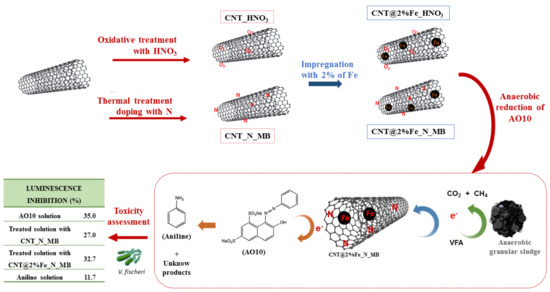
Tailoring Carbon Nanotubes to Enhance their Efficiency as Electron Shuttle on the Biological Removal of Acid Orange 10 Under Anaerobic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Carbon Materials
2.3. Dye Biodegradation Assays
2.4. Analytical Techniques
2.5. Toxicity Assessment with Vibrio Fischeri
3. Results and Discussion
3.1. Characterization of Carbon Nanomaterials
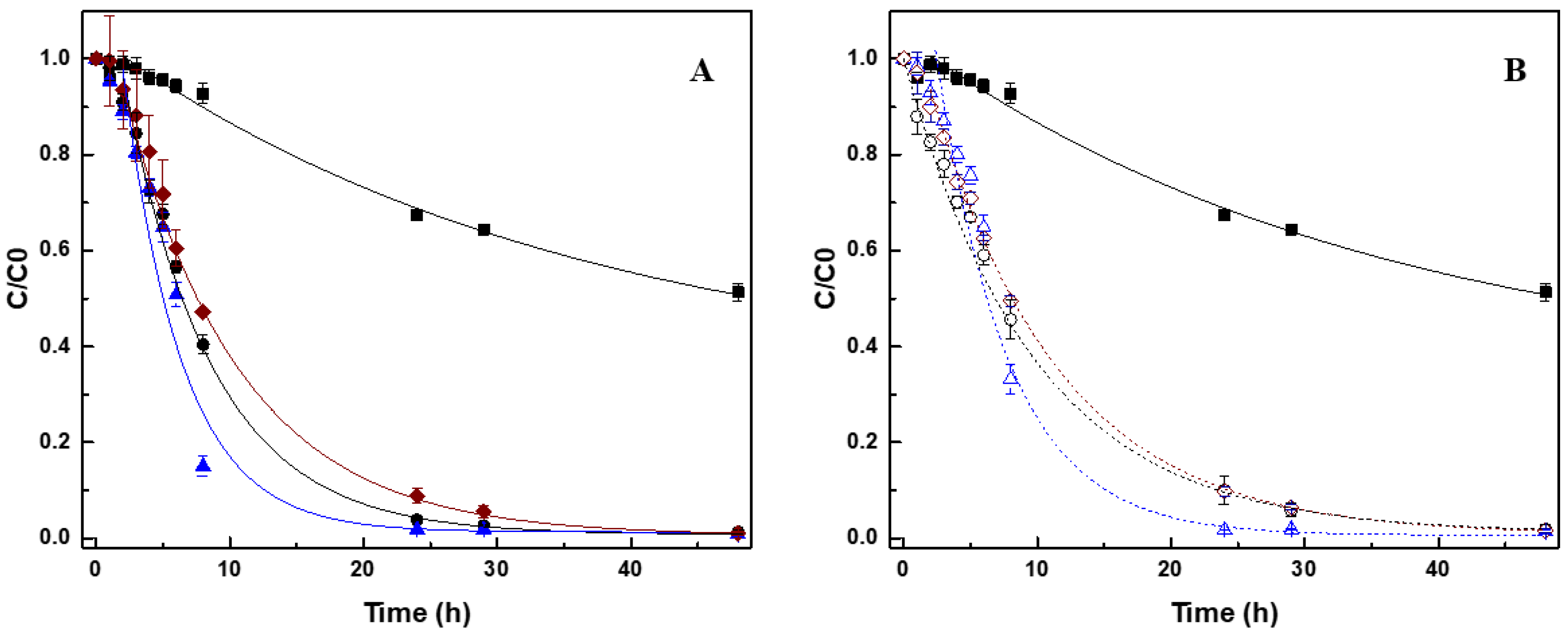
3.2. Effect of CNM on Biological Reduction of AO10
3.3. Biological Activity During AO10 Reduction
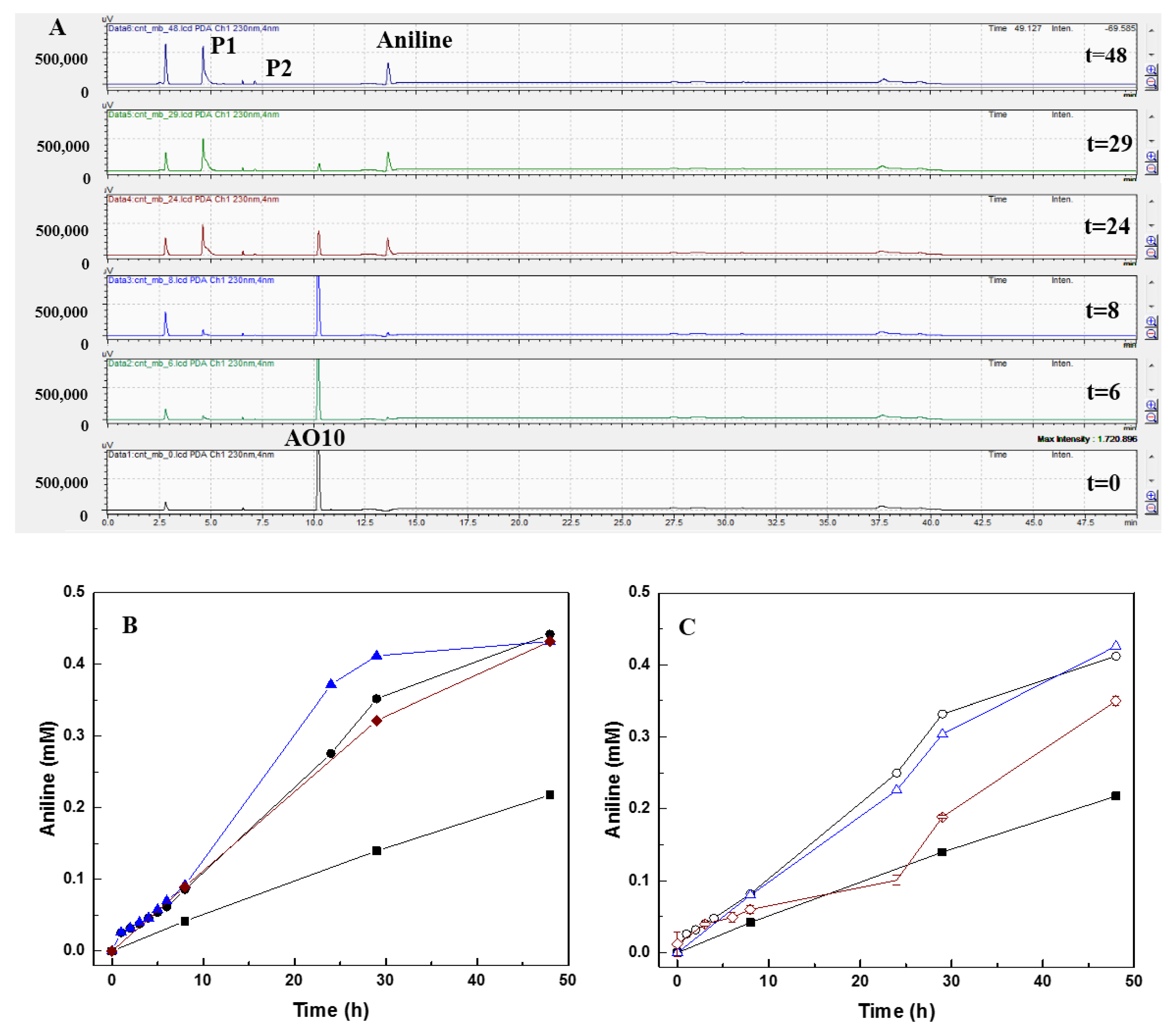
3.4. Products and Mechanism of Azo Dye Reduction
3.5. Toxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- van der Zee, F.P.; Bouwman, R.H.M.; Strik, D.P.B.T.B.; Lettinga, G.; Field, J.A. Application of redox mediators to accelerate the transformation of reactive azo dyes in anaerobic bioreactors. Biotechnol. Bioeng. 2001, 75, 691–701. [Google Scholar] [CrossRef]
- van der Zee, F.P.; Cervantes, F.J. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: A review. Biotechnol. Adv. 2009, 27, 256–277. [Google Scholar] [CrossRef]
- van der Zee, F.P.; Bisschops, I.A.E.; Lettinga, G.; Field, J.A. Activated Carbon as an Electron Acceptor and Redox Mediator during the Anaerobic Biotransformation of Azo Dyes. Environ. Sci. Technol. 2003, 37, 402–408. [Google Scholar] [CrossRef]
- Pereira, R.A.; Pereira, M.F.R.; Alves, M.M.; Pereira, L. Carbon based materials as novel redox mediators for dye wastewater biodegradation. Appl. Catal. B Environ. 2014, 144, 713–720. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Influence of the surface chemistry of multi-walled carbon nanotubes on their activity as ozonation catalysts. Carbon N. Y. 2010, 48, 4369–4381. [Google Scholar] [CrossRef]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21. [Google Scholar] [CrossRef]
