On the Primary Water Radicals’ Production in the Presence of Gold Nanoparticles: Electron Pulse Radiolysis Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electron Pulse Radiolysis
2.2. Gold Nanoparticles Synthesis
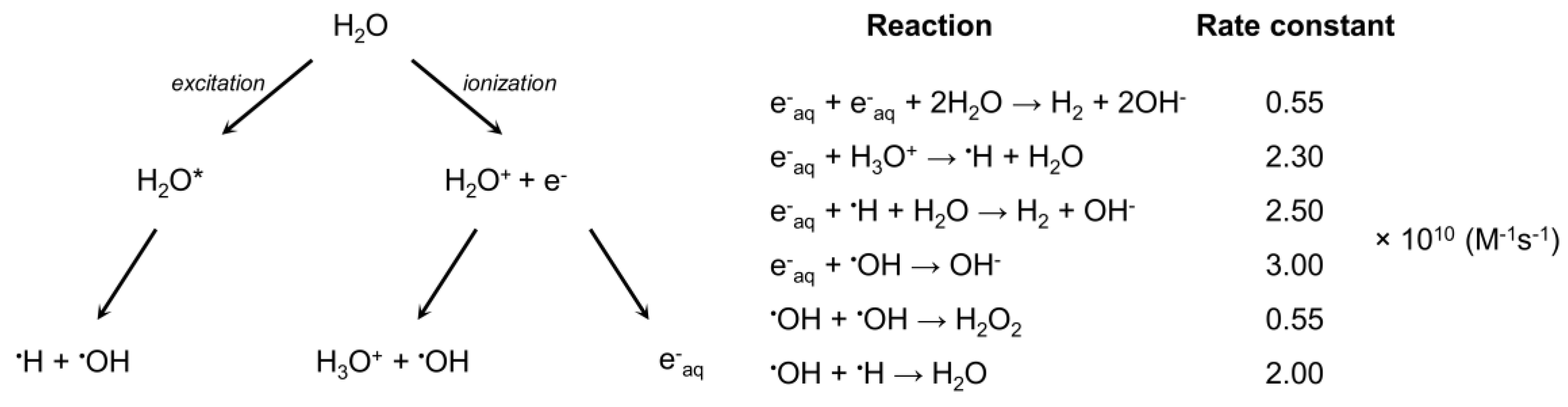
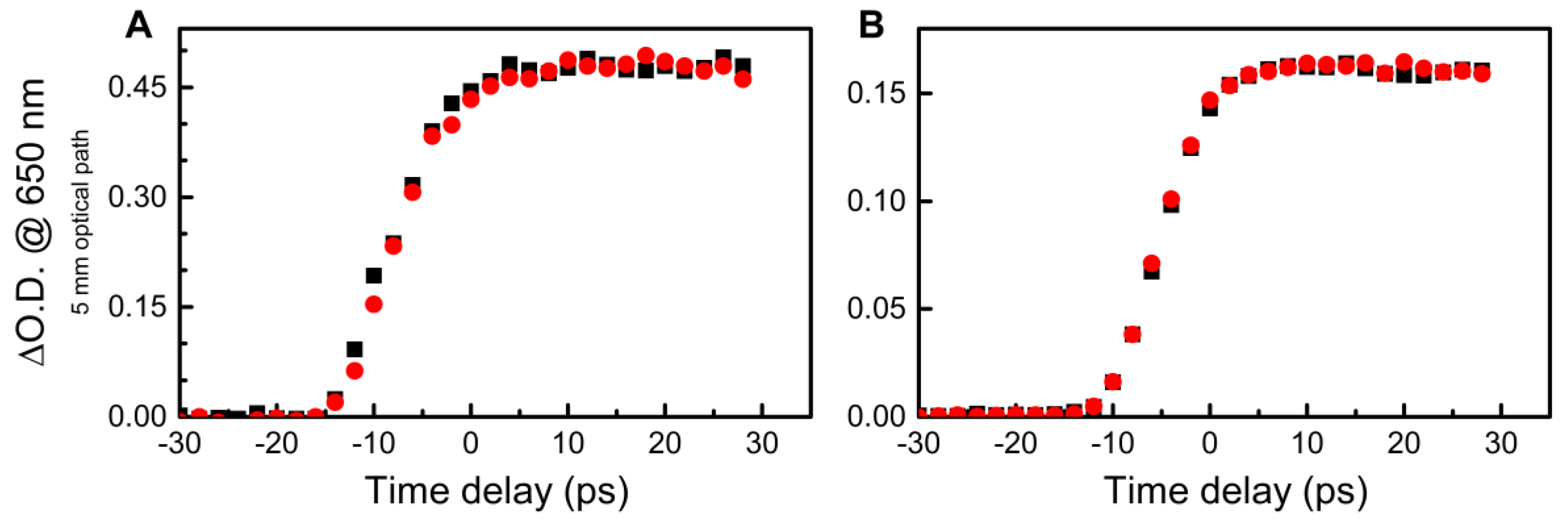
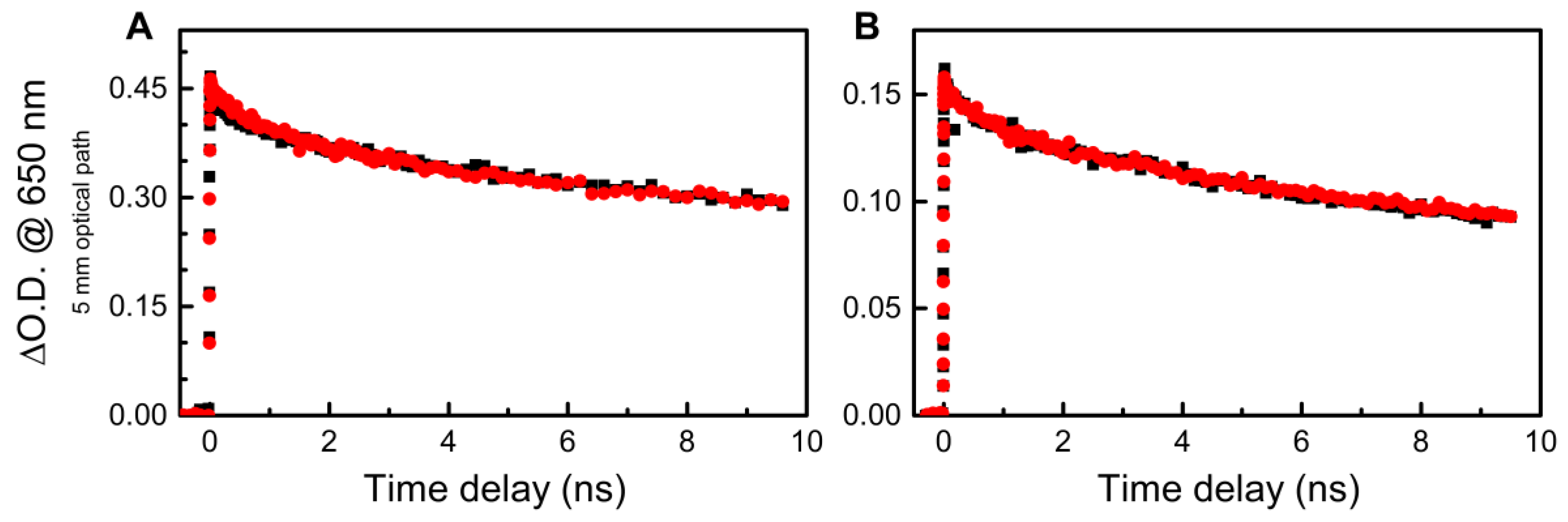
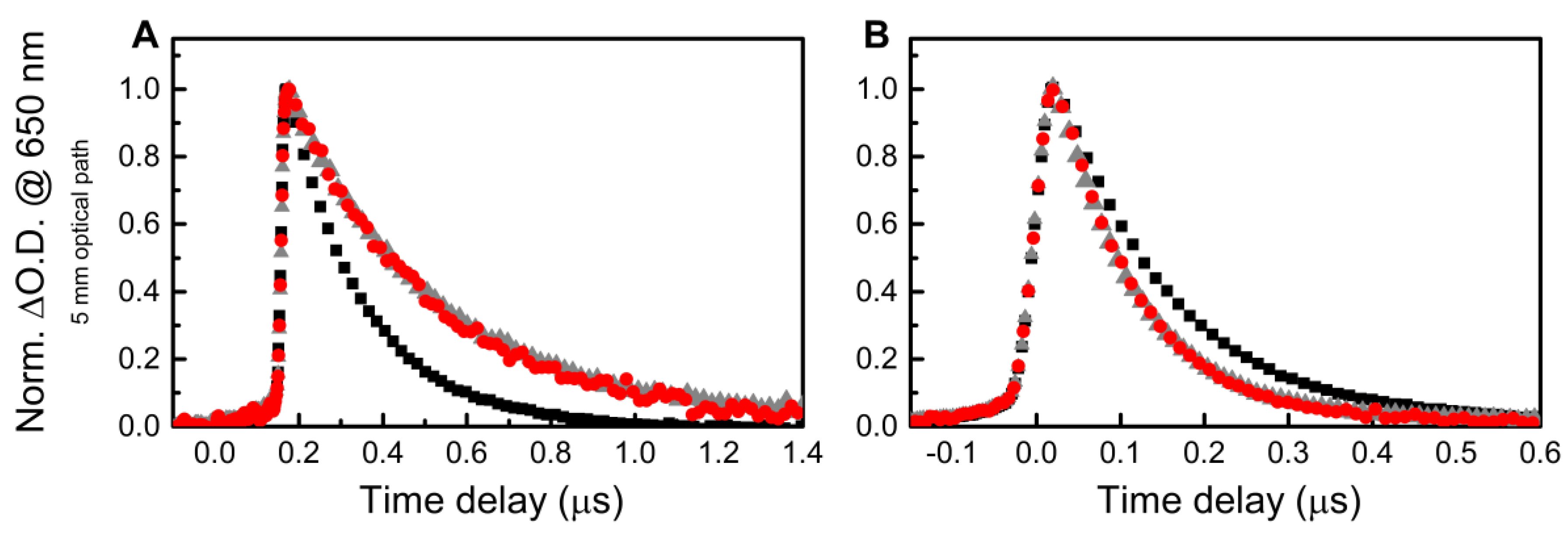
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Subiel, A.; Ashmore, R.; Schettino, G. Standards and methodologies for characterizing radiobiological impact of high-Z nanoparticles. Theranostics 2016, 6, 1651–1671. [Google Scholar] [CrossRef] [PubMed]
- Regulla, D.F.; Hiebert, L.B.; Seidenbusch, M. Physical and Biological Interface Dose Effects in Tissue due to X-Ray- Induced Release of Secondary Radiation from Metallic Gold Surfaces. Radiat. Res. 1998, 150, 92–100. [Google Scholar] [CrossRef]
- Herold, D.M.; Das, I.J.; Stobbe, C.C.; Iyer, R.V.; Chapman, J.D. Gold microspheres: A selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000, 76, 1357–1364. [Google Scholar]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Howard, D.; Sebastian, S.; Le, Q.V.C.; Thierry, B.; Kempson, I. Chemical mechanisms of nanoparticle radiosensitization and radioprotection: A review of structure-function relationships influencing reactive oxygen species. Int. J. Mol. Sci. 2020, 21, 579. [Google Scholar] [CrossRef] [Green Version]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Brun, E.; Butterworth, K.T.; Coulter, J.A.; Douki, T.; Hirst, D.G.; Jain, S.; Kavanagh, A.P.; Krpetic, Z.; et al. Energy dependence of gold nanoparticle radiosensitization in plasmid DNA. J. Phys. Chem. C 2011, 115, 20160–20167. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Turnbull, T.; Douglass, M.; Williamson, N.H.; Howard, D.; Bhardwaj, R.; Lawrence, M.; Paterson, D.J.; Bezak, E.; Thierry, B.; Kempson, I.M. Cross-Correlative Single-Cell Analysis Reveals Biological Mechanisms of Nanoparticle Radiosensitization. ACS Nano 2019, 13, 5077–5090. [Google Scholar] [CrossRef] [PubMed]
