Enhanced Photocatalytic Hydrogen Production of the Polyoxoniobate Modified with RGO and PPy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
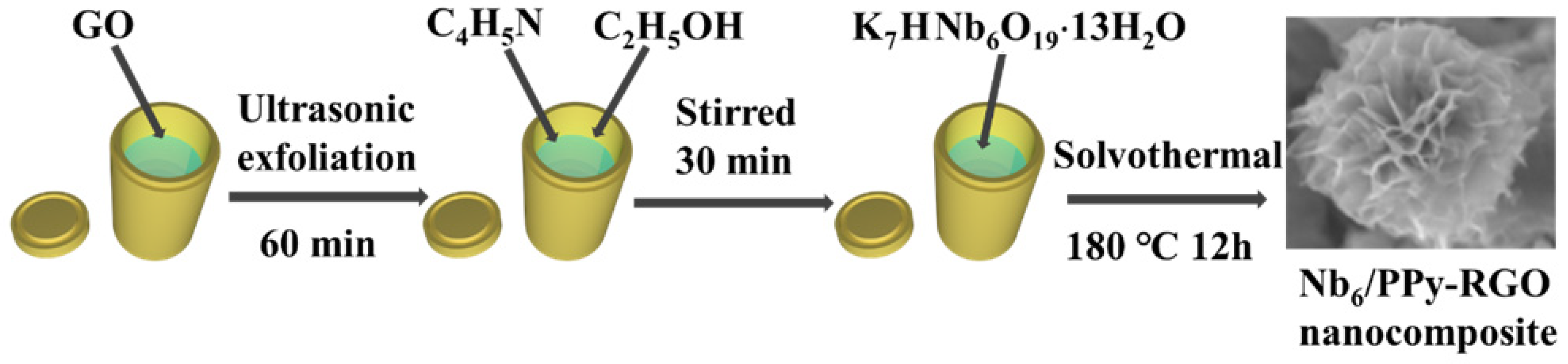
2.2. Synthesis of Nb6/PPy-RGO
2.3. Characterization
2.4. Photocatalytic Hydrogen Production
2.5. Photocatalytic Measurement
3. Results and Discussion
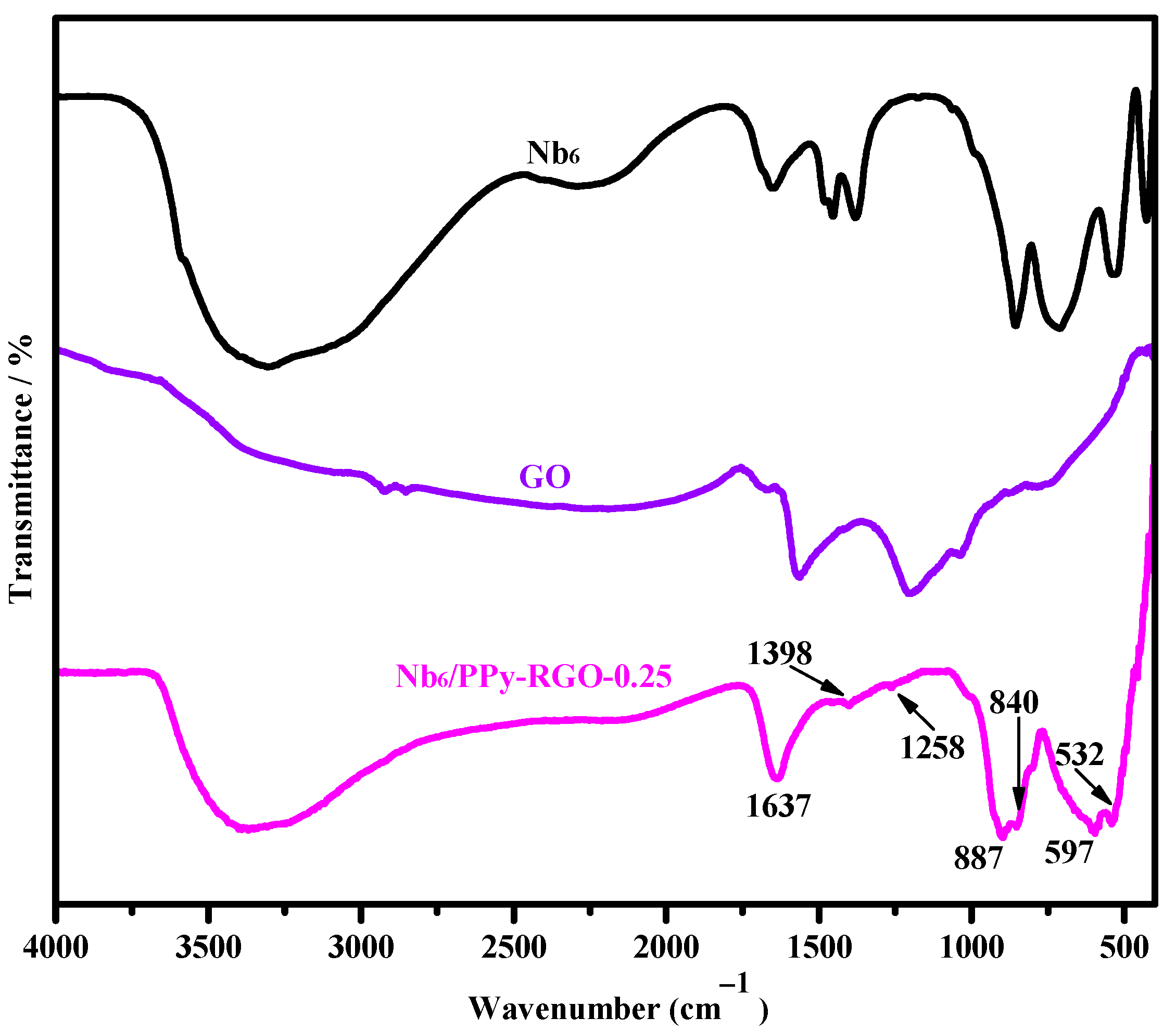
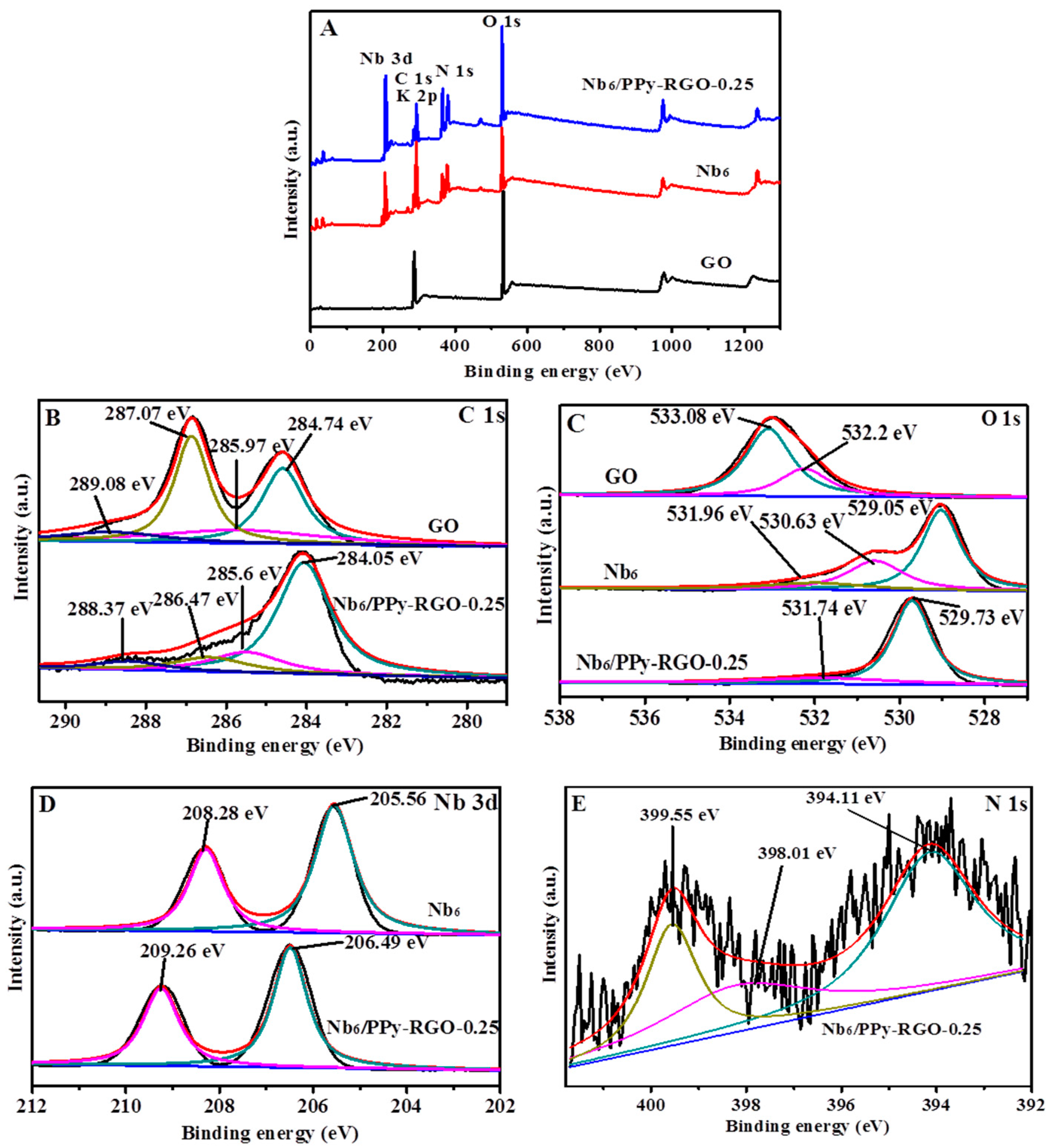
3.1. Structural Characterizations
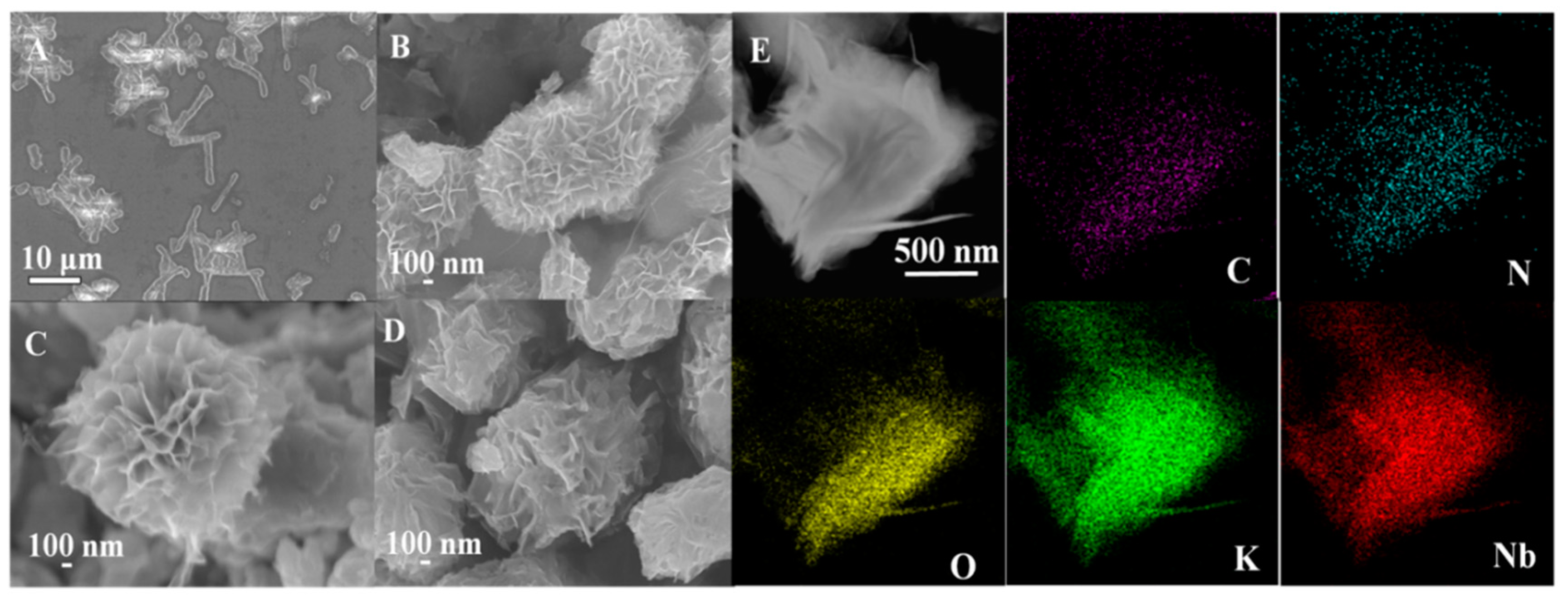
3.2. Nanosphere Morphologies
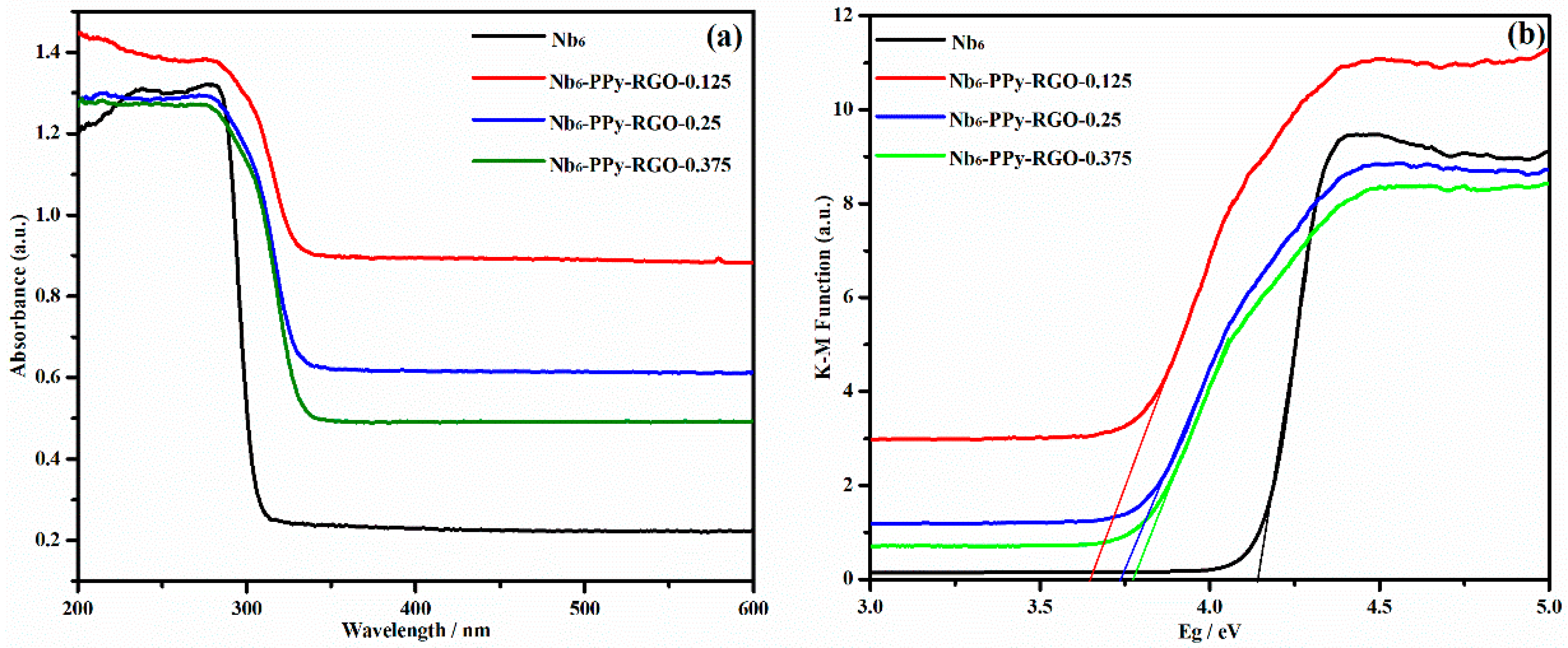
3.3. Photochemistry and Electrochemistry
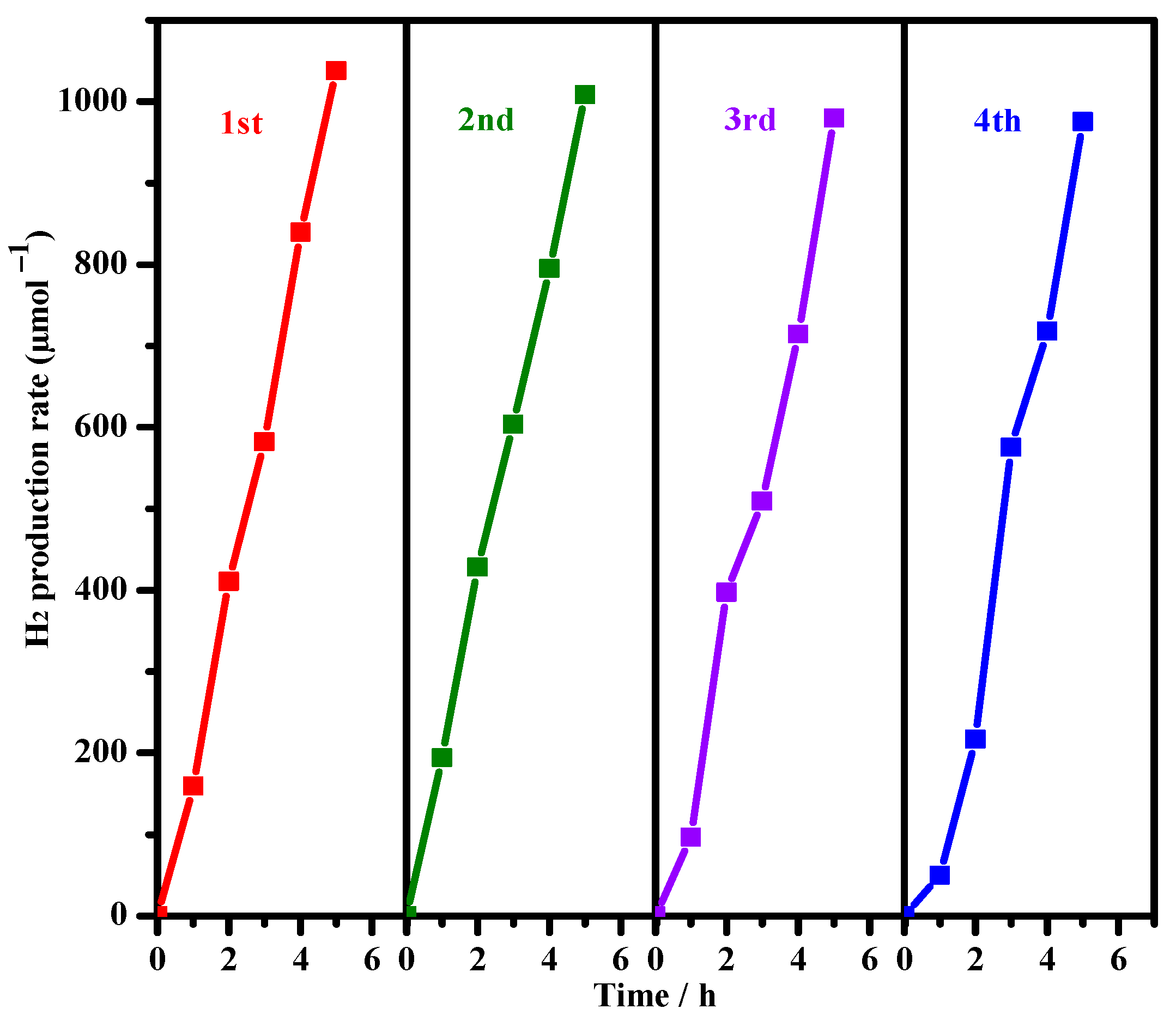
3.4. Photocatalytic Hydrogen Evolution
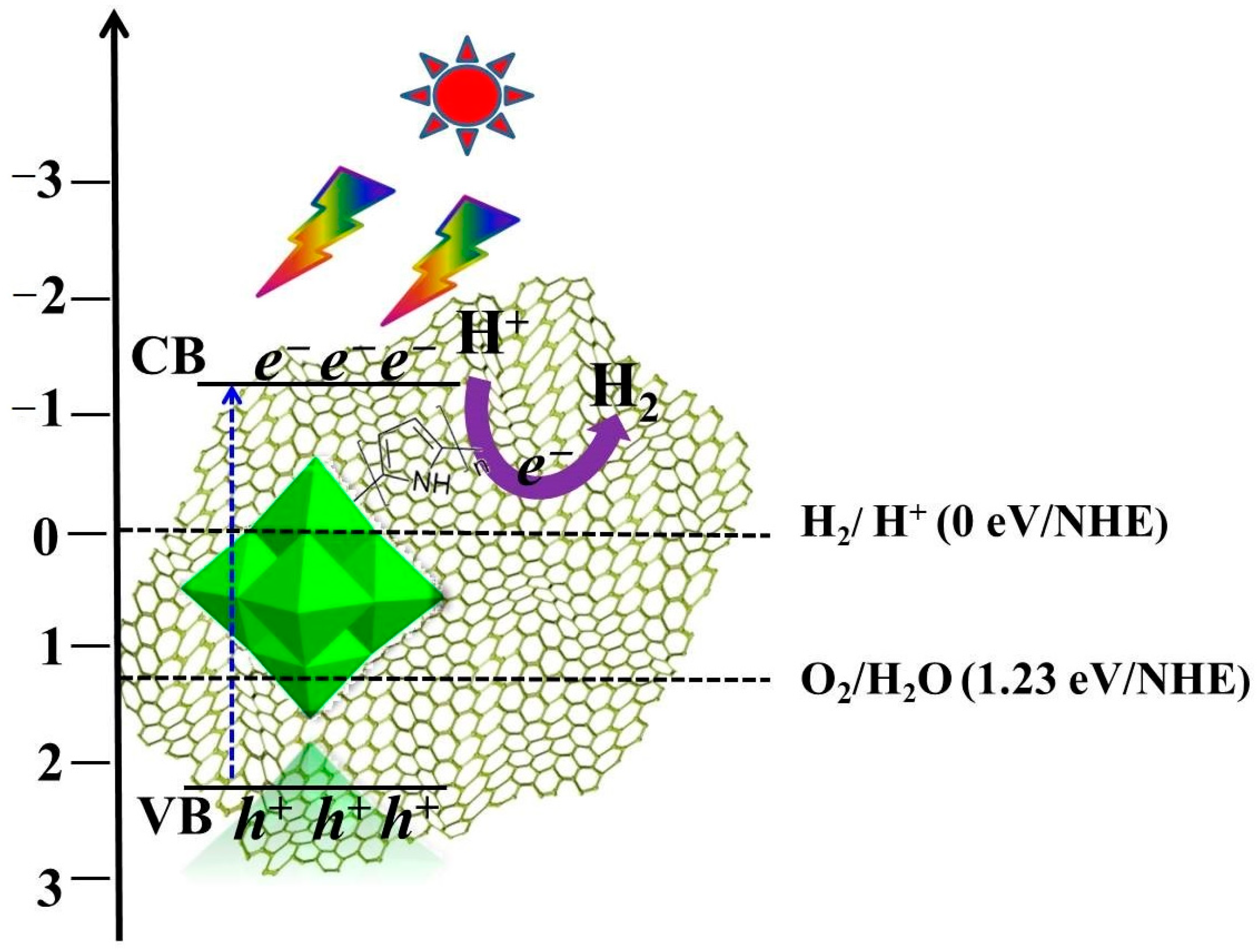
3.5. Proposed Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Sun, Y.; Yuan, Z.Y.; Zhu, Y.P.; Ma, T.Y. Titanium Phosphonate Based Metal-Organic Frameworks with Hierarchical Porosity for Enhanced Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 3222–3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yu, J.G.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, G.; Liu, Y.; Liu, H.; Qu, J.; Li, J. Highly Active and Stable Catalysts of Phytic Acid-Derivative Transition Metal Phosphides for Full Water Splitting. J. Am. Chem. Soc. 2016, 138, 14686–14693. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.M.; Zhu, L.L.; Ong, W.L.; Wang, L.; Ho, G.W. Structural design of TiO2-based photocatalyst for H2 production and degradation applications. Catal. Sci. Technol. 2015, 5, 4703–4726. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Li, L.; Zhu, S.; Hao, R.; Wang, J.J.; Yang, E.C.; Zhao, X.J. Amino group promoted photocatalytic hydrogen evolution activity observed in two copper(ii)–based layered complexes. Dalton Trans. 2018, 47, 12726–12733. [Google Scholar] [CrossRef]
- Zhao, X.X.; Feng, J.R.; Liu, J.W.; Lu, J.; Shi, W.; Yang, G.M.; Wang, G.C.; Feng, P.Y.; Cheng, P. Metal-Organic Framework-Derived ZnO/ZnS Heteronanostructures for Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. Adv. Sci. 2018, 5, 1700590–1700598. [Google Scholar] [CrossRef]