- Pereira, L.; Pereira, R.; Pereira, M.F.R.; van der Zee, F.P.; Cervantes, F.J.; Alves, M.M. Thermal modification of activated carbon surface chemistry improves its capacity as redox mediator for azo dye reduction. J. Hazard. Mater. 2010, 183, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Salvador, A.F.; Dias, P.; Pereira, M.F.R.; Alves, M.M.; Pereira, L. Perspectives on carbon materials as powerful catalysts in continuous anaerobic bioreactors. Water Res. 2016, 101, 441–447. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Jabbari, V.; Imam, M.A.; Castro, E.; Kim, H.; Curry, M.L.; Valles-Rosales, D.J.; Noveron, J.C. Nanoscale nickel metal organic framework decorated over graphene oxide and carbon nanotubes for water remediation. Sci. Total Environ. 2020, 698, 134214. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhu, D. Graphene Oxide-Facilitated Reduction of Nitrobenzene in Sulfide-Containing Aqueous Solutions. Environ. Sci. Technol. 2013, 47, 4204–4210. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Pereira, R.; Pereira, M.F.R.; Alves, M.M. Effect of different carbon materials as electron shuttles in the anaerobic biotransformation of nitroanilines. Biotechnol. Bioeng. 2016, 113, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.B.; Cervantes, F.J.; van Lier, J.B. Azo dye reduction by thermophilic anaerobic granular sludge, and the impact of the redox mediator anthraquinone-2,6-disulfonate (AQDS) on the reductive biochemical transformation. Appl. Microbiol. Biotechnol. 2004, 64, 62–69. [Google Scholar] [CrossRef]
- Cadena Ramírez, A.; Texier, A.-C.; Martínez, I.G.; Hernández, J.G. Inhibitory effects of quinoid redox mediators on a denitrifying culture. Environ. Technol. 2019, 40, 1306–1315. [Google Scholar] [CrossRef]
- Mezohegyi, G.; Gonçalves, F.; Órfão, J.J.M.; Fabregat, A.; Fortuny, A.; Font, J.; Bengoa, C.; Stuber, F. Tailored activated carbons as catalysts in biodecolourisation of textile azo dyes. Appl. Catal. B Environ. 2010, 94, 179–185. [Google Scholar] [CrossRef]
