Organic–Inorganic Ternary Nanohybrids of Single-Walled Carbon Nanohorns for Room Temperature Chemiresistive Ethanol Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of Experiments for Organic–Inorganic Ternary Nanohybrids of Ox-SWCNH Fabrication
2.3. Synthesis of the ZnO Nanopowder by Sonochemistry
2.4. Synthesis of the Organic–Inorganic Ternary Nanohybrids of Ox-SWCNHs
- A solution of polyvinylpyrrolidone is prepared by dissolving 0.1 g polymer in 50 mL isopropyl alcohol under stirring in the ultrasonic bath (for one hour at a temperature of 50 °C).
- An amount of 0.1 g of ox-SWCNH powder is added to the previously prepared solution, while continued stirring is performed in the ultrasound bath for 2 h at room temperature.
- An amount of 0.1 g of SnO2 nanometric powder is added to the previously prepared suspension and continued stirring is performed in the ultrasound bath for 2 h at room temperature.
2.5. Morphological and Compositional Characterization of the Sensing Films
3. Results and Discussion
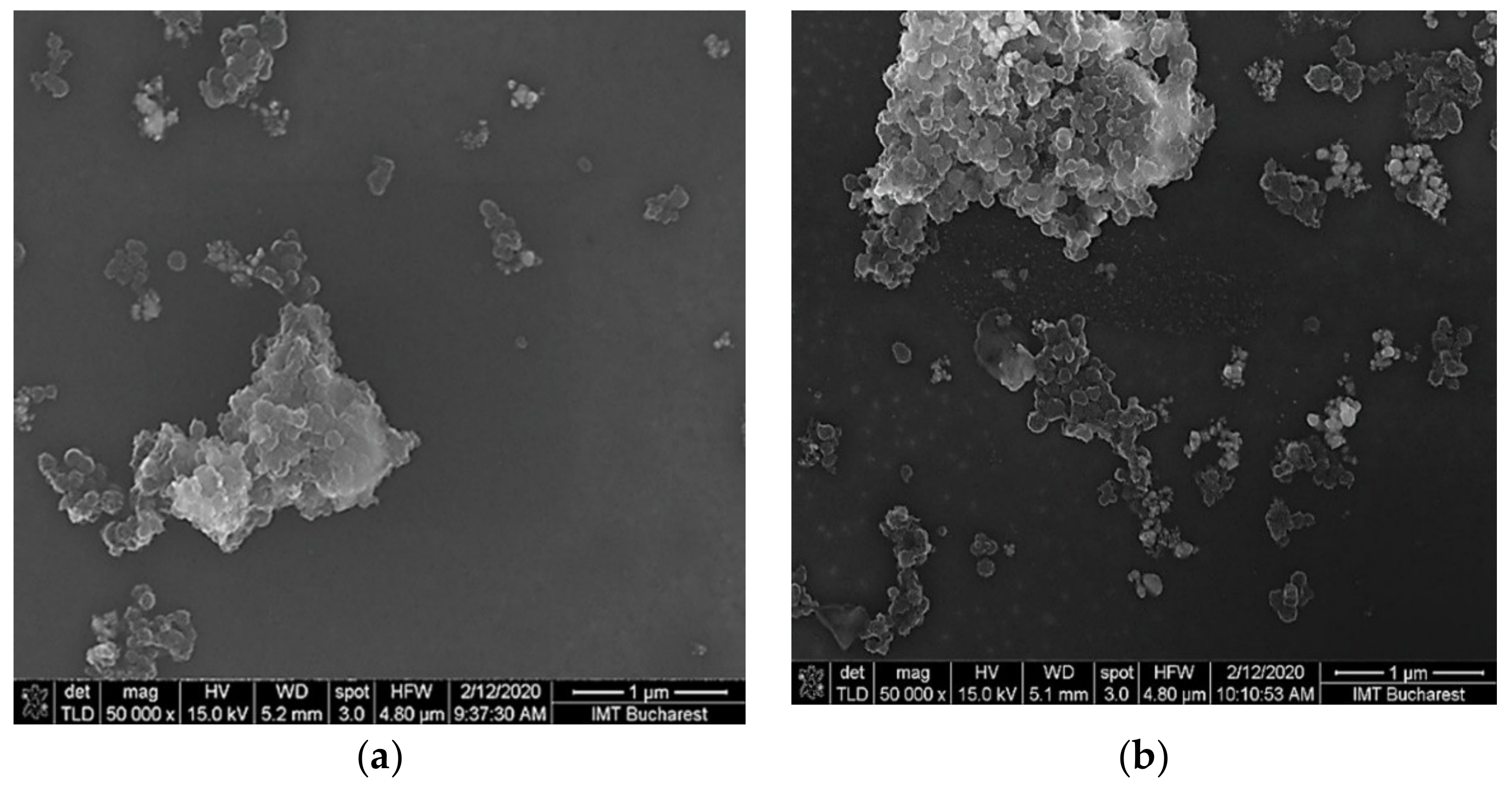
3.1. Structural and Topographical Characterization of Ternary Nanohybrids of SWCNHs
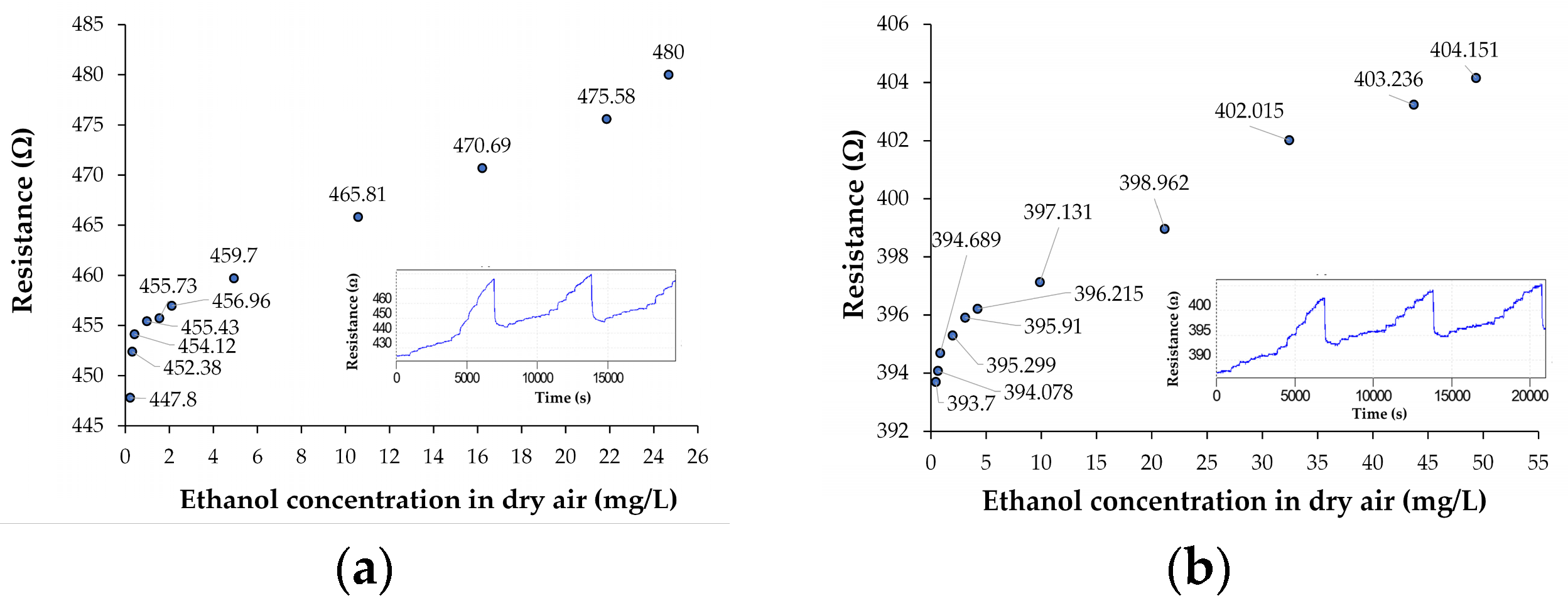
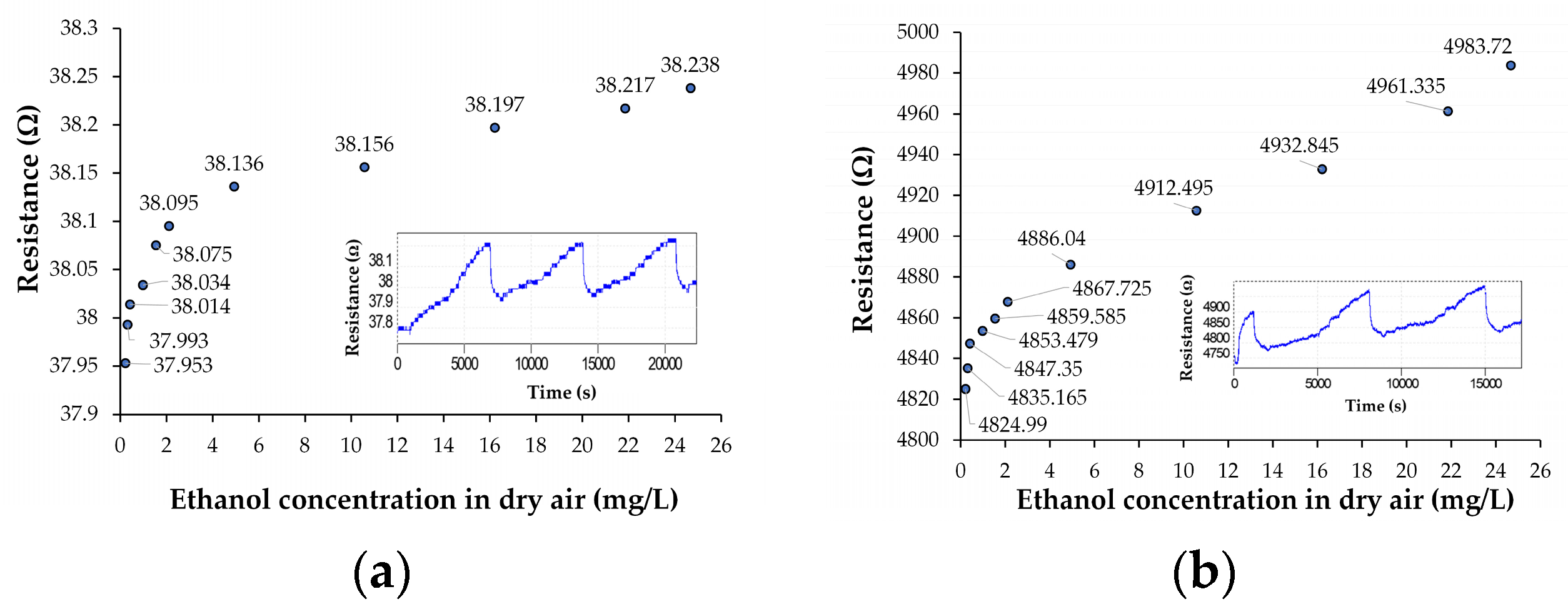
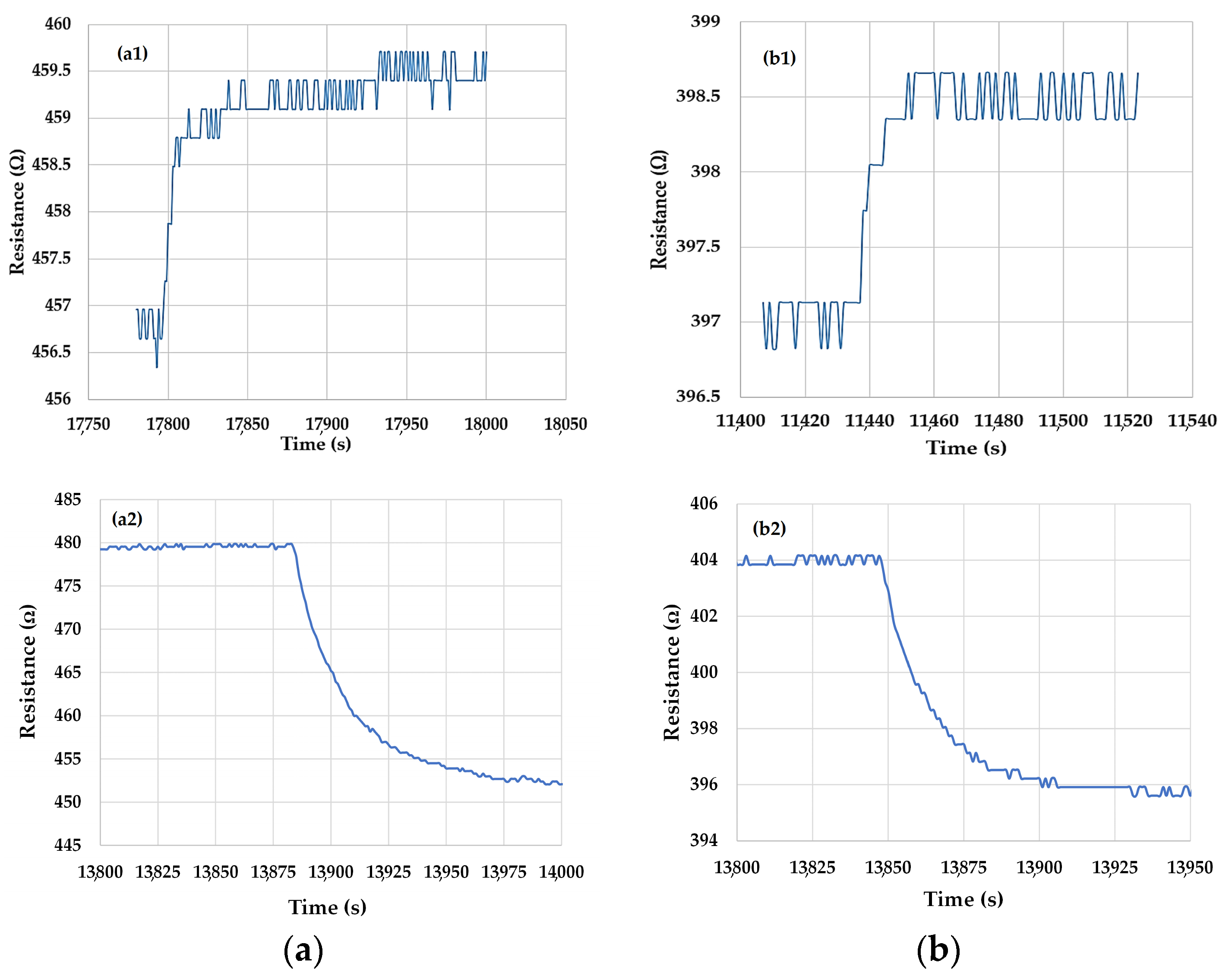
