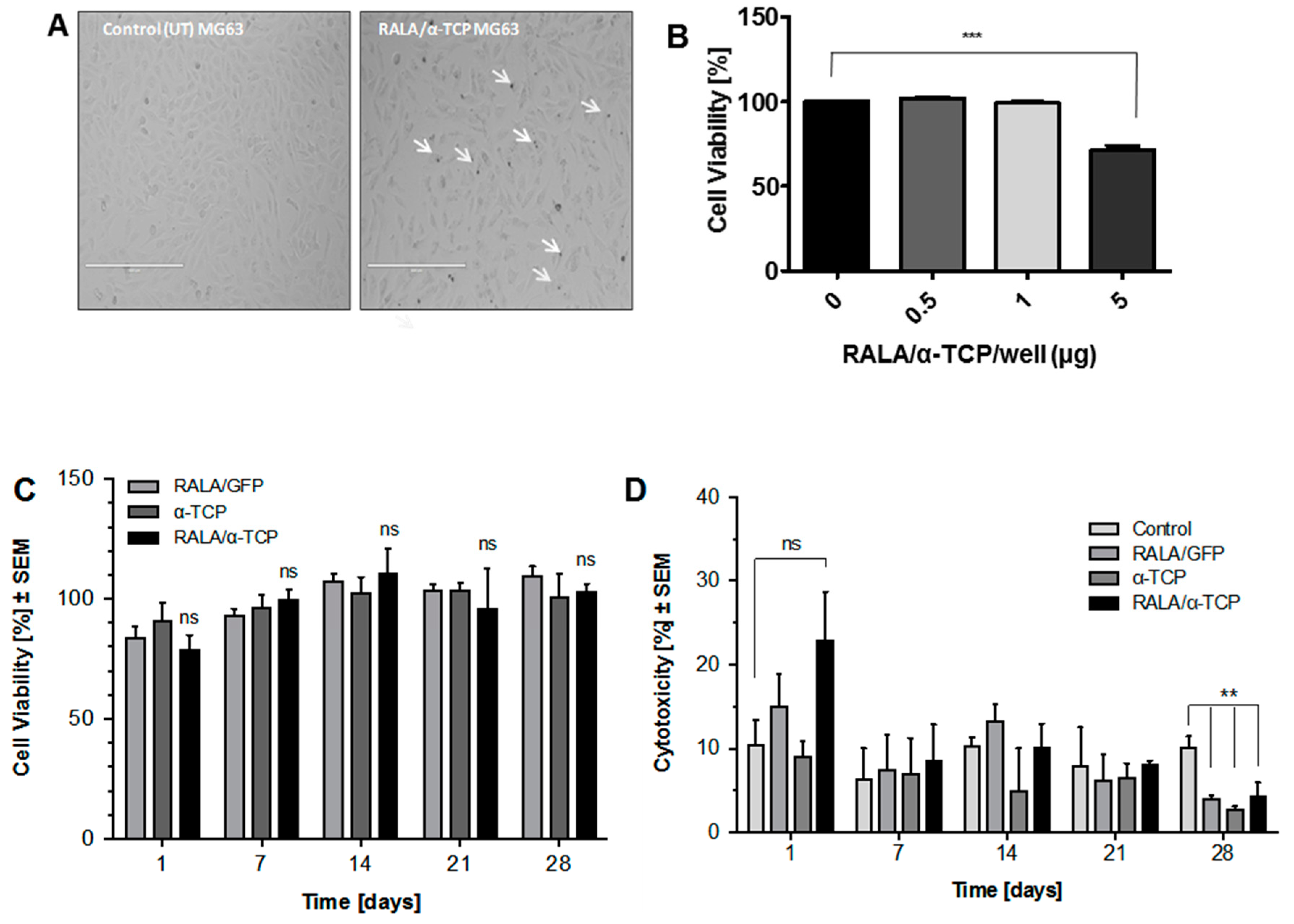
3.4. Cytotoxicity of RALA/α-TCP Nanoparticles
3.5. Effects of RALA/α-TCP Nanoparticles on Collagen Production in MG-63 Cells
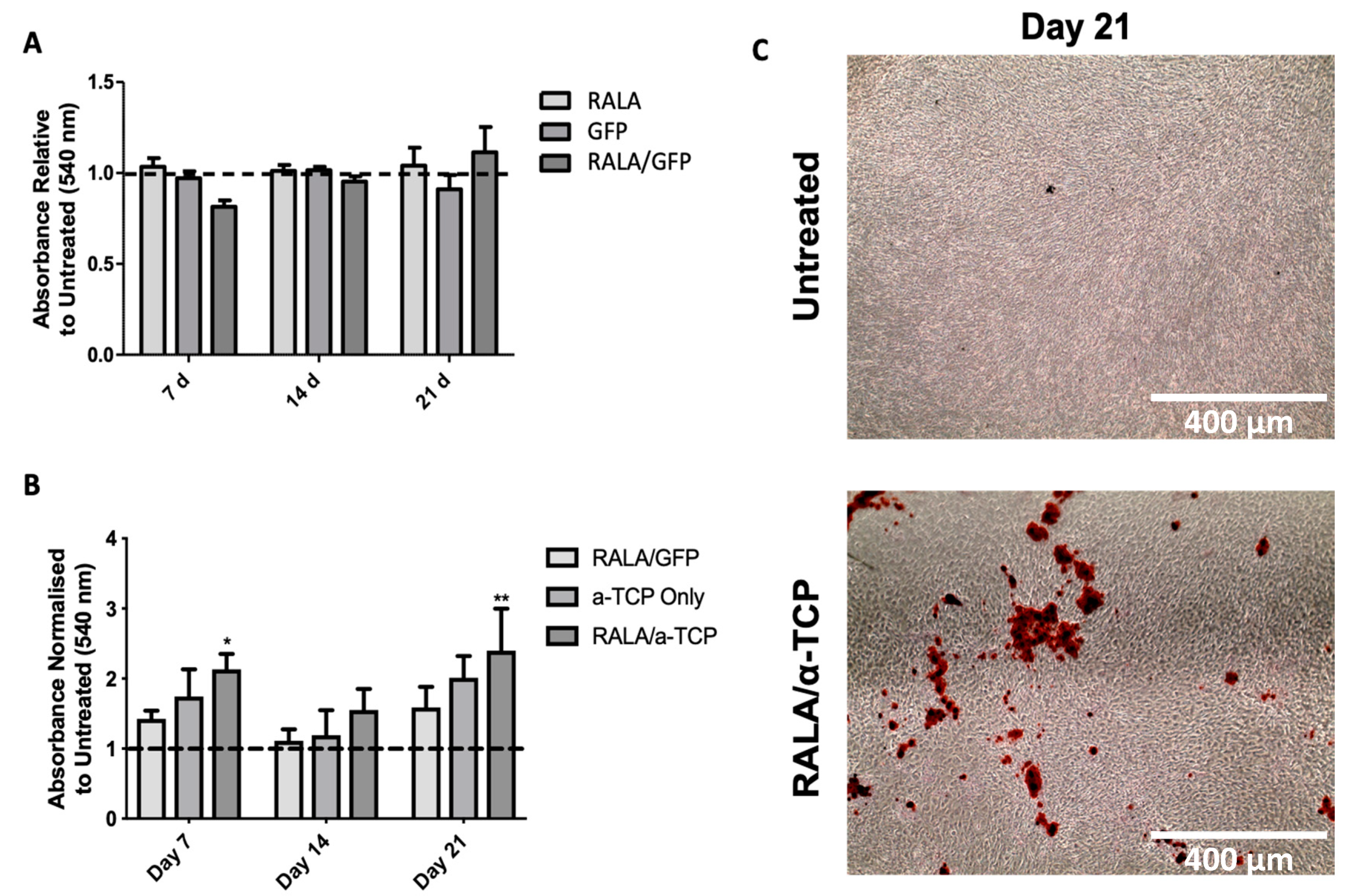
3.6. Effects of RALA/α-TCP Nanoparticles on Calcium Deposition and Matrix Mineralization in pMSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Ziminska, M.; Chalanqui, M.J.; Chambers, P.; Acheson, J.G.; McCarthy, H.O.; Dunne, N.; Hamilton, A.R. Nanocomposite-coated porous templates for engineered bone scaffolds: A parametric study of layer-by-layer assembly conditions. Biomed. Mater. 2019, 14, 065008. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Saunders, L.C.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Melo-Fonseca, F.; Miranda, G.; Domingues, H.S.; Pinto, I.M.; Gasik, M.; Silva, F.S. Reengineering Bone-Implant Interfaces for Improved Mechanotransduction and Clinical Outcomes. Stem Cell Rev. Rep. 2020, 16, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Solano-Umaña, V.; Vega-Baudrit, J.R.; González-Paz, R. The new field of the nanomedicine. Int. J. Appl. Sci. Tech. 2015, 5, 79–88. [Google Scholar]
- Mulholland, E.J.; Ali, A.; Robson, T.; Dunne, N.J.; McCarthy, H.O. Delivery of RALA/siFKBPL nanoparticles via electrospun bilayer nanofibres: An innovative angiogenic therapy for wound repair. J. Control Release 2019, 316, 53–65. [Google Scholar] [CrossRef]
- Mallick, S.P.; Beyene, Z.; Suman, D.K.; Madhual, A.; Singh, B.N.; Srivastava, P. Strategies towards Orthopaedic Tissue Engineered Graft Generation: Current Scenario and Application. Biotechnol. Bioprocess Eng. 2019, 24, 854–869. [Google Scholar] [CrossRef]
- Hutchings, G.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Sibiak, R.; Bryja, A.; Jankowski, M.; Mozdziak, P.; Bukowska, D.; Antosik, P.; et al. Bone Regeneration, Reconstruction and Use of Osteogenic Cells; from Basic Knowledge, Animal Models to Clinical Trials. J. Clin. Med. 2020, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Westhri, M.; Xie, M.; Olderøy, M.Ø.; Sikorski, P.; Strand, B.L.; Standal, T. Osteogenic Differentiation of Human Mesenchymal Stem Cells in Mineralized Alginate Matrices. PLoS ONE 2015, 10, e0120374. [Google Scholar]
