Highly Sensitive Gas Sensing Material for Environmentally Toxic Gases Based on Janus NbSeTe Monolayer
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
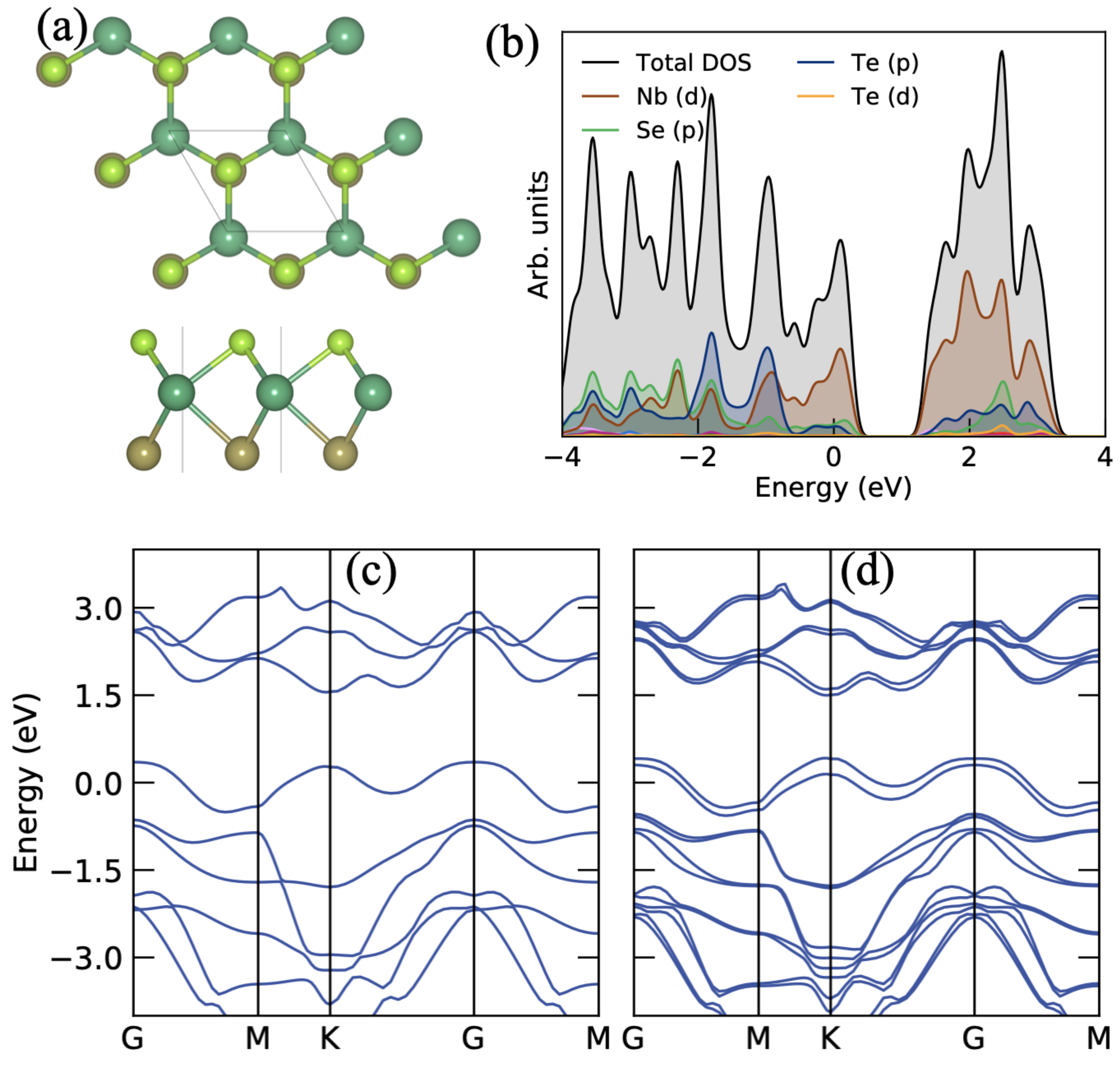
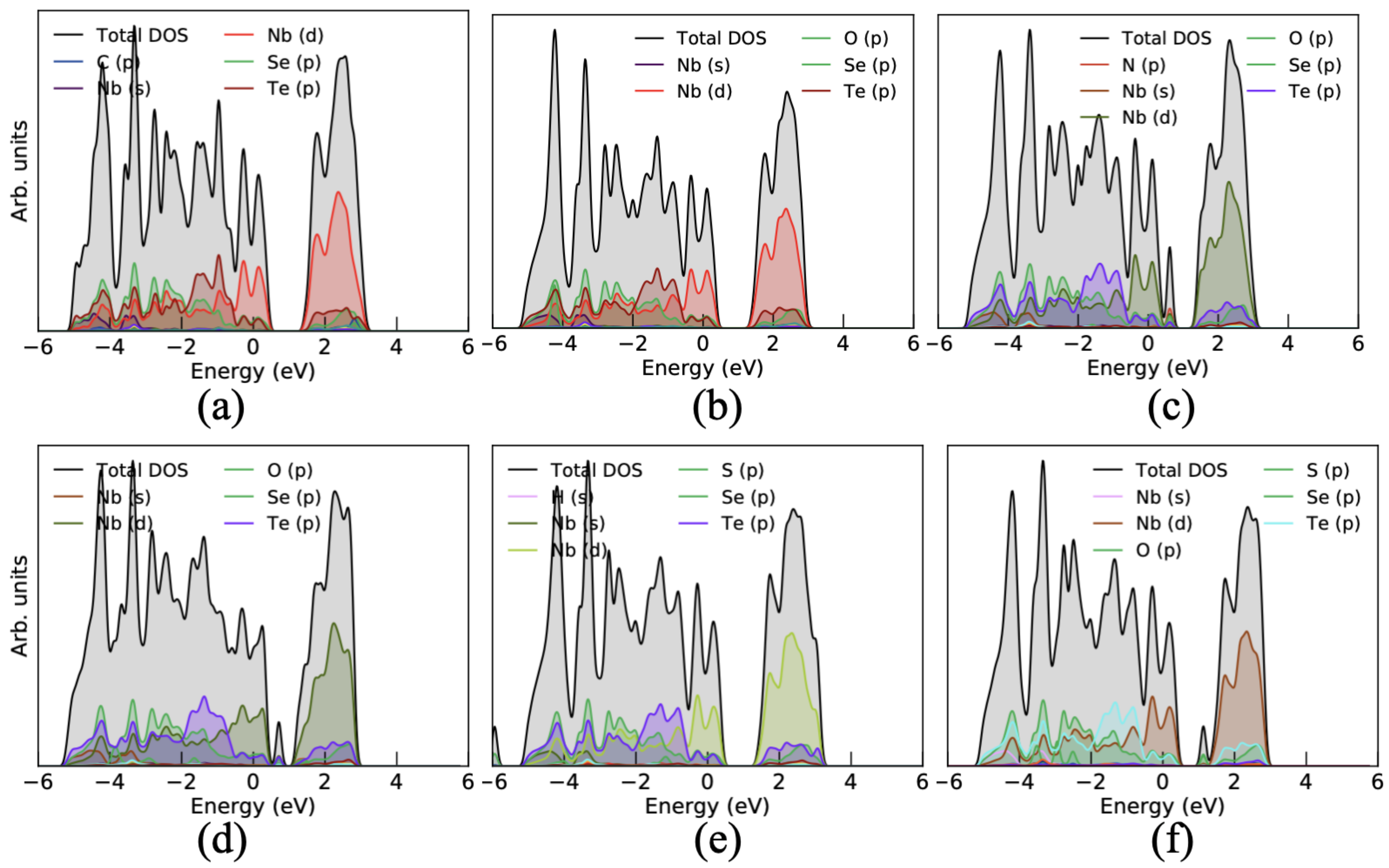
3.1. Structural and Electronic Properties
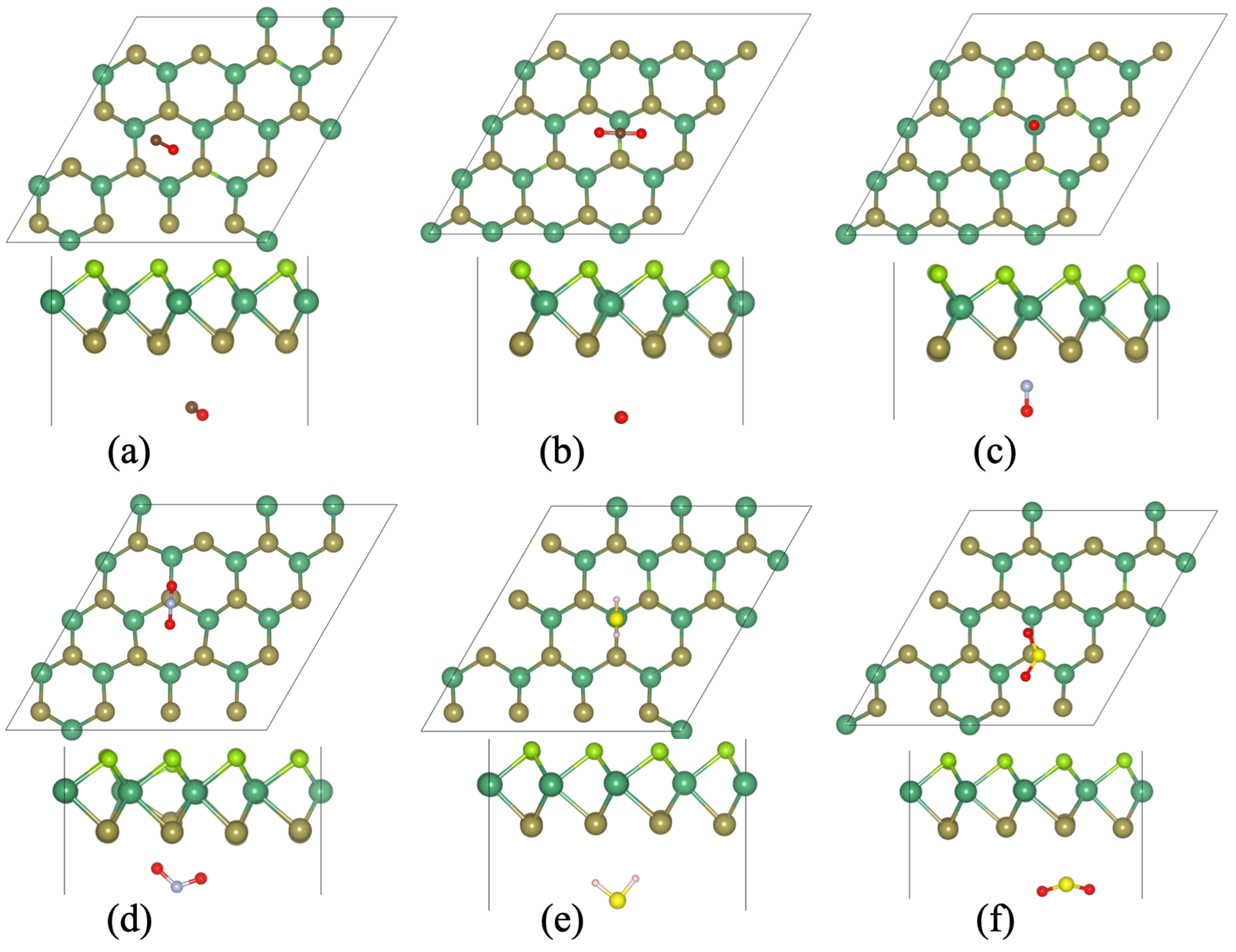
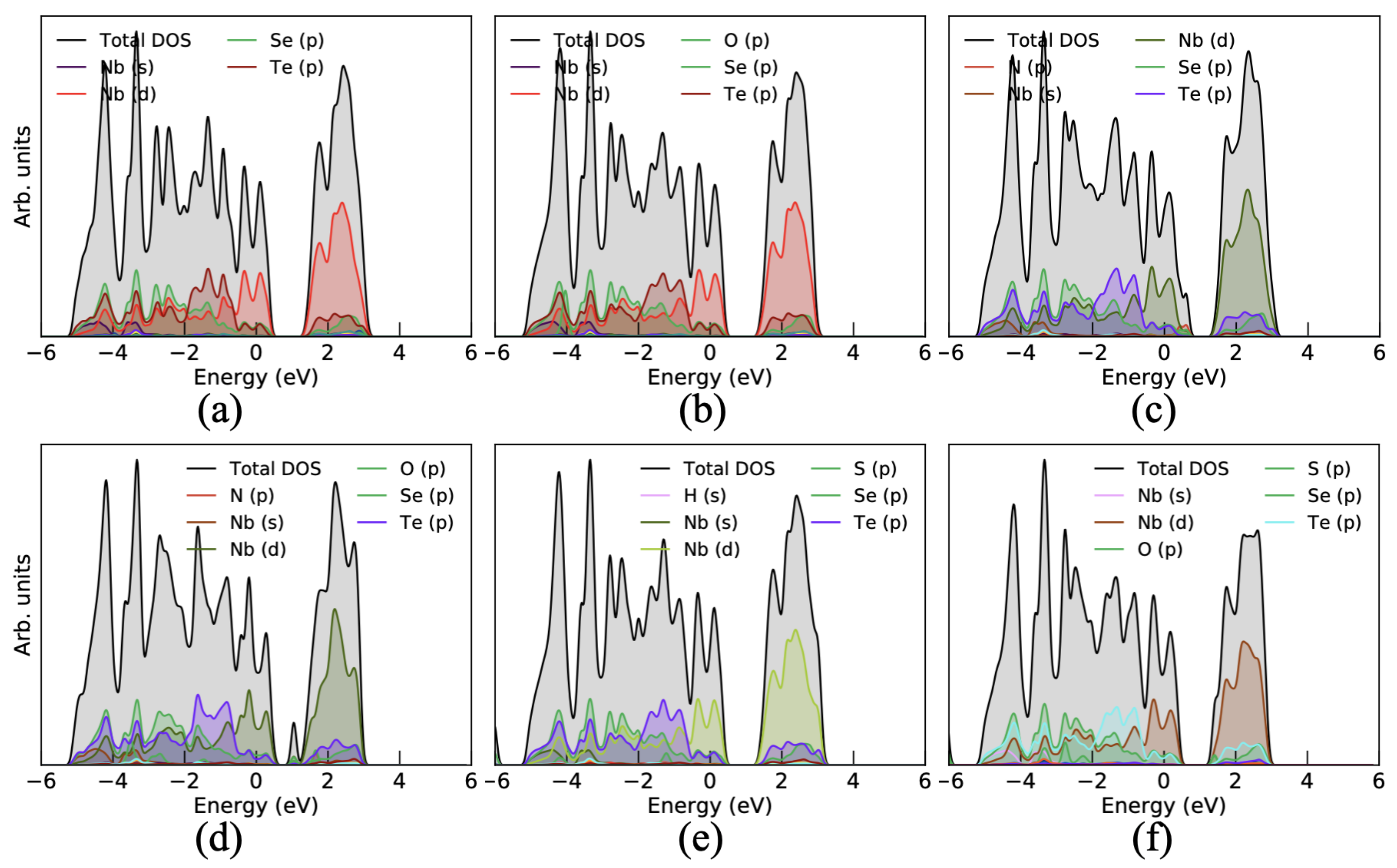
3.2. Binding of Toxic Gases on Monolayer
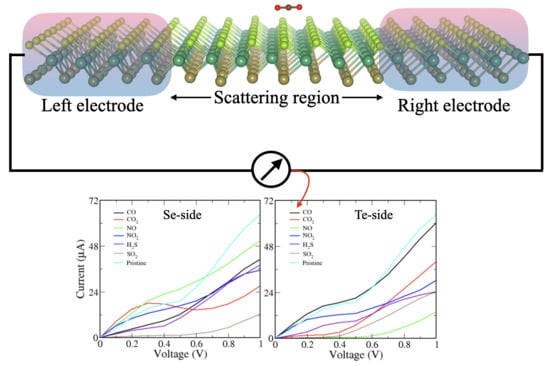
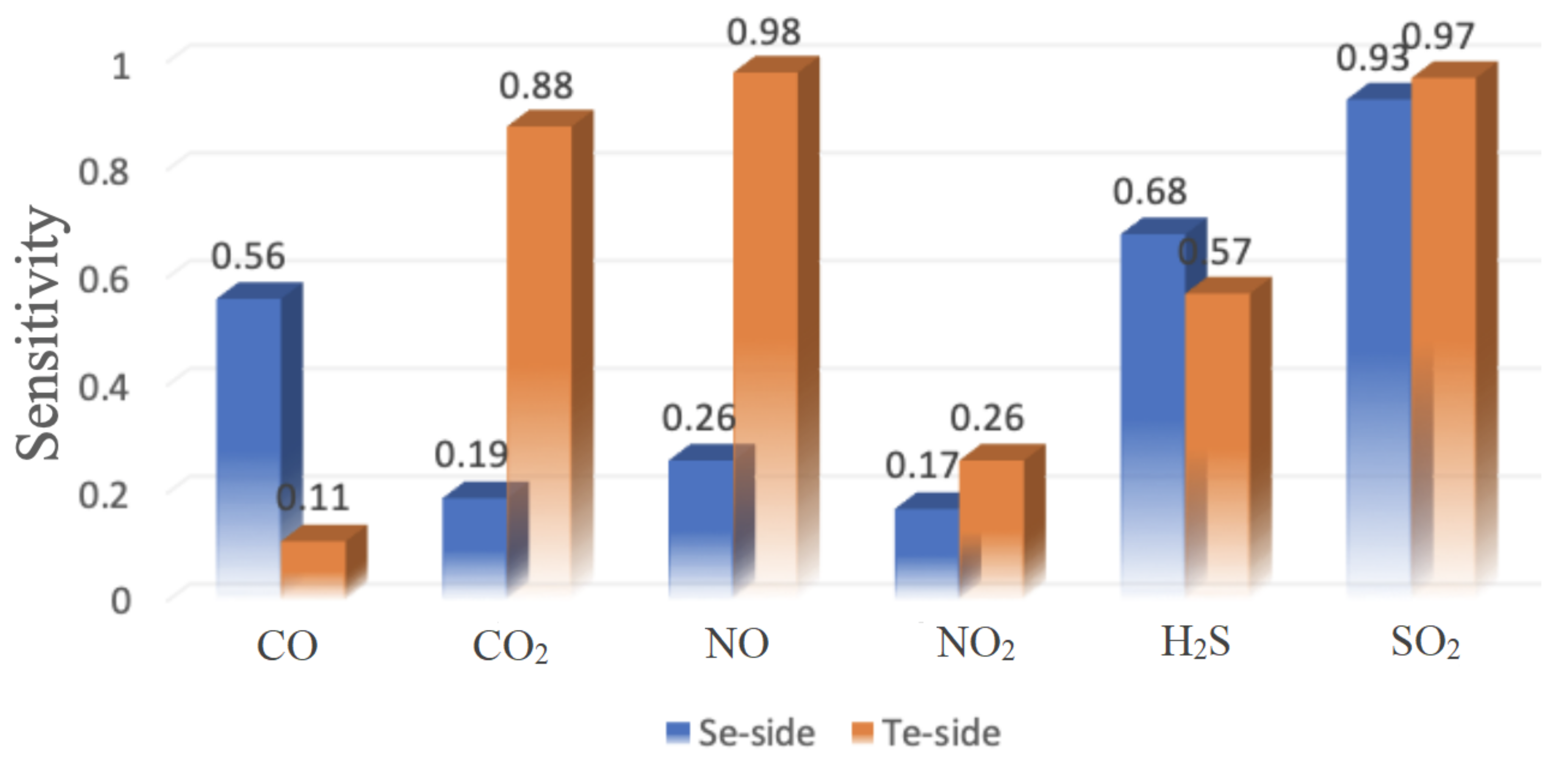
3.3. Transport Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Billings, P. Emission of hazardous air pollutants from coal-fired power plants. Environ. Health Eng. 2007. [Google Scholar] [CrossRef] [Green Version]
- American Lung Association. Toxic Air: The Case for Cleaning Up Coal-Fired Power Plants; American Lung Association (March 2011) Archived; American Lung Association: Chicago, IL, USA, 2012; Volume 26. [Google Scholar]
- Hong, M.K.; Bero, L.A. How the tobacco industry responded to an influential study of the health effects of secondhand smoke. BMJ 2002, 325, 1413–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrill, J.B.; Montgomery, R.R.; Reinhardt, C.F. Toxic gases from fires. Science 1978, 200, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Bessac, B.F.; Jordt, S.E. Sensory detection and responses to toxic gases: Mechanisms, health effects, and countermeasures. Proc. Am. Thorac. Soc. 2010, 7, 269–277. [Google Scholar] [CrossRef]
- Fisher, A.B. Oxygen therapy: Cide effects and toxicity. Am. Rev. Respir. Dis. 1980, 122, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Assael, M.J.; Kakosimos, K.E. Fires, Explosions, and Toxic Gas Dispersions: Effects Calculation and Risk Analysis; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Late, D.J.; Doneux, T.; Bougouma, M. Single-layer MoSe2 based NH3 gas sensor. Appl. Phys. Lett. 2014, 105, 233103. [Google Scholar] [CrossRef]
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx sensors based on semiconducting metal oxide nanostructures: Progress and perspectives. Sens. Actuators Chem. 2012, 171, 25–42. [Google Scholar] [CrossRef]