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Kudaibergenov, S.; Nuraje, N. A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials. J. Mater. Chem. A 2018, 6, 21696–21718. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N. Solar light harvesting with multinary metal chalcogenide nanocrystals. Chem. Soc. Rev. 2018, 47, 5354–5422. [Google Scholar] [CrossRef]
- Kumar, P.; Boukherroub, R.; Shankar, K. Sunlight-driven water-splitting using two-dimensional carbon based semiconductors. J. Mater. Chem. A 2018, 6, 12876–12931. [Google Scholar] [CrossRef]
- Li, S.J.; Liu, S.M.; Liu, S.X.; Liu, Y.W.; Tang, Q.; Shi, Z.; Ou yang, S.X.; Ye, J.H. {Ta12}/{Ta16} Cluster-Containing Polytantalotungstates with Remarkable Photocatalytic H2 Evolution Activity. J. Am. Chem. Soc. 2012, 138, 19716–19721. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.S.; Du, D.Y.; Guan, W.; Bo, X.J.; Li, Y.F.; Guo, L.P.; Su, Z.M.; Wang, Y.Y.; Lan, Y.Q.; Zhou, H.C. Ultrastable Polymolybdate-Based Metal-Organic Frameworks as Highly Active Electrocatalysts for Hydrogen Generation from Water. J. Am. Chem. Soc. 2015, 137, 7169–7177. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, Y.; Zheng, M. Polyoxometalate-based manganese clusters as catalysts for efficient photocatalytic and electrochemical water oxidation. Appl. Catal. B Environ. 2017, 209, 45–52. [Google Scholar] [CrossRef]
- Geletii, Y.V.; Botar, B.; Kögerler, P.; Hillesheim, D.A.; Musaev, D.G.; Hill, C.L. An All-Inorganic, Stable, and Highly Active Tetraruthenium Homogeneous Catalyst for Water Oxidation. Angew. Chem. Int. Ed. 2008, 47, 3896–3899. [Google Scholar] [CrossRef]
- Du, X.Q.; Zhao, J.L.; Mi, J.Q.; Ding, Y.; Zhou, P.P.; Ma, B.C.; Zhao, J.W.; Song, J. Efficient photocatalytic H2 evolution catalyzed by an unprecedented robust molecular semiconductor {Fe11} nanocluster without cocatalysts at neutral conditions. Nano Energy 2015, 16, 247–255. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.L.; Wang, X.L.; Li, Y.G.; Su, Z.M.; Wang, E.B. Polyoxometalates in dye-sensitized solar cells. Chem. Soc. Rev. 2019, 48, 260–284. [Google Scholar] [CrossRef]
- Troupis, A.; Hiskia, A.; Papaconstantinou, E. Photocatalytic Reduction and Recovery of Copper by Polyoxometalates. Environ. Sci. Technol. 2002, 36, 5355–5362. [Google Scholar] [CrossRef]