- Verrier, S.; Perogli, M.; Voisar, C.; Lechman, B.; Alini, M. The osteogenic differentiation of human osteoprogenitor cells on Anodic-Plasma-Chemical treated Ti6Al7Nb. Biomaterials 2011, 32, 672. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Bergemann, C.; Duske, K.; Nebe, J.B.; Schöne, A.; Bulnheim, U.; Seitz, H.; Fischer, J. Microstructured zirconia surfaces modulate osteogenic marker genes in human primary osteoblasts. J. Mater. Sci. Mater. Med. 2015, 26, 5350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Wu, S.; Li, K.; Fan, Y.; Dunne, N.; Li, X. Peptide-modified bone repair materials: Factors influencing osteogenic activity. J. Biomed. Mater. Res. Part A 2019, 107, 1491–1512. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509. [Google Scholar] [CrossRef] [Green Version]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.A. Bioceramics for implant coatings. Mater. Today 2003, 6, 26–30. [Google Scholar] [CrossRef]
- Macha, I.J.; Cazalbou, S.; Ben-Nissan, B.; Harvey, K.L.; Milthorpe, B. Marine structure derived calcium phosphate-polymer biocomposites for local antibiotic delivery. Mar. Drugs 2015, 13, 666. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.H. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y.-R.V.; Hwang, Y.; Phadke, A.; Kang, H.; Hwang, N.S.; Caro, E.J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.C.; et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhao, L.; Liu, J.; Weir, M.D.; Zhou, X.; Xu, H.H.K. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014, 2, 14017. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.-X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, L.; Ni, L.; Qiao, C.; Li, D.; Sun, H.; Zhang, Z. The effect of synthetic α-tricalcium phosphate on osteogenic differentiation of rat bone mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 1588. [Google Scholar] [PubMed]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, H.O.; McCaffrey, J.; McCrudden, C.M.; Zholobenko, A.; Ali, A.A.; McBride, J.W.; Massey, A.S.; Pentlavalli, S.; Chen, K.