- Novoselov, K.; Jiang, D.; Schedin, F.J.; Booth, T.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652. [Google Scholar] [CrossRef]
- Kaewmaraya, T.; Ngamwongwan, L.; Moontragoon, P.; Jarernboon, W.; Singh, D.; Ahuja, R.; Karton, A.; Hussain, T. Novel green phosphorene as a superior chemical gas sensing material. J. Hazard. Mater. 2020, 401, 123340. [Google Scholar] [CrossRef]
- Vogt, P.; De Padova, P.; Quaresima, C.; Avila, J.; Frantzeskakis, E.; Asensio, M.C.; Resta, A.; Ealet, B.; Le Lay, G. Silicene: Compelling experimental evidence for graphenelike two-dimensional silicon. Phys. Rev. Lett. 2012, 108, 155501. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Singh, D.; Rajput, K.; Sonvane, Y. Germanene: A new electronic gas sensing material. RSC Adv. 2016, 6, 102264–102271. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, S.K.; Sonvane, Y.; Lukačević, I. Antimonene: A monolayer material for ultraviolet optical nanodevices. J. Mater. Chem. C 2016, 4, 6386–6390. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Gupta, S.K.; Lukačević, I.; Sonvane, Y. Indiene 2D monolayer: A new nanoelectronic material. RSC Adv. 2016, 6, 8006–8014. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, S.K.; Sonvane, Y.; Sahoo, S. Modulating the electronic and optical properties of monolayer arsenene phases by organic molecular doping. Nanotechnology 2017, 28, 495202. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single Layer MoS2 Transistors. Nat. Nano 2011, 6, 147. [Google Scholar] [CrossRef]
- Pandey, K.; Yadav, P.; Singh, D.; Gupta, S.K.; Sonvane, Y.; Lukačević, I.; Kim, J.; Kumar, M. First step to investigate nature of electronic states and transport in flower-like MoS2: Combining experimental studies with computational calculations. Sci. Rep. 2016, 6, 32690. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, S.K.; Sonvane, Y.; Kumar, A.; Ahuja, R. 2D-HfS2 as an efficient photocatalyst for water splitting. Catal. Sci. Technol. 2016, 6, 6605–6614. [Google Scholar] [CrossRef]