- Mezohegyi, G.; Kolodkin, A.; Castro, U.I.; Bengoa, C.; Stuber, F.; Font, J.; Fabregat, A.; Fortuny, A. Effective Anaerobic Decolorization of Azo Dye Acid Orange 7 in Continuous Upflow Packed-Bed Reactor Using Biological Activated Carbon System. Ind. Eng. Chem. Res. 2007, 46, 6788–6792. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. Carbon as catalyst. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 1–579. ISBN 9780470178850. [Google Scholar]
- Rios-Del Toro, E.E.; Celis, L.B.; Cervantes, F.J.; Rangel-Mendez, J.R. Enhanced microbial decolorization of methyl red with oxidized carbon fiber as redox mediator. J. Hazard. Mater. 2013, 260, 967–974. [Google Scholar] [CrossRef]
- Amezquita-Garcia, H.J.; Rangel-Mendez, J.R.; Cervantes, F.J.; Razo-Flores, E. Activated carbon fibers with redox-active functionalities improves the continuous anaerobic biotransformation of 4-nitrophenol. Chem. Eng. J. 2016, 286, 208–215. [Google Scholar] [CrossRef]
- Amezquita-Garcia, H.J.; Razo-Flores, E.; Cervantes, F.J.; Rangel-Mendez, J.R. Activated carbon fibers as redox mediators for the increased reduction of nitroaromatics. Carbon N. Y. 2013, 55, 276–284. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon N. Y. 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Thavorn-amornsri, T.; Pereira, M.F.R.; Serp, P.; Figueiredo, J.L. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Cho, H.-H.; Huang, H.; Schwab, K. Effects of Solution Chemistry on the Adsorption of Ibuprofen and Triclosan onto Carbon Nanotubes. Langmuir 2011, 27, 12960–12967. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, P.; Soares, O.S.G.P.; Ramalho, P.S.F.; Pereira, M.F.R.; Alves, M.M. Synthesis, characterization and application of magnetic carbon materials as electron shuttles for the biological and chemical reduction of the azo dye Acid Orange 10. Appl. Catal. B Environ. 2017, 212, 175–184. [Google Scholar] [CrossRef]
- ISO 11348-1 International Standard. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part1: Method Using Freshly Prepared Bacteria. 1998. Available online: https://www.iso.org/standard/19308.html (accessed on 1 November 2016).