-H.; Cole, G.; et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J. Control. Release 2014, 189, 141. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.; Yakkundi, A.; McKeen, H.D.; McClements, L.; McKeogh, T.J.; McCrudden, C.M.; Arthur, K.; Robson, T.; McCarthy, H.O. RALA-mediated delivery of FKBPL nucleic acid therapeutics. Nanomedicine 2015, 10, 2989. [Google Scholar] [CrossRef] [Green Version]
- McCaffrey, J.; McCrudden, C.M.; Ali, A.A.; Massey, A.S.; McBride, J.W.; McCrudden, M.T.C.; Vicente-Perez, E.M.; Coulter, J.A.; Robson, T.; Donnelly, R.F.; et al. Transcending epithelial and intracellular biological barriers; a prototype DNA delivery device. J. Control. Release 2016, 226, 238. [Google Scholar] [CrossRef]
- Massey, A.S.; Pentlavalli, S.; Cunningham, R.; McCrudden, C.M.; McErlean, E.M.; Redpath, P.; Ali, A.A.; Annett, S.; McBride, J.W.; McCaffrey, J.; et al. Potentiating the anticancer properties of bisphosphonates by nanocomplexation with the cationic amphipathic peptide, RALA. Mol. Pharm. 2016, 13, 1217. [Google Scholar] [CrossRef]
- Jack, V.; Buchanan, F.J.; Dunne, N.J. Particle attrition of alpha-tricalcium phosphate: Effect on mechanical, handling, and injectability properties of calcium phosphate cements. Proc. Inst. Mech. Eng. H 2008, 222, 19. [Google Scholar] [CrossRef]
- O’Hara, R.M.; Dunne, N.J.; Orr, J.F.; Buchanan, F.J.; Wilcox, R.X.; Barton, D.C. Optimisation of the mechanical and handling properties of an injectable calcium phosphate cement. J. Mater. Sci. Mater. Med. 2010, 21, 2299. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Fernández, E.; De Maeyer, E.A.; Verbeeck, R.M.; Boltong, M.G.; Ginebra, J.; Driessens, F.C.; Planell, J.A. Setting Reaction and Hardening of an Apatitic Calcium Phosphate Cement. J. Dent. Res. 1997, 76, 905. [Google Scholar] [CrossRef]
- Dunfield, D.; Sayer, M.; Shurvell, H.F. Total Attenuated Reflection Infrared Analysis of Silicon-Stabilized Tri-Calcium Phosphate. J. Phys. Chem. B 2005, 109, 19579. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.W.; Wu, T.; Wang, Q.; Pan, H.H. Molecular simulation of protein adsorption and desorption on hydroxyapatite surfaces. Biomaterials 2008, 29, 513. [Google Scholar] [CrossRef] [PubMed]