- Singh, D.; Kansara, S.; Gupta, S.K.; Sonvane, Y. Single layer of carbon phosphide as an efficient material for optoelectronic devices. J. Mater. Sci. 2018, 53, 8314–8327. [Google Scholar]
- Singh, D.; Gupta, S.K.; Sonvane, Y.; Ahuja, R. High performance material for hydrogen storage: Graphenelike Si2BN solid. Int. J. Hydrogen Energy 2017, 42, 22942–22952. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, D.; Sonvane, Y.; Ahuja, R. Two-dimensional boron monochalcogenide monolayer for thermoelectric material. Sustain. Energy Fuels 2020, 4, 2363–2369. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Chu, J.; Wang, Z.; Zhang, J.; Yang, A.; Li, X.; Gao, C.; Huang, H.; Wang, X.; Cheng, Y.; et al. Chemisorption of NO2 to MoS2 Nanostructures and its Effects for MoS2 Sensors. ChemNanoMat 2019, 5, 1123–1130. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent Advances in Emerging 2D Material-Based Gas Sensors: Potential in Disease Diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar] [CrossRef]
- Singh, D.; Shukla, V.; Panda, P.K.; Mishra, Y.K.; Rubahn, H.G.; Ahuja, R. Carbon-phosphide monolayer with high carrier mobility and perceptible I–V response for superior gas sensing. New J. Chem. 2020, 44, 3777–3785. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, Y.; Wu, H.; Zhang, Y.; Zhang, Y.; Wang, Y.; Zhang, Y.; Zhu, W.; Li, J.; Wu, D.; et al. Multifunctional Janus Microplates Arrays Actuated by Magnetic Fields for Water/Light Switches and Bio-Inspired Assimilatory Coloration. Adv. Mater. 2019, 31, 1807507. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Zhu, H.; Li, Y.; Alsaid, Y.; Fong, K.Y.; Zhou, Y.; Wang, S.; Shi, W.; Wang, Y.; et al. Structural phase transition in monolayer MoTe2 driven by electrostatic doping. Nature 2017, 550, 487–491. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, X. Two-dimensional magnetic crystals and emergent heterostructure devices. Science 2019, 363, 6428. [Google Scholar] [CrossRef]