- ISO 11348-3 International Standard. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part3: Method Using Freeze-Dried Bacteria. 2007. Available online: https://www.iso.org/standard/40518.html (accessed on 1 October 2015).
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Rocha, R.P.; Soares, O.S.G.P.; Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Different methodologies for synthesis of nitrogen doped carbon nanotubes and their use in catalytic wet air oxidation. Appl. Catal. A Gen. 2017, 548, 62–70. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon N. Y. 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Yue, X.; Zhou, H.; Yang, J.; Wang, Y.; Ma, L.; Chen, K. Facile synthesis of magnetically separable reduced graphene oxide/magnetite/silver nanocomposites with enhanced catalytic activity. J. Colloid Interface Sci. 2015, 459, 79–85. [Google Scholar] [CrossRef]
- Toral-Sánchez, E.; Rangel-Mendez, J.R.; Hurt, R.H.; Ascacio Valdés, J.A.; Aguilar, C.N.; Cervantes, F.J. Novel application of magnetic nano-carbon composite as redox mediator in the reductive biodegradation of iopromide in anaerobic continuous systems. Appl. Microbiol. Biotechnol. 2018, 102, 8951–8961. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shah, A.-H.; Mian, S.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C 2019, 5, 3. [Google Scholar] [CrossRef]
- Carabineiro, S.; Pereira, M.; Nunes-Pereira, J.; Caparros, C.; Sencadas, V.; Lanceros-Méndez, S. Effect of the carbon nanotube surface characteristics on the conductivity and dielectric constant of carbon nanotube/poly(vinylidene fluoride) composites. Nanoscale Res. Lett. 2011, 6, 302. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Thavorn-Amornsri, T.; Pereira, M.F.R.; Figueiredo, J.L. Adsorption of ciprofloxacin on surface-modified carbon materials. Water Res. 2011, 45, 4583–4591. [Google Scholar] [CrossRef] [PubMed]