- Kornarakis, I.; Lykakis, I.N.; Vordos, N.; Armatas, G.S. Efficient visible-light photocatalytic activity by band alignment in mesoporous ternary polyoxometalate–Ag2S–CdS semiconductors. Nanoscale 2014, 6, 8694–8703. [Google Scholar] [CrossRef]
- Hu, B.; Wang, P.F.; Hou, J.; Wang, C.; Qian, J.; Zhang, N.N.; Yuan, Q.S. Effects of titanium dioxide (TiO2) nanoparticles on the photodissolution of particulate organic matter: Insights from fluorescence spectroscopy and environmental implications. Environ. Pollut. 2017, 229, 19–28. [Google Scholar] [CrossRef]
- Park, H.; Choi, W. Photocatalytic conversion of benzene to phenol using modified TiO2 and polyoxometalates. Catal. Today 2005, 101, 291–297. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, X. Polyoxometalates-derived metal oxides incorporated into graphitic carbon nitride framework for photocatalytic hydrogen peroxide production under visible light. J. Catal. 2018, 366, 98–106. [Google Scholar] [CrossRef]
- Li, K.X.; Yan, L.S.; Zeng, Z.X.; Luo, S.L.; Luo, X.B.; Liu, X.M.; Guo, H.Q.; Guo, Y.H. Fabrication of H3PW12O40-doped carbon nitride nanotubes by one–step hydrothermal treatment strategy and their efficient visible-light photocatalytic activity toward representative aqueous persistent organic pollutants degradation. Appl. Catal. B Environ. 2014, 156–157, 141–152. [Google Scholar] [CrossRef]
- Gamelas, J.A.F.; Evtuguin, D.V.; Esculcas, A.P. Transition metal substituted polyoxometalates supported on amine-functionalized silica. Transit. Met. Chem. 2007, 32, 1061–1067. [Google Scholar] [CrossRef]
- Sajjad, S.; Leghari, S.A.K.; Iqbal, A. Study of Graphene Oxide Structural Features for Catalytic, Antibacterial, Gas Sensing, and Metals Decontamination Environmental Applications. ACS Appl. Mater. Inter. 2017, 9, 43393–43414. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Graphene-Based Nanoassemblies for Energy Conversion. J. Phys. Chem. Lett. 2011, 2, 242–251. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.H.; Xu, Y.J. Recent progress on graphene-based photocatalysts: Current status and future perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.C.; Tang, D.; Zhang, X.; Yan, L.K.; Han, Y.Z.; Huang, H.; Liu, Y.; Kang, Z.H. Carbon Quantum Dot/Silver Nanoparticle/Polyoxometalate Composites as Photocatalysts for Overall Water Splitting in Visible Light. ChemCatChem 2014, 6, 2634–2641. [Google Scholar] [CrossRef]