- Yu, X.-F.; Li, Y.-C.; Cheng, J.-B.; Liu, Z.-B.; Li, Q.-Z.; Li, W.-Z.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A promising candidate for NH3 sensor or capturer with high sensitivity and selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef]
- Ai, W.; Kou, L.; Hu, X.; Wang, Y.; Krasheninnikov, A.V.; Sun, L.; Shen, X. Enhanced sensitivity of MoSe2 monolayer for gas adsorption induced by electric field. J. Phys. Condens. Matter 2019, 31, 445301. [Google Scholar] [CrossRef]
- Lu, A.Y.; Zhu, H.; Xiao, J.; Chuu, C.P.; Han, Y.; Chiu, M.H.; Cheng, C.C.; Yang, C.W.; Wei, K.H.; Yang, Y.; et al. Janus monolayers of transition metal dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jia, S.; Kholmanov, I.; Dong, L.; Er, D.; Chen, W.; Guo, H.; Jin, Z.; Shenoy, V.B.; Shi, L.; et al. Janus monolayer transition-metal dichalcogenides. ACS Nano 2017, 11, 8192–8198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Singh, D.; Xu, Z.; Wang, Z.; Ahuja, R. An emerging Janus MoSeTe material for potential applications in optoelectronic devices. J. Mater. Chem. C 2019, 7, 12312–12320. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Singh, D.; Xu, Z.; Ahuja, R. Sensing the polar molecules MH3 (M = N, P, or As) with a Janus NbTeSe monolayer. New J. Chem. 2020, 44, 7932–7940. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wu, X.; Wang, H.; Wang, Y. A Janus MoSSe monolayer: A potential wide solar-spectrum water-splitting photocatalyst with a low carrier recombination rate. J. Mater. Chem. A 2018, 6, 2295–2301. [Google Scholar] [CrossRef]
- Li, F.; Wei, W.; Zhao, P.; Huang, B.; Dai, Y. Electronic and optical properties of pristine and vertical and lateral heterostructures of Janus MoSSe and WSSe. J. Phys. Chem. Lett. 2017, 8, 5959–5965. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Li, T.; Li, S.; Liu, K. Ferromagnetism induced by point defect in Janus monolayer MoSSe regulated by strain engineering. J. Phys. Appl. Phys. 2018, 51, 105004. [Google Scholar] [CrossRef]