- Aquino, S.F.; Chernicharo, C.A.L. Build up of volatile fatty acids (VFA) in anaerobic reactors under stress conditions: Causes and control strategies. Eng. Sanit. Ambient 2005, 10, 152–161. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; Wiley: Weinheim, Germany, 2008; Volume 91, ISBN 9783527318414. [Google Scholar]
- dos Santos, A.B.; de Madrid, M.P.; Stams, A.J.M.; Van Lier, J.B.; Cervantes, F.J. Azo Dye Reduction by Mesophilic and Thermophilic Anaerobic Consortia. Biotechnol. Prog. 2005, 21, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.J.; dos Santos, A.B. Reduction of azo dyes by anaerobic bacteria: Microbiological and biochemical aspects. Rev. Environ. Sci. Bio/Technol. 2011, 10, 125–137. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Liu, G.; Jin, R.; Lu, H. Enhanced nitrobenzene biotransformation by graphene-anaerobic sludge composite. J. Chem. Technol. Biotechnol. 2014, 89, 750–755. [Google Scholar] [CrossRef]
- Mendes, S.; Pereira, L.; Batista, C.; Martins, L.O. Molecular determinants of azo reduction activity in the strain Pseudomonas putida MET94. Appl. Microbiol. Biotechnol. 2011, 92, 393–405. [Google Scholar] [CrossRef]
- Pereira, L.; Coelho, A.V.; Viegas, C.A.; dos Santos, M.M.C.; Robalo, M.P.; Martins, L.O. Enzymatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase. J. Biotechnol. 2009, 139, 68–77. [Google Scholar] [CrossRef]
- Fernández-Alba, A.R.; Hernando, M.D.; Piedra, L.; Chisti, Y. Toxicity evaluation of single and mixed antifouling biocides measured with acute toxicity bioassays. Anal. Chim. Acta 2002, 456, 303–312. [Google Scholar] [CrossRef]
- Mendonça, E.; Picado, A.; Paixão, S.M.; Silva, L.; Cunha, M.A.; Leitão, S.; Moura, I.; Cortez, C.; Brito, F. Ecotoxicity tests in the environmental analysis of wastewater treatment plants: Case study in Portugal. J. Hazard. Mater. 