3.2. Results on Chemiresistive Ethanol Detection at Room Temperature by Ternary Nanohybrids of Ox-SWCNHs
4. Conclusions
5. Patents
- Bogdan-Cătălin Șerban, Octavian Buiu, Cornel Cobianu, Niculae Dumbravescu, Viorel Marian Avramescu, “Strat senzitiv ternar pentru senzor rezistiv de etanol” (Ternary sensitive layer for ethanol resistive sensor), application patent, OSIM, Romania, A/00477, 31 July 2020.
- Bogdan-Cătălin Șerban, Octavian Buiu, Cornel Cobianu, Niculae Dumbravescu, Viorel Marian Avramescu, “Senzor rezistiv de etanol”, (Ethanol resistive sensor) application patent, OSIM, Romania, A/00480, 31 July 2020.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ho, J.J.; Fang, Y.K.; Wu, K.H.; Hsieh, W.T.; Chen, C.H.; Ju, M.S.; Lin, J.J.; Hwang, S.B. High sensitivity ethanol gas sensor integrated with a solid-state heater and thermal isolation improvement structure for legal drink-drive limit detecting. Sens. Actuators B 1998, 50, 227–233. [Google Scholar] [CrossRef]
- Kieser, B.; Dieterle, F.; Gauglitz, G. Discrimination of methanol and ethanol vapors by the use of a single optical sensor with a microporous sensitive layer. Anal. Chem. 2002, 74, 4781–4787. [Google Scholar] [CrossRef] [PubMed]
- The National Institute for Occupational Safety and Health (NIOSH). Available online: https://www.cdc.gov/niosh/idlh/64175.html (accessed on 15 July 2020).