- Guo, S.D. Phonon transport in Janus monolayer MoSSe: A first-principles study. Phys. Chem. Chem. Phys. 2018, 20, 7236–7242. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Guo, Y.; Gao, H.; Guo, W. Tribo-piezoelectricity in Janus transition metal dichalcogenide bilayers: A first-principles study. Nano Energy 2019, 56, 33–39. [Google Scholar] [CrossRef]
- Long, C.; Gong, Z.R.; Jin, H.; Dai, Y. Observation of intrinsic dark exciton in Janus-MoSSe heterosturcture induced by intrinsic electric field. J. Phys. Condens. Matter 2018, 30, 395001. [Google Scholar] [CrossRef]
- Wang, M.; Pang, Y.; Liu, D.Y.; Zheng, S.H.; Song, Q.L. Tuning magnetism by strain and external electric field in zigzag Janus MoSSe nanoribbons. Comput. Mater. Sci. 2018, 146, 240–247. [Google Scholar] [CrossRef]
- Jin, C.; Tang, X.; Tan, X.; Smith, S.C.; Dai, Y.; Kou, L. A Janus MoSSe monolayer: A superior and strain-sensitive gas sensing material. J. Mater. Chem. A 2019, 7, 1099–1106. [Google Scholar] [CrossRef]
- Wang, D.; Lan, T.; Pan, J.; Liu, Z.; Yang, A.; Yang, M.; Chu, J.; Yuan, H.; Wang, X.; Li, Y.; et al. Janus MoSSe monolayer: A highly strain-sensitive gas sensing material to detect SF6 decompositions. Sens. Actuators A Phys. 2020, 311, 112049. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, L.; Ang, Y.S.; Ang, L.K. Superior and tunable gas sensing properties of Janus PtSSe monolayer. Nano Express 2020. [Google Scholar] [CrossRef]
- Chaurasiya, R.; Dixit, A. Defect engineered MoSSe Janus monolayer as a promising two dimensional material for NO2 and NO gas sensing. Appl. Surf. Sci. 2019, 490, 204–219. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.Y.; Hussain, T.; Wärnå, J.P.A.; Xu, Z.; Ahuja, R. Exploring Janus MoSSe monolayer as a workable media for SOF6 decompositions sensing based on DFT calculations. Comput. Mater. Sci. 2021, 186, 109976. [Google Scholar] [CrossRef]
- Chaurasiya, R.; Dixit, A. Ultrahigh sensitivity with excellent recovery time for NH3 and NO2 in pristine and defect mediated Janus WSSe monolayer. Phys. Chem. Chem. Phys. 2020, 22, 13903–13922. [Google Scholar] [CrossRef]