2009, 163, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Olivares, C.I.; Wang, J.; Luna, C.D.S.; Field, J.A.; Abrell, L.; Sierra-Alvarez, R. Continuous treatment of the insensitive munitions compound N-methyl-p-nitro aniline (MNA) in an upflow anaerobic sludge blanket (UASB) bioreactor. Chemosphere 2016, 144, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, F.P.; Villaverde, S. Combined anaerobic–aerobic treatment of azo dyes—A short review of bioreactor studies. Water Res. 2005, 39, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Donlon, B.; Razo-Flores, E.; Luijten, M.; Swarts, H.; Lettinga, G.; Field, J. Detoxification and partial mineralization of the azo dye mordant orange 1 in a continuous upflow anaerobic sludge-blanket reactor. Appl. Microbiol. Biotechnol. 1997, 47, 83–90. [Google Scholar] [CrossRef]
- Sponza, D.T.; Kuscu, Ö.S. Relationships between acute toxicities of para nitrophenol (p-NP) and nitrobenzene (NB) to Daphnia magna and Photobacterium phosphoreum: Physicochemical properties and metabolites under anaerobic/aerobic sequentials. J. Hazard. Mater. 2011, 185, 1187–1197. [Google Scholar] [CrossRef]
- Arias, L.R.; Yang, L. Inactivation of Bacterial Pathogens by Carbon Nanotubes in Suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef]
- Pasquini, L.M.; Hashmi, S.M.; Sommer, T.J.; Elimelech, M.; Zimmerman, J.B. Impact of Surface Functionalization on Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. Environ. Sci. Technol. 2012, 46, 6297–6305. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.-A.; Chanéac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.-Y. Relation between the Redox State of Iron-Based Nanoparticles and Their Cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal Effect of Zero-Valent Iron Nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, A.; Togo, C.; Mutanda, T.; Slatter, C.; Whiteley, C. Decolourisation and degradation of reactive blue 2 by sulphate reducing bacteria (SRB) and zero valent iron in a biosulphidogenic reactor. Afr. J. Biotechnol. 2011, 10, 584–588. [Google Scholar] [CrossRef]