- Liu, G.D.; Zhao, X.F.; Zhang, J.; Liu, S.J.; Sha, J.Q. Z-scheme Ag3PO4/POM/GO heterojunction with enhanced photocatalytic performance for degradation and water splitting. Dalton Trans. 2018, 47, 6225–6232. [Google Scholar] [CrossRef]
- Tang, Y.J.; Wang, Y.; Wang, X.L.; Li, S.L.; Huang, W.; Dong, L.Z.; Liu, C.H.; Li, Y.F.; Lan, Y.Q. Molybdenum Disulfide/Nitrogen-Doped Reduced Graphene Oxide Nanocomposite with Enlarged Interlayer Spacing for Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1600116. [Google Scholar] [CrossRef]
- Tang, Y.J.; Liu, C.H.; Huang, W.; Wang, X.L.; Dong, L.Z.; Li, S.L.; Lan, Y.Q. Bimetallic Carbides-Based Nanocomposite as Superior Electrocatalyst for Oxygen Evolution Reaction. ACS Appl. Mater. Inter. 2017, 9, 16977–16985. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Yoshimi, I.; Kudo, A.; Amal, R. Reduced Graphene Oxide as a Solid-State Electron Mediator in Z-Scheme Photocatalytic Water Splitting under Visible Light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Zhang, M.; Wu, P.; Tang, Y.J.; Li, S.L.; Shen, F.C.; Wang, X.L.; Zhou, X.P.; Lan, Y.Q. POM-based metal-organic framework/reduced graphene oxide nanocomposites with hybrid behavior of battery-supercapacitor for superior lithium storage. Nano Energy 2017, 34, 205–214. [Google Scholar] [CrossRef]
- Li, H.L.; Gupta, J.; Wang, S.; Zhang, N.; Bubeck, C. Photoreduction of graphene oxide with polyoxometalate clusters and its enhanced saturable absorption. J. Colloid Interface Sci. 2014, 427, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Xiao, A.X.; Hou, G.H.; Li, H.T.; Guo, T.; Guan, B.O. Photocatalysis in an evanescent field: An in situ approach to studying photocatalytic performance by tracing interfacial refractive index changes and kinetics. J. Mater. Chem. A 2018, 6, 20513–20522. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.Q.; Liu, S.Q.; Sun, Y.G.; Xu, Y.J. Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Wang, Z.L.; Tan, H.Q.; Chen, W.L.; Li, Y.G.; Wang, E.B. A copper(ii)-ethylenediamine modified polyoxoniobate with photocatalytic H2 evolution activity under visible light irradiation. Dalton Trans. 2012, 41, 9882–9884. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.Q.; Zhang, Y.; Zhang, Z.M.; Li, Y.G.; Gao, Y.Q.; Wang, E.B. Polyoxoniobate-based 3D framework materials with photocatalytic hydrogen evolution activity. Chem. Commun. 2014, 50, 6017–6019. [Google Scholar] [CrossRef]
- Geng, Q.H.; Liu, Q.S.; Ma, P.T.; Wang, J.P.; Niu, J.Y. Synthesis crystal structure and photocatalytic properties of an unprecedented arsenic-disubstituted Lindqvist-type peroxopolyoxoniobate ion: {As2Nb4(O2)4O14H1.5}4.5−. Dalton Trans. 2014, 43, 9843–9846. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Wang, Y.; Zhang, X.N.; Chi, Y.N.; Yang, S.; Li, J.K.; Hu, C.W. Controllable Assembly of Vanadium-Containing Polyoxoniobate-Based Three-Dimensional Organic-Inorganic Hybrid Compounds and Their Photocatalytic Properties. Inorg. Chem. 2016, 55, 7501–7507. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.Y.; Li, F.; Zhao, J.W.; Ma, P.T.; Zhang, D.D.; Bassil, B.; Kortz, U.; Wang, J.P. Tetradecacobalt(II)-Containing 36-Niobate [Co14(OH)16(H2O)8Nb36O106]20− and Its Photocatalytic H2 Evolution Activity. Chem. Eur. J. 2014, 20, 9852–9857. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xie, A.M.; Sun, M.X.; Wang, Y.; Wang, M.Y. Reduced graphene oxide (RGO) modified spongelike polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Abaci, U.; Guney, H.Y.; Kadiroglu, U. Morphological and electrochemical properties of PPy, PAni bilayer films and enhanced stability of their electrochromic devices (PPy/PAni–PEDOT, PAni/PPy–PEDOT). Electrochim. Acta 2013, 96, 214–224. [Google Scholar] [CrossRef]