- Farbod, K.; Nejadnik, M.R.; Jansen, J.A.; Leeuwenburgh, S.C. Interactions between inorganic and organic phases in bone tissue as a source of inspiration for design of novel nanocomposites. Tissue Eng. Part B Rev. 2014, 20, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levingstone, T.J.; Herbaj, S.; Redmond, J.; McCarthy, H.O.; Dunne, N.J. Calcium Phosphate Nanoparticles-Based Systems for RNAi Delivery: Applications in Bone Tissue Regeneration. Nanomaterials 2020, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, V.; Kozlova, D.; Knuschke, T.; Buer, J.; Westendorf, A.M.; Epple, M. Mechanism of the uptake of cationic and anionic calcium phosphate nanoparticles by cells. Acta Biomater. 2013, 9, 7527. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of nanoparticles in medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Pareta, R.A. Calcium Phosphate Nanoparticles: Toxicology and Lymph Node Targeting for Cancer Metastasis Prevention. In Safety of Nanoparticles. Nanostructure Science and Technology; Webster, T., Ed.; Springer: New York, NY, USA, 2009; p. 189. [Google Scholar]
- Sommer, A.P. Cytotoxicity of Calcium Phosphate Crystals and Human-Derived Nanoparticles: An Overlooked Link. Circ. Res. 2010, 106, 10. [Google Scholar] [CrossRef] [Green Version]
- Qing, F.; Wang, Z.; Hong, Y.; Liu, M.; Guo, B.; Luo, H.; Zhang, X. Selective effects of hydroxyapatite nanoparticles on osteosarcoma cells and osteoblasts. J. Mater. Sci. Mater. Med. 2012, 23, 2245. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, G.; Liu, H.; Niu, X.; Han, J.; Zheng, L.; Fan, Y. Nano-hydroxyapatite particles induce apoptosis on MC3T3-E1 cells and tissue cells in SD rats. Nanoscale 2012, 4, 2894. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338. [Google Scholar] [CrossRef]
- Ooi, J.P.; Kasim, S.R.B.; Saidin, N.A. Cytotoxicity of Nano β–Tricalcium Phosphate (β-TCP) on Human Osteoblast (hFOB1.19). Int. J. Med. Health Sci. 2015, 9, 12. [Google Scholar]