- Belyakov, O.V.; Mitchell, S.A.; Parikh, D.; Randers-Pehrson, G.; Marino, S.A.; Amundson, S.A.; Geard, C.R.; Brenner, D.J. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc. Natl. Acad. Sci. USA 2005, 102, 14203–14208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Ruiz, M.E.; Vitale, I.; Harrington, K.J.; Melero, I.; Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 2019. [Google Scholar] [CrossRef]
- Gilles, M.; Brun, E.; Sicard-Roselli, C. Quantification of hydroxyl radicals and solvated electrons produced by irradiated gold nanoparticles suggests a crucial role of interfacial water. J. Colloid Interface Sci. 2018, 525, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.; Takahashi, J. Generation of reactive oxygen species induced by gold nanoparticles under X-ray and UV Irradiations. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Belloni, J.; Monard, H.; Gobert, F.; Larbre, J.P.; Demarque, A.; De Waele, V.; Lampre, I.; Marignier, J.L.; Mostafavi, M.; Bourdon, J.C.; et al. ELYSE—A picosecond electron accelerator for pulse radiolysis research. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2005, 539, 527–539. [Google Scholar] [CrossRef]
- Marignier, J.-L.; de Waele, V.; Monard, H.; Gobert, F.; Larbre, J.-P.; Demarque, A.; Mostafavi, M.; Belloni, J. Time-resolved spectroscopy at the picosecond laser-triggered electron accelerator ELYSE. Radiat. Phys. Chem. 2006, 75, 1024–1033. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in New Robes: Key Questions Answered for the Most Common Gold Nanoparticle Synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef]