- Filowitz, M.; Ho, R.K.C.; Klemperer, W.G.; Shum, W. 17O nuclear magnetic resonance spectroscopy of polyoxometalates. 1. Sensitivity and resolution. Inorg. Chem. 1979, 18, 93–103. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Bao, C.L.; Song, L.; Xing, W.Y.; Yuan, B.H.; Wilkie, C.A.; Huang, J.L.; Guo, Y.Q.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Suvina, V.; Krishna, S.M.; Nagaraju, D.H.; Melo, J.S.; Balakrishna, R.G. Polypyrrole-reduced graphene oxide nanocomposite hydrogels: A promising electrode material for the simultaneous detection of multiple heavy metal ions. Mater. Lett. 2018, 232, 209–212. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, H.T.; Huang, P.R.; Xiang, C.L.; Zou, Y.J.; Xu, F.; Sun, L.X. Inducement of nanoscale Cu–BTC on nanocomposite of PPy–rGO and its performance in ammonia sensing. Mater. Res. Bull. 2018, 99, 152–160. [Google Scholar] [CrossRef]
- Liu, R.J.; Li, S.W.; Yu, X.L.; Zhang, G.J.; Zhang, S.J.; Yao, J.N.; Keita, B.; Nadjo, L.; Zhi, L.J. Facile Synthesis of Au-Nanoparticle/Polyoxometalate/Graphene Tricomponent Nanohybrids: An Enzyme-Free Electrochemical Biosensor for Hydrogen Peroxide. Small 2012, 8, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Q.; Zhang, Z.L.; Yang, M.; Li, T.X.; Wang, Y.M.; Cao, A.P.; Chen, Z.X. Facile preparation of reduced graphene oxide/polypyrrole nanocomposites with urchin-like microstructure for wide-potential-window supercapacitors. Electrochim. Acta 2018, 289, 238–247. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Gu, Y.; Nie, G.D.; Chi, M.Q.; Yang, Z.Z.; Wang, C.; Wei, Y.; Lu, X.F. Synthesis of RGO/Cu8S5/PPy Composite Nanosheets with Enhanced Peroxidase-Like Activity for Sensitive Colorimetric Detection of H2O2 and Phenol. Part. Part. Syst. Charact. 2017, 3, 1600233–1600240. [Google Scholar] [CrossRef]
- Gan, Q.Y.; Shi, W.L.; Xing, Y.J.; Hou, Y.A. Polyoxoniobate/g-C3N4 Nanoporous Material with High Adsorption Capacity of Methylene Blue from Aqueous Solution. Front. Chem. 2018, 6, 7–16. [Google Scholar] [CrossRef]
- Aureliano, M.; Ohlin, C.A.; Vieira, M.O.; Marques, M.P.M.; Casey, W.H.; Batista de Carvalho, L.A.E. Characterization of decavanadate and decaniobate solutions by Raman spectroscopy. Dalton Trans. 2016, 45, 7391–7399. [Google Scholar] [CrossRef] [Green Version]