- Ippolito, S.J.; Ponzoni, A.; Kalantar-Zadeh, K.; Wlodarski, W.; Comini, E.; Faglia, G.; Sberveglieri, G. Layered WO3/ZnO/36° LiTaO3 SAW gas sensor sensitive towards ethanol vapour and humidity. Sens. Actuators B 2006, 117, 442–450. [Google Scholar] [CrossRef]
- Balcerzak, A.; Aleksiejuk, M.; Zhavnerko, G.; Agabekov, V. Sensing properties of two-component Langmuir–Blodgett layer and its porous derivative in SAW sensor for vapors of methanol and ethanol. Thin Solid Film. 2010, 518, 3402–3406. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Liu, J.H.; He, Y.N.; Yu, L.M.; Liu, W.H. ZnO nanomaterials-based surface acoustic wave ethanol gas sensor. J. Nanosci. Nanotechnol. 2012, 12, 6505–6509. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, M.; Mayes, A.G.; Melendi-Espina, S. Graphene oxide in lossy mode resonance-based optical fiber sensors for ethanol detection. Sensors 2018, 18, 58. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Kumar, R.; Wei, F.; Han, W.; Mallik, A.K.; Yuan, J.; Yu, C. High sensitivity optical fiber sensors for simultaneous measurement of methanol and ethanol. Sens. Actuators B Chem. 2018, 271, 1–8. [Google Scholar] [CrossRef]
- Kudo, H.; Sawai, M.; Suzuki, Y.; Wang, X.; Gessei, T.; Takahashi, D.; Arakawa, T.; Mitsubayashi, K. Fiber-optic bio-sniffer (biochemical gas sensor) for high-selective monitoring of ethanol vapor using 335 nm UV-LED. Sens. Actuators B 2010, 147, 676–680. [Google Scholar] [CrossRef]
- Tianshu, Z.; Hing, P.; Li, Y.; Jiancheng, Z. Selective detection of ethanol vapor and hydrogen using Cd-doped SnO2-based sensors. Sens. Actuators B Chem. 1999, 60, 208–215. [Google Scholar] [CrossRef]
- Raksa, P.; Gardchareon, A.; Chairuangsri, T.; Mangkorntong, P.; Mangkorntong, N.; Choopun, S. Ethanol sensing properties of CuO nanowires prepared by an oxidation reaction. Ceram. Int. 2009, 35, 649–652. [Google Scholar] [CrossRef]
- Illyaskutty, N.; Kohler, H.; Trautmann, T.; Schwotzer, M.; Pillai, V.M. Enhanced ethanol sensing response from nanostructured MoO3: ZnO thin films and their mechanism of sensing. J. Mater. Chem. C 2013, 1, 3976–3984. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, C.; Wang, T. The enhanced ethanol sensing properties of multi-walled carbon nanotubes/SnO2 core/shell nanostructures. Nanotechnology 2006, 17, 3012. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Gao, Y.; Wang, L.; Rao, J.; Yin, G. Mild Synthesis of Pt/SnO2/Graphene Nanocomposites with Remarkably Enhanced Ethanol Electro-oxidation Activity and Durability. Chem. A Eur. J. 2016, 22, 193–198. [Google Scholar] [CrossRef]
- Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Impact of reduced graphene oxide on the ethanol sensing performance of hollow SnO2 nanoparticles under humid atmosphere. Sens. Actuators B Chem. 2017, 244, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, F.K. Nanoaggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Yudasaka, M.; Iijima, S.; Crespi, V.H. Single-Wall Carbon Nanohorns and Nanocones. Top. Appl. Phys. 2007, 111, 605–629. [Google Scholar] [CrossRef]
- Sano, N.; Kinugasa, M.; Otsuki, F.; Suehiro, J. Gas sensor using single-wall carbon nanohorns. Adv. Powder Technol. 2007, 18, 455–466. [Google Scholar] [CrossRef]
- Suehiro, J.; Sano, N.; Zhou, G.B. Application of dielectrophoresis to fabrication of carbon nanohorn gas sensor. J. Electrost. 2006, 64, 408–415. [Google Scholar] [CrossRef]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Bumbac, M.; Nicolescu, C.M. Oxidized Carbon Nanohorns as Novel Sensing Layer for Resistive Humidity Sensor. Acta Chim. Slov. 2020, 67, 1–7. [Google Scholar] [CrossRef]