- Dong, H.; Li, L.; Wang, Y.; Ning, Q.; Wang, B.; Zeng, G. Aging of zero-valent iron-based nanoparticles in aqueous environment and the consequent effects on their reactivity and toxicity. Water Environ. Res. 2019, 92. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef]
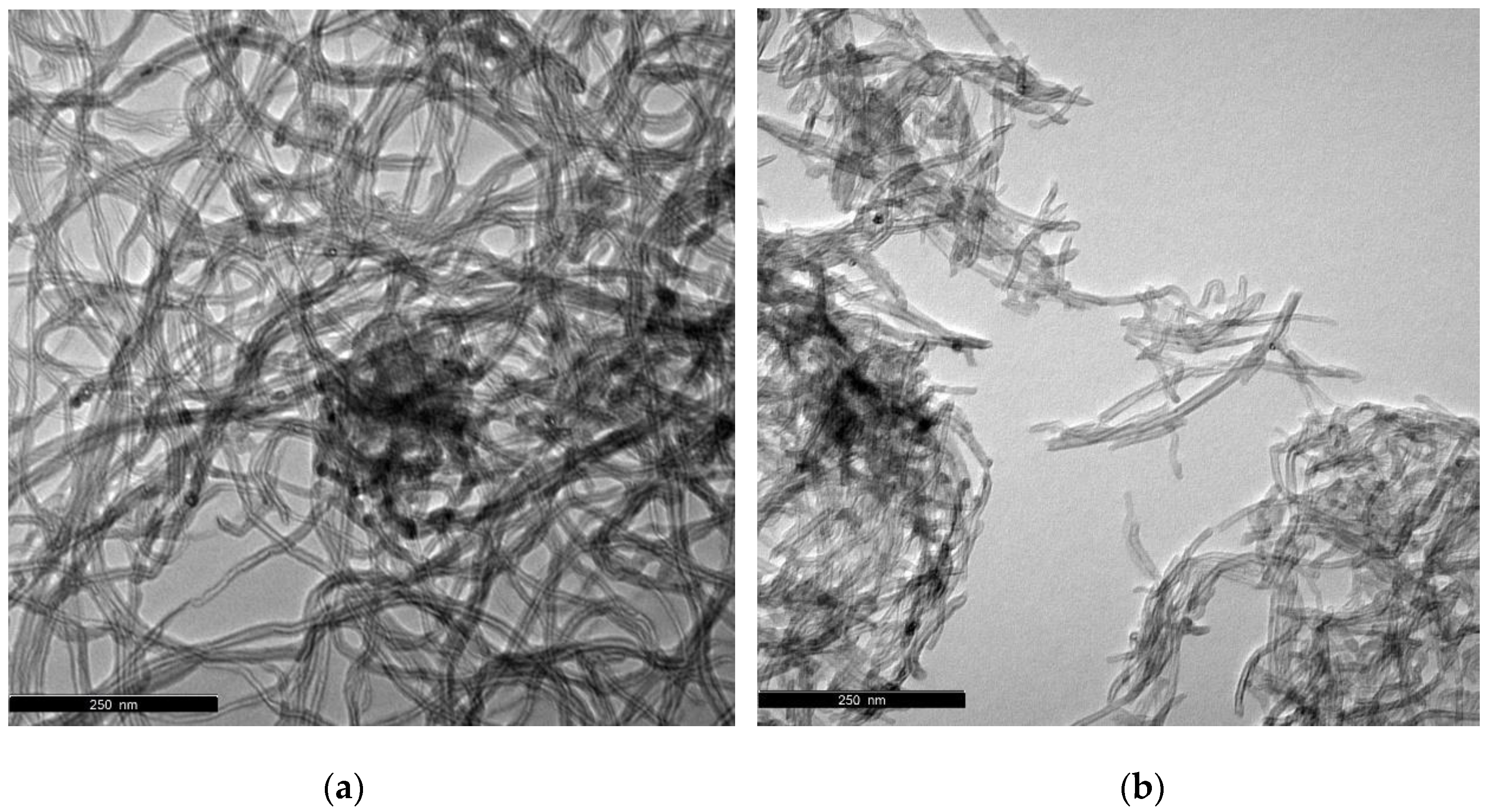
| Sample | SBET (±10 m2/g) | Vp p/p0 = 0.95 (±0.005 cm3/g) |
|---|---|---|
| CNT | 201 | 0.416 |
| CNT_N_MB | 225 | 0.503 |
| CNT_HNO3 | 223 | 0.448 |
| CNT@2%Fe | 196 | 0.440 |
| CNT@2%Fe_N_MB | 243 | 0.581 |
| CNT@2%Fe_HNO3 | 208 | 0.444 |
| Sample | (CO) TPD (±20 μmol/g) | (CO2) TPD (±20 μmol/g) | pHPZC | CEA (wt.%) | HEA (wt.%) | NEA (wt.%) | SEA (wt.%) | OEA (wt.%) |
|---|---|---|---|---|---|---|---|---|
| CNT | 189 | 81 | 6.6 | 99.8 | 0.11 | 0.00 | 0.00 | 0.06 |
| CNT_N_MB | 366 | 53 | 6.7 | 96.4 | 0.18 | 1.69 | 0.00 | 0.39 |
| CNT_HNO3 | 1340 | 841 | 2.2 | 98.0 | 0.19 | 0.00 | 0.15 | 1.25 |
| AO10 Reduction | Aniline Formation | ||||
|---|---|---|---|---|---|
| Condition | Extent (%) | Rate (d−1) | Extent (%) | Rate (mmol d−1) | |
| Biotic assays | Control | 32.4 ± 0.3 | 0.27 ± 0.03 | 43.6 | 0.11 |
| CNT | 97.3 ± 0.2 | 2.64 ± 0.16 | 88.4 | 0.28 | |
| CNT_N_MB | 98.2 ± 0.1 | 2.94 ± 0.18 | 86.4 | 0.35 | |
| CNT_HNO3 | 94.4 ± 1.2 | 2.32 ± 0.14 | 86.4 | 0.27 | |
| CNT@2%Fe | 94.1 ± 1.4 | 2.00 ± 0.18 | 82.4 | 0.26 | |
| CNT@2%Fe_N_MB | 98.1 ± 0.1 | 2.50 ± 0.11 | 85.2 | 0.34 | |
| CNT@2%Fe_HNO3 | 93.5 ± 0.6 | 1.59 ± 0.23 | 70.0 | 0.16 | |
| Blank control | Control | 19.2 ± 1.1 | 0.09 ± 0.06 | n.d. | n.d. |
| CNT | 43.3 ± 4.3 | 0.2 ± 0.02 | n.d. | n.d. | |
| CNT_N_MB | 38.7 ± 3.8 | 0.16 ± 0.03 | n.d. | n.d. | |
| CNT_HNO3 | 41.5 ± 2.6 | 0.17 ± 0.01 | n.d. | n.d. | |
| CNT@2%Fe | 43.3 ± 2.9 | 0.18 ± 0.02 | n.d. | n.d. | |
| CNT@2%Fe_N_MB | 48.6 ± 1.8 | 0.2 ± 0.02 | n.d. | n.d. | |
| CNT@2%Fe_HNO3 | 45.0 ± 3.2 | 0.17 ± 0.01 | n.d. | n.d. | |
| Abiotic | CNT | 6.6 ± 1.3 | 0 | n.d. | n.d. |
| CNT_N_MB | 0.6 ± 0.7 | 0 | n.d. | n.d. | |
| CNT_HNO3 | 3.8 ± 1.8 | 0 | n.d. | n.d. | |
| CNT@2%Fe | 2.6 ± 1.9 | 0 | n.d. | n.d. | |
| CNT@2%Fe_N_MB | 3.5 ± 2.3 | 0 | n.d. | n.d. | |