- Fullmer, L.B.; Malmberg, C.E.; Fast, D.B.; Wills, L.A.; Cheong, P.H.Y.; Dolgos, M.R.; Nyman, M. Aqueous tantalum polyoxometalate reactivity with peroxide. Dalton Trans. 2017, 46, 8486–8493. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Ma, J.; He, Y.; Shi, H.; Fan, Y.; Yu, H.; Li, Y.B. Stable graphene oxide-based composite membranes intercalated with montmorillonite nanoplatelets for water purification. J. Mater. Sci. 2019, 54, 2241–2255. [Google Scholar] [CrossRef]
- Zhang, L.B.; Li, X.T.; Wang, X.H.; Han, L.; Liu, R.Y.; Zheng, B.B.; Li, W.T.; Liu, X.L.; Liu, Y.C. Photo-assisted preparation and patterning of large-area reduced graphene oxide-TiO2 conductive thin film. Chem. Commun. 2010, 46, 3499–3501. [Google Scholar]
- Liu, Q.Q.; Shen, J.Y.; Yang, X.F.; Zhang, T.R.; Tang, H. 3D reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B Environ. 2018, 232, 562–573. [Google Scholar] [CrossRef]
- Bai, X.; Du, Y.Y.; Hu, X.Y.; He, Y.D.; He, C.L.; Liu, E.Z.; Fan, J. Synergy removal of Cr (VI) and organic pollutants over RP-MoS2/rGO photocatalyst. Appl. Catal. B Environ. 2018, 239, 204–213. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.H.; Fan, D.Y.; Chen, S.H. First-principles investigation on the electronic property and bonding configuration of NbC (111)/NbN (111) interface. J. Alloys Compd. 2016, 689, 874–884. [Google Scholar] [CrossRef]
- Kulkarni, A.K.; Praveen, C.S.; Sethi, Y.A.; Panman, R.P.; Arbuj, S.S.; Naik, S.D.; Ghule, A.V.; Kale, B.B. Nanostructured N-doped orthorhombic Nb2O5 as an efficient stable photocatalyst for hydrogen generation under visible light. Dalton Trans. 2017, 46, 14859–14868. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.F.; Li, F.Y.; Sun, Z.X.; Liu, M.M.; Wang, Y.H.; Xu, L. Synergetic effect of polyoxoniobate and NiS as cocatalysts for enhanced photocatalytic H2 evolution on Cd0.65Zn0.35S. RSC Adv. 2014, 4, 21369–21372. [Google Scholar] [CrossRef]
- Li, T.; Wang, K.; Fang, Q.T.; Zhang, Y.; Wang, B.; Li, R.; Lin, Y.Z.; Liu, K.C.; Xie, H.Q.; Li, K. Conductive polymer supported and confined iron phosphide nanocrystals for boosting the photocatalytic hydrogen production of graphitic carbon nitride. J. Mater. Chem. C 2020, 8, 14540–14547. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Shen, C.; Lu, G.X.; Ni, Y.R.; Lu, C.H.; Xu, Z.Z. Synthesis of PPy/RGO-based hierarchical material with super-paramagnetic behavior and understanding its robust photo current driven by visible light. Synth. Met. 2018, 241, 17–25. [Google Scholar] [CrossRef]