- Dou, K.P.; Hu, H.H.; Wang, X.; Wang, X.; Jin, H.; Zhang, G.P.; Shi, X.Q.; Kou, L. Asymmetrically flexoelectric gating effect of Janus transition-metal dichalcogenides and their sensor applications. J. Mater. Chem. C 2020, 8, 11457–11467. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundqvist, B.I. Van der Waals density functional for general geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Serrano, Á.; Hine, N.D.; Skylaris, C.K. Pulay forces from localized orbitals optimized in situ using a psinc basis set. J. Chem. Phys. 2012, 136, 234101. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Sun, L.; Benassi, E.; Shen, Z.; Zhao, X.; Sanvito, S.; Hou, S. Spin filter effect of manganese phthalocyanine contacted with single-walled carbon nanotube electrodes. J. Chem. Phys. 2010, 132, 054703. [Google Scholar] [CrossRef]
- Rocha, A.R.; García-Suárez, V.M.; Bailey, S.; Lambert, C.; Ferrer, J.; Sanvito, S. Spin and molecular electronics in atomically generated orbital landscapes. Phys. Rev. B 2006, 73, 085414. [Google Scholar] [CrossRef] [Green Version]
- Román-Pérez, G.; Soler, J.M. Efficient implementation of a van der Waals density functional: Application to double-wall carbon nanotubes. Phys. Rev. Lett. 2009, 103, 096102. [Google Scholar] [CrossRef] [Green Version]
- Datta, S. Electronic Transport in Mesoscopic Systems; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Yang, X.; Banerjee, A.; Ahuja, R. Structural Insight of the Frailty of 2D Janus NbSeTe as an Active Photocatalyst. ChemCatChem 2020, 12, 6013–6023. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Ni, J.; Shi, L.; Shi, S.; Tang, W. First principles study of structural, vibrational and electronic properties of graphene-like MX2 (M = Mo, Nb, W, Ta; X = S, Se, Te) monolayers. Phys. B Condens. Matter 2011, 406, 2254–2260. [Google Scholar] [CrossRef]