- Buxton, G. The Radiation Chemistry of Liquid Water. In Charge Particle and Photon Interactions with Matter; CRC Press: Boca Raton, FL, USA, 2003; pp. 333–363. ISBN 978-0-8247-4623-0. [Google Scholar]
- Le Caër, S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef] [Green Version]
- Torche, F.; Marignier, J.L. Direct Evaluation of the Molar Absorption Coefficient of Hydrated Electron by the Isosbestic Point Method. J. Phys. Chem. B 2016, 120, 7201–7206. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Schmidhammer, U.; Larbre, J.-P.; Zong, Z.; Marignier, J.-L.; Mostafavi, M. Time-dependent yield of the hydrated electron and the hydroxyl radical in D2O: A picosecond pulse radiolysis study. Phys. Chem. Chem. Phys. 2018, 20, 15671–15679. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Al Gharib, S.; Marignier, J.L.; El Omar, A.K.; Naja, A.; Le Caer, S.; Mostafavi, M.; Belloni, J. Key Role of the Oxidized Citrate-Free Radical in the Nucleation Mechanism of the Metal Nanoparticle Turkevich Synthesis. J. Phys. Chem. C 2019, 123, 22624–22633. [Google Scholar] [CrossRef] [Green Version]
- Ghandi, K.; Wang, F.; Landry, C. Mehran Mostafavi Naked Gold Nanoparticles and Hot Electrons in Water. Sci. Rep. 2018, 8, 7258–7264. [Google Scholar] [CrossRef] [Green Version]
- Ghandi, K.; Findlater, A.D.; Mahimwalla, Z.; MacNeil, C.S.; Awoonor-Williams, E.; Zahariev, F.; Gordon, M.S. Ultra-fast electron capture by electrosterically-stabilized gold nanoparticles. Nanoscale 2015, 7, 11545–11551. [Google Scholar] [CrossRef]
- Hermannsdörfer, J.; De Jonge, N.; Verch, A. Electron beam induced chemistry of gold nanoparticles in saline solution. Chem. Commun. 2015, 51, 16393–16396. [Google Scholar] [CrossRef]