- Serban, B.-C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Bumbac, M.; Pachiu, C.; Nicolescu, C.M. Oxidized carbon nanohorn-hydrophilic polymer nanocomposite for the resistive sensing of relative humidity. Anal. Lett. 2020. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Zahab, A.; Spina, L.; Poncharal, P.; Marlier, C. Water vapor effect on the electrical conductivity of a single-walled carbon nanotube mat. Phys. Rev. B 2000, 62, 10000–10003. [Google Scholar] [CrossRef]
- Șerban, B.-C.; Buiu, O.; Cobianu, C.; Avramescu, V.; Dumbrăvescu, N.; Brezeanu, M.; Marinescu, R. Ternary Carbon-Based Nanocomposite as Sensing Layer for Resistive Humidity Sensor. Multidiscip. Digit. Publ. Inst. Proc. 2019, 29, 114. [Google Scholar] [CrossRef] [Green Version]
- Șerban, B.-C.; Buiu, O.; Cobianu, C.; Ionescu, O.; Varsescu, D.; Marinescu, R.; Dumbravescu, N. Ethanol Sensor and Process for Preparing the Same. Romanian Patent Application 2019 RO133637 (A2), 30 September 2019. [Google Scholar]
- Șerban, B.-C.; Buiu, O.; Cobianu, C.; Ionescu, O.; Varsescu, D.; Marinescu, R.; Dumbravescu, N. Chemiresistive Ethanol Sensor. Romanian Patent Application 2019, RO133636 (A2), 30 September 2019. [Google Scholar]
- Șerban, B.-C.; Buiu, O.; Cobianu, C.; Ionescu, O.; Varsescu, D.; Avramescu, V.; Marinescu, R.; Dumbravescu, N. Sensitive Layer for Ethanol Sensor and Process for Manufacturing the Same. Romanian Patent Application 2019 RO133635 (A2), 30 September 2019. [Google Scholar]
- Cobianu, C.; Dumbravescu, N.; Serban, B.-C.; Buiu, O.; Romanitan, C.; Comanescu, F.; Danila, M.; Marinescu, R.; Avramescu, V.; Ionescu, O. Facile 3D Nanostructured ZnO-Graphene Hybrids for Gas Sensing Applications. In Proceedings of the IEEE International Conference of Semiconductors, CAS 2019, Sinaia, Romania, 9–11 October 2019; pp. 27–30. [Google Scholar]
- Cobianu, C.; Dumbravescu, N.; Serban, B.-C.; Buiu, O.; Romanitan, C.; Comanescu, F.; Danila, M.; Marinescu, R.; Avramescu, V.; Ionescu, O. Sonochemically synthetized ZnO-Graphene nanohybrids and its characterization. Rev. Adv. Mater. Sci. 2020, 59, 176–187. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B., III; Mdleleni, M.M. Acoustic cavitation and its chemical consequences. Phil. Trans. R. Soc. Lond. 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Yang, C.-M.; Noguchi, H.; Murata, K.; Yudasaka, M.; Hashimoto, A.; Iijima, S.; Kaneko, K. Highly ultramicroporous single-walled carbon nanohorn assemblies. Adv. Mater. 2005, 17, 866–870. [Google Scholar] [CrossRef]
- Pena-Alvarez, M.; del Corro, E.; Langa, F.; Baonza, V.G.; Taravillo, M. Morphological changes in carbon nanohorns under stress: A combined Raman spectroscopy and TEM study. RSC Adv. 2016, 6, 49543–49550. [Google Scholar] [CrossRef] [Green Version]
- Ferralis, N. Probing mechanical properties of graphene with Raman spectroscopy. J. Mater. Sci. 2010. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.F. Raman Spectroscopy of SnO2. J. Chem. Phys. 1970, 53, 852–853. [Google Scholar] [CrossRef]
- Ajito, K.; Sukamto, J.P.H.; Nagahara, L.A.; Hashimoto, K.; Fujishima, A. Strain imaging analysis of Si using Raman microscopy. J. Vac. Sci. Technol. A 1995, 13, 1234–1238. [Google Scholar] [CrossRef]