- Hussain, T.; Singh, D.; Gupta, S.K.; Karton, A.; Sonvane, Y.; Ahuja, R. Efficient and selective sensing of nitrogen-containing gases by Si2BN nanosheets under pristine and pre-oxidized conditions. Appl. Surf. Sci. 2019, 469, 775–780. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wilcox, J. CO2 adsorption on carbon models of organic constituents of gas shale and coal. Environ. Sci. Technol. 2011, 45, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, Y.; Wang, H.; Zhao, J.; Cai, Q.; Wang, X. Gas adsorption on silicene: A theoretical study. Comput. Mater. Sci. 2014, 87, 218–226. [Google Scholar] [CrossRef]
- Xia, W.; Hu, W.; Li, Z.; Yang, J. A first-principles study of gas adsorption on germanene. Phys. Chem. Chem. Phys. 2014, 16, 22495–22498. [Google Scholar] [CrossRef]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a superior gas sensor: Selective adsorption and distinct I–V response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef] [Green Version]
- Shokri, A.; Salami, N. Gas sensor based on MoS2 monolayer. Sens. Actuators B Chem. 2016, 236, 378–385. [Google Scholar] [CrossRef]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.J.; Park, S.G.; Kwon, J.D.; Kim, C.S.; Song, M.; et al. Charge-transfer-based gas sensing using atomic-layer MoS2. Sci. Rep. 2015, 5, 8052. [Google Scholar] [CrossRef]
- Singh, D.; Panda, P.K.; Mishra, Y.K.; Ahuja, R. Van der Waals induced molecular recognition of canonical DNA nucleobases on a 2D GaS monolayer. Phys. Chem. Chem. Phys. 2020, 22, 6706–6715. [Google Scholar] [CrossRef]
- Bano, A.; Krishna, J.; Pandey, D.K.; Gaur, N. An ab initio study of sensing applications of MoB2 monolayer: A potential gas sensor. Phys. Chem. Chem. Phys. 2019, 21, 4633–4640. [Google Scholar] [CrossRef]
- Kumar, V.; Azhikodan, D.; Roy, D.R. 2D Sb2C3 monolayer: A promising material for recyclable gas sensor for environmentally toxic nitrogen-containing gases (NCGs). J. Hazard. Mater. 2020, 124168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rajput, K.; Roy, D.R. Monolayer Bi2C3: A promising sensor for environmentally toxic NCGs with high sensitivity and selectivity. Appl. Surf. Sci. 2020, 534, 147609. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, W.; Lu, N.; Zhuo, Z.; Zeng, X.C.; Wu, X.; Yang, J. CO2 capture on h-BN sheet with high selectivity controlled by external electric field. J. Phys. Chem. C 2015, 119, 6912–6917. [Google Scholar] [CrossRef]