- Yuan, X.J.; Dragoe, D.; Beaunier, P.; Uribe, D.B.; Ramos, L.; Méndez-Medrano, M.G.; Remita, H. Polypyrrole nanostructures modified with monoand bimetallic nanoparticles for photocatalytic H2 generation. J. Mater. Chem. A 2020, 8, 268–277. [Google Scholar] [CrossRef]
- Wang, K.L.; Endo-Kimura, M.; Belchi, R.; Zhang, D.; Habert, A.; Bouclé, J.; Ohtani, B.; Kowalska, E.; Herlin-Boime, N. Carbon/Graphene-Modified Titania with Enhanced Photocatalytic Activity under UV and Vis Irradiation. Materials 2019, 12, 4158. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.J.; Floresyona, D.; Aubert, P.H.; Bui, T.T.; Remita, S.; Ghosh, S.; Brisset, F.; Fabrice, G.; Remita, H. Photocatalytic degradation of organic pollutant with polypyrrole nanostructures under UV and visible light. Appl. Catal. B Environ. 2019, 242, 284–292. [Google Scholar] [CrossRef]




| Samples | Nb6 | Nb6/PPy-RGO-0.125 | Nb6/PPy-RGO-0.25 | Nb6/PPy-RGO-0.375 |
|---|---|---|---|---|
| Crystalite sizes (nm) | 26 | 6 | 12 | 14 |
| Samples | Nb6 | Nb6/PPy-RGO-0.125 | Nb6/PPy-RGO-0.25 | Nb6/PPy-RGO-0.375 |
|---|---|---|---|---|
| TOF (h−1) | 137 × 10−3 | 562.6 × 10−3 | 830.4 × 10−3 | 368 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heng, S.; Li, L.; Li, W.; Li, H.; Pang, J.; Zhang, M.; Bai, Y.; Dang, D. Enhanced Photocatalytic Hydrogen Production of the Polyoxoniobate Modified with RGO and PPy. Nanomaterials 2020, 10, 2449. https://doi.org/10.3390/nano10122449
Heng S, Li L, Li W, Li H, Pang J, Zhang M, Bai Y, Dang D. Enhanced Photocatalytic Hydrogen Production of the Polyoxoniobate Modified with RGO and PPy. Nanomaterials. 2020; 10(12):2449. https://doi.org/10.3390/nano10122449
Chicago/Turabian StyleHeng, Shiliang, Lei Li, Weiwei Li, Haiyan Li, Jingyu Pang, Mengzhen Zhang, Yan Bai, and Dongbin Dang. 2020. "Enhanced Photocatalytic Hydrogen Production of the Polyoxoniobate Modified with RGO and PPy" Nanomaterials 10, no. 12: 2449. https://doi.org/10.3390/nano10122449