- Nowicka, A.M.; Hasse, U.; Hermes, M.; Scholz, F. Hydroxyl radicals attack metallic gold. Angew. Chem. Int. Ed. 2010, 49, 1061–1063. [Google Scholar] [CrossRef]
- McMahon, S.J.; Paganetti, H.; Prise, K.M. Optimising element choice for nanoparticle radiosensitisers. Nanoscale 2016, 8, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Meisel, D. Catalysis of Hydrogen Production in Irradiated Aqueous Solutions by Gold Sols. J. Am. Chem. Soc. 1979, 101, 6133–6135. [Google Scholar] [CrossRef]
- Henglein, A. Reactions of organic free radicals at colloidal silver in aqueous solution. Electron pool effect and water decomposition. J. Phys. Chem. 1979, 83, 2209–2216. [Google Scholar] [CrossRef]
- Zidki, T.; Cohen, H.; Meyerstein, D. Reactions of alkyl-radicals with gold and silver nanoparticles in aqueous solutions. Phys. Chem. Chem. Phys. 2006, 8, 3552–3556. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbakov, V.; Denisov, S.A.; Mostafavi, M. On the Primary Water Radicals’ Production in the Presence of Gold Nanoparticles: Electron Pulse Radiolysis Study. Nanomaterials 2020, 10, 2478. https://doi.org/10.3390/nano10122478
Shcherbakov V, Denisov SA, Mostafavi M. On the Primary Water Radicals’ Production in the Presence of Gold Nanoparticles: Electron Pulse Radiolysis Study. Nanomaterials. 2020; 10(12):2478. https://doi.org/10.3390/nano10122478
Chicago/Turabian StyleShcherbakov, Viacheslav, Sergey A. Denisov, and Mehran Mostafavi. 2020. "On the Primary Water Radicals’ Production in the Presence of Gold Nanoparticles: Electron Pulse Radiolysis Study" Nanomaterials 10, no. 12: 2478. https://doi.org/10.3390/nano10122478
APA StyleShcherbakov, V., Denisov, S. A., & Mostafavi, M. (2020). On the Primary Water Radicals’ Production in the Presence of Gold Nanoparticles: Electron Pulse Radiolysis Study. Nanomaterials, 10(12), 2478. https://doi.org/10.3390/nano10122478