- Dieguez, A.; Romano-Rodrigues, A.; Vila, A.; Morante, J.R. The complete Raman spectrum of nanometric SnO2 particles. J. Appl. Phys. 2001, 90, 1550–1557. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Feng, J.; Ma, X.; Wu, C.; Zhao, X. One-dimensional silver nanowires synthesized by self-seeding polyol process. J. Nanoparticle Res. 2012, 14. [Google Scholar] [CrossRef]
- Jabbarnia, A.; Asmatulu, R. Synthesis and Characterization of PVdF/PVP-Based Electrospun Membranes as Separators for Supercapacitor Applications. J. Mater. Sci. Technol. Res. 2015, 2, 43–51. [Google Scholar] [CrossRef]
- Serban, B.-C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Nicolescu, C.-M.; Cobianu, C. Electrical Percolation Threshold In Oxidized Single Wall Carbon Nanohorn-Polyvinylpyrrolidone Nanocomposite: A Possible Application For High Sensitivity Resistive Humidity Sensor. In Proceedings of the IEEE International Semiconductor Conference (CAS-2020), Sinaia, Romania, 7–9 October 2020; pp. 239–242. [Google Scholar]
- Zhang, H.; Liu, J.; Chang, H.; Liu, A.; Xia, B. Characterization of a hybrid composite of SnO2 nanocrystal-decorated reduced graphene oxide for acetone gas sensor based on hydrothermal synthetized SnO2-reduced graphene oxide hybrid composite. RSC Adv. 2015, 5, 1866–18672. [Google Scholar]
- Barsan, N.; Simion, C.; Heine, T.; Pockhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide-based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Cobianu, C.; Serban, B.C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C. Room Temperature Chemiresistive Ethanol Detection by Ternary Nanocomposites of Oxidized Single Wall Carbon Nanohorn (ox-SWCNH). In Proceedings of the IEEE International Semiconductor Conference (CAS-2020), Sinaia, Romania, 7–9 October 2020; pp. 13–16. [Google Scholar]


| Sensor Parameters | Sensor Types | |||
|---|---|---|---|---|
| Ox-SWCNH/SnO2/PVP 1/1/1 | Ox-SWCNH/SnO2/PVP 2/1/1 | Ox-SWCNH/ZnO/PVP 5/2/1 | Ox-SWCNH/ZnO/PVP 5/3/2 | |
| Resistance, Ro [Ω] | 447.8 | 393.7 | 37.953 | 4824.99 |
| Sensitivity, S = ΔR/ΔC [Ω/(mg/L)] | 1.003 | 0.3638 | 0.0032 | 2.4213 |
| Relative sensitivity, Sr = S/Ro [1/(mg/L)] | 0.0022 | 0.00092 | 0.000084 | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobianu, C.; Serban, B.-C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Pachiu, C.; Bita, B.; Bumbac, M.; Nicolescu, C.-M.; Cobianu, C. Organic–Inorganic Ternary Nanohybrids of Single-Walled Carbon Nanohorns for Room Temperature Chemiresistive Ethanol Detection. Nanomaterials 2020, 10, 2552. https://doi.org/10.3390/nano10122552
Cobianu C, Serban B-C, Dumbravescu N, Buiu O, Avramescu V, Pachiu C, Bita B, Bumbac M, Nicolescu C-M, Cobianu C. Organic–Inorganic Ternary Nanohybrids of Single-Walled Carbon Nanohorns for Room Temperature Chemiresistive Ethanol Detection. Nanomaterials. 2020; 10(12):2552. https://doi.org/10.3390/nano10122552
Chicago/Turabian StyleCobianu, Cornel, Bogdan-Catalin Serban, Niculae Dumbravescu, Octavian Buiu, Viorel Avramescu, Cristina Pachiu, Bogdan Bita, Marius Bumbac, Cristina-Mihaela Nicolescu, and Cosmin Cobianu. 2020. "Organic–Inorganic Ternary Nanohybrids of Single-Walled Carbon Nanohorns for Room Temperature Chemiresistive Ethanol Detection" Nanomaterials 10, no. 12: 2552. https://doi.org/10.3390/nano10122552
APA StyleCobianu, C., Serban, B. -C., Dumbravescu, N., Buiu, O., Avramescu, V., Pachiu, C., Bita, B., Bumbac, M., Nicolescu, C. -M., & Cobianu, C. (2020). Organic–Inorganic Ternary Nanohybrids of Single-Walled Carbon Nanohorns for Room Temperature Chemiresistive Ethanol Detection. Nanomaterials, 10(12), 2552. https://doi.org/10.3390/nano10122552