- Ewence, A.E.; Bootman, M.; Roderick, H.L.; Skepper, J.N.; McCarthy, G.; Epple, M.; Neumann, M.; Shanahan, C.M.; Proudfoot, D. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: A potential mechanism in atherosclerotic plaque destabilization. Circ. Res. 2008, 103, e28-34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43. [Google Scholar]
- Taylor, S.E.; Shah, M.; Orriss, I.R. Generation of rodent and human osteoblasts. Bonekey Rep. 2014, 3, 585. [Google Scholar] [CrossRef] [Green Version]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, 131. [Google Scholar] [CrossRef] [Green Version]
- Bonjour, J.-P. Calcium and phosphate: A duet of ions playing for bone health. J. Am. Coll. Nutr. 2011, 30, 438S–448S. [Google Scholar] [CrossRef]
- Chang, Y.L.; Stanford, C.M.; Keller, J.C. Calcium and phosphate supplementation promotes bone cell mineralization: Implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000, 52, 270. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Doherty, M.; Mulholland, E.J.; Chambers, P.; Pentlavalli, S.; Ziminska, M.; Chalanqui, M.J.; Pauly, H.M.; Sathy, B.N.; Donahue, T.H.; Kelly, D.J.; et al. Improving the Intercellular Uptake and Osteogenic Potency of Calcium Phosphate via Nanocomplexation with the RALA Peptide. Nanomaterials 2020, 10, 2442. https://doi.org/10.3390/nano10122442
O’Doherty M, Mulholland EJ, Chambers P, Pentlavalli S, Ziminska M, Chalanqui MJ, Pauly HM, Sathy BN, Donahue TH, Kelly DJ, et al. Improving the Intercellular Uptake and Osteogenic Potency of Calcium Phosphate via Nanocomplexation with the RALA Peptide. Nanomaterials. 2020; 10(12):2442. https://doi.org/10.3390/nano10122442
Chicago/Turabian StyleO’Doherty, Michelle, Eoghan J. Mulholland, Philip Chambers, Sreekanth Pentlavalli, Monika Ziminska, Marine J. Chalanqui, Hannah M. Pauly, Binulal N. Sathy, Tammy H. Donahue, Daniel J. Kelly, and et al. 2020. "Improving the Intercellular Uptake and Osteogenic Potency of Calcium Phosphate via Nanocomplexation with the RALA Peptide" Nanomaterials 10, no. 12: 2442. https://doi.org/10.3390/nano10122442