| CNT@2%Fe_HNO3 | 2.6 ± 1.4 | 0 | n.d. | n.d. | |
| Samples | INH (%) | |
|---|---|---|
| AO10 (0.25 mmol L−1) | 35 ± 2.3 | |
| Biological degradation of AO10 | Control | 23.6 ± 1.8 |
| CNT | 28.4 ± 0.1 | |
| CNT_N_MB | 27.0 ± 2.2 | |
| CNT_HNO3 | 39.7 ± 9.8 | |
| CNT@2%Fe | 37.9 ± 2.0 | |
| CNT@2%Fe_N_MB | 32.7 ± 5.6 | |
| CNT@2%Fe_HNO3 | 57.5 ± 9.2 | |
| Positive control (K2Cr2O7) | 86 ± 0.1 | |
| Aniline (0.4 mmol L−1) | 11.7 ± 1.5 | |
| Anaerobic medium | 7.5 ± 2.9 | |
| Ascorbic acid (200 mg L−1) | 3.4 ± 0.5 | |
| Medium after incubation with 0.1 g L−1 of CNM for 48 h | CNT | 6.8 ± 0.3 |
| CNT_N_MB | 10.8 ± 5.3 | |
| CNT_HNO3 | 7.8 ± 2.3 | |
| CNT@2%Fe | 22.3 ± 5.4 | |
| CNT@2%Fe_N_MB | 10 ± 2.1 | |
| CNT@2%Fe_HNO3 | 13.0 ± 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.R.; Soares, O.S.G.P.; Pereira, M.F.R.; Alves, M.M.; Pereira, L. Tailoring Carbon Nanotubes to Enhance their Efficiency as Electron Shuttle on the Biological Removal of Acid Orange 10 Under Anaerobic Conditions. Nanomaterials 2020, 10, 2496. https://doi.org/10.3390/nano10122496
Silva AR, Soares OSGP, Pereira MFR, Alves MM, Pereira L. Tailoring Carbon Nanotubes to Enhance their Efficiency as Electron Shuttle on the Biological Removal of Acid Orange 10 Under Anaerobic Conditions. Nanomaterials. 2020; 10(12):2496. https://doi.org/10.3390/nano10122496
Chicago/Turabian StyleSilva, Ana Rita, O. Salomé G.P. Soares, M. Fernando R. Pereira, M. Madalena Alves, and Luciana Pereira. 2020. "Tailoring Carbon Nanotubes to Enhance their Efficiency as Electron Shuttle on the Biological Removal of Acid Orange 10 Under Anaerobic Conditions" Nanomaterials 10, no. 12: 2496. https://doi.org/10.3390/nano10122496
APA StyleSilva, A. R., Soares, O. S. G. P., Pereira, M. F. R., Alves, M. M., & Pereira, L. (2020). Tailoring Carbon Nanotubes to Enhance their Efficiency as Electron Shuttle on the Biological Removal of Acid Orange 10 Under Anaerobic Conditions. Nanomaterials, 10(12), 2496. https://doi.org/10.3390/nano10122496