- Rajput, K.; Kumar, V.; Roy, D.R. Heterobilayer CaS/CaSe: A promising sensor for environmental toxic NO2 gas with high selectivity and sensitivity. Appl. Surf. Sci. 2020, 528, 146996. [Google Scholar] [CrossRef]



| System | ESe) | ETe | ||||
|---|---|---|---|---|---|---|
| CO | −0.15 | −0.12 | −0.0159 | −0.0065 | 4.946 | 5.083 |
| CO | −0.20 | −0.19 | −0.0157 | −0.0075 | 5.092 | 5.092 |
| NO | −0.55 | −0.53 | −0.1297 | 0.024 | 4.99 | 5.044 |
| NO | −0.59 | −0.68 | −0.284 | −0.533 | 5.253 | 5.098 |
| HS | −0.18 | −0.13 | −0.092 | 0.0235 | 5.157 | 5.267 |
| SO | −0.26 | −0.25 | −0.1614 | −0.135 | 5.338 | 5.366 |
| System | ||||||
|---|---|---|---|---|---|---|
| CO | 4.95 | 5.08 | 2.8 ps | 1.4 ps | 0.58 (0.2 V) | 0.39 (0.1 V) |
| CO | 5.09 | 5.09 | 23.1 ps | 13.3 ps | 0.72 (0.1 V) | 0.88 (0.3 V) |
| NO | 4.99 | 5.04 | 17.49 s | 8.07 s | 0.34 (0.5 V) | 0.98 (0.3 V) |
| NO | 5.25 | 5.10 | 75.74 s | 3015.54 s | 0.45 (1 V) | 0.56 (0.9 V) |
| HS | 5.17 | 5.27 | 11.9 ps | 1.78 ps | 0.68 (0.3 V) | 0.70 (0.2 V) |
| SO | 5.34 | 5.36 | 251.9 ps | 204.6 ps | 0.94 (0.5 V) | 0.97 (0.3 V) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.; Ahuja, R. Highly Sensitive Gas Sensing Material for Environmentally Toxic Gases Based on Janus NbSeTe Monolayer. Nanomaterials 2020, 10, 2554. https://doi.org/10.3390/nano10122554
Singh D, Ahuja R. Highly Sensitive Gas Sensing Material for Environmentally Toxic Gases Based on Janus NbSeTe Monolayer. Nanomaterials. 2020; 10(12):2554. https://doi.org/10.3390/nano10122554
Chicago/Turabian StyleSingh, Deobrat, and Rajeev Ahuja. 2020. "Highly Sensitive Gas Sensing Material for Environmentally Toxic Gases Based on Janus NbSeTe Monolayer" Nanomaterials 10, no. 12: 2554. https://doi.org/10.3390/nano10122554
APA StyleSingh, D., & Ahuja, R. (2020). Highly Sensitive Gas Sensing Material for Environmentally Toxic Gases Based on Janus NbSeTe Monolayer. Nanomaterials, 10(12), 2554. https://doi.org/10.3390/nano10122554