Multifunctional Nanocarriers for Lung Drug Delivery
Abstract
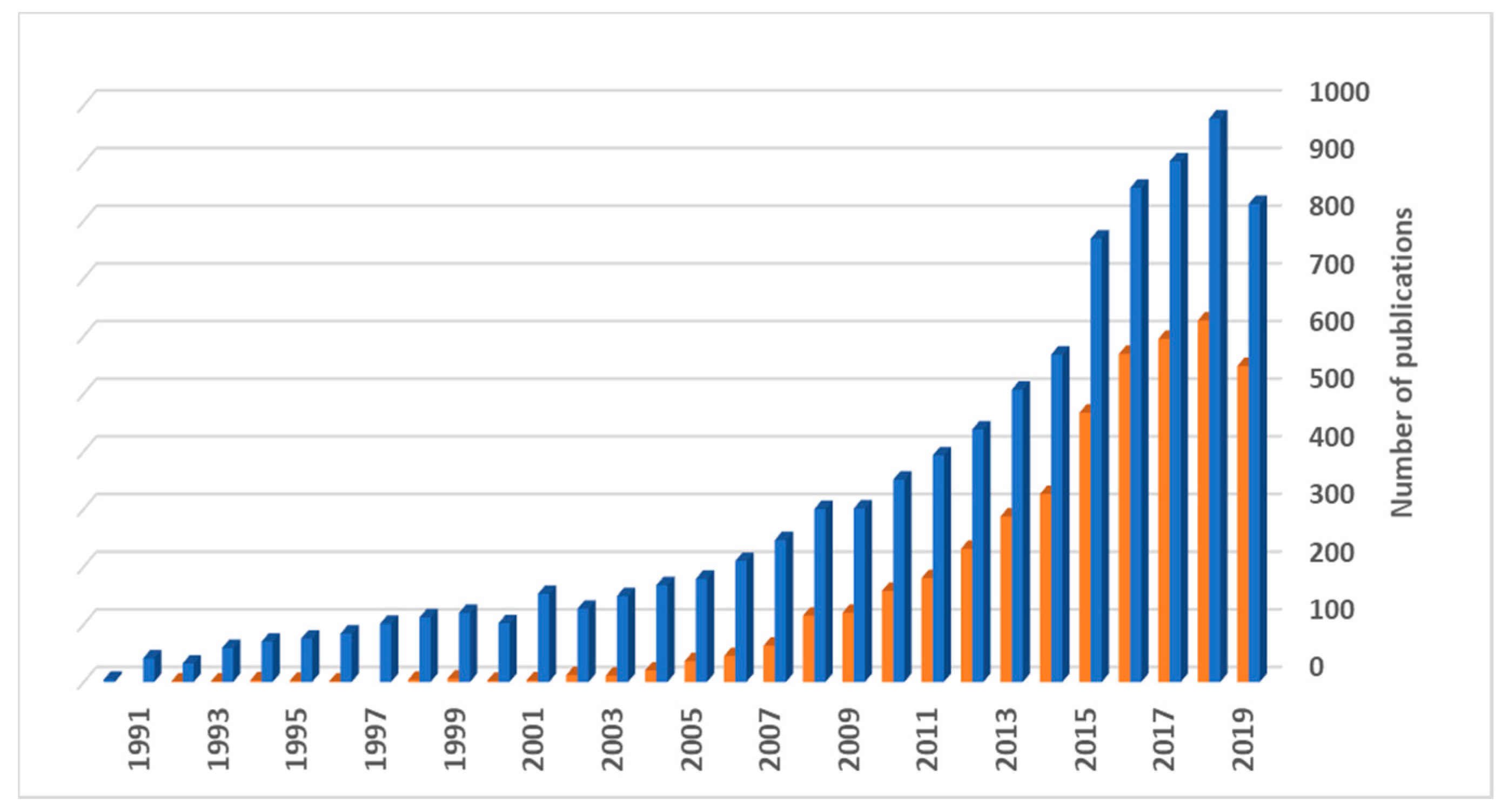
:1. Introduction
2. Lung Drug Delivery Mediated by Multifunctional Nanocarriers
2.1. Delivery of Proteins and Protein-Based Materials
2.2. Delivery of Antibiotics
2.3. Applications in Cancer Therapy
3. Expectations for the Future
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, L.; Kruger, H.G.; Maguire, G.E.M.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Peres, C.; Matos, A.I.; Conniot, J.; Sainz, V.; Zupančič, E.; Silva, J.M.; Graça, L.; Sá Gaspar, R.; Préat, V.; Florindo, H.F. Poly(lactic acid)-based particulate systems are promising tools for immune modulation. Acta Biomater. 2017, 48, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, F.; Li, L.; Gu, W.; Xu, Z.P. Nanoformulations of albendazole as effective anticancer and antiparasite agents. Nanomedicine 2017, 12, 2555–2574. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P. Delivering drugs to the lungs: The history of repurposing in the treatment of respiratory diseases. Adv. Drug Deliv. Rev. 2018, 133, 5–18. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef]
- Pacheco, R.M. (Ed.) Tratado de Tecnología Farmacéutica, Volumen II: Operaciones Básicas; Editorial Síntesis, S.A.: Madrid, Spain, 2016; p. 458. [Google Scholar]
- Edgar, J.Y.C.; Wang, H. Introduction for design of nanoparticle based drug delivery systems. Curr. Pharm. Des. 2017, 23, 2108–2112. [Google Scholar] [CrossRef]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.; Alves, A.D.; Cavaco, J.S.; Pontes, J.F.; Guerreiro, F.; Rosa da Costa, A.M.; Buttini, F.; Grenha, A. Dual antibiotherapy of tuberculosis mediated by inhalable locust bean gum microparticles. Int. J. Pharm. 2017, 529, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Rodrigues, S.; Rosa da Costa, A.M.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable fucoidan microparticles combining two antitubercular drugs with potential application in pulmonary tuberculosis therapy. Polymers 2018, 10, 636. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Mi, G.; Hickey, D.; Li, Y.; Tu, J.; Webster, T.J.; Shen, Y. Azithromycin-loaded respirable microparticles for targeted pulmonary delivery for the treatment of pneumonia. Biomaterials 2018, 160, 107–123. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Zhang, M.; Jin, Y. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. Int. J. Pharm. 2018, 551, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Rivera Gil, P.; Hühn, D.; del Mercato, L.L.; Sasse, D.; Parak, W.J. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol. Res. 2010, 62, 115–125. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). Nanotechnologies—Vocabulary—Part 2: Nano-Objects; International Organization for Standardization (ISO): Geneva, Switzerland, 2015; Volume ISO/TS 80004-2-2015. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for Industry—Considering whether an FDA-Regulated Product Involves the Application of Nanotechnology; U.S. Department of Health and Human Services, Ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2014; Volume FDA-2010-D-0530.
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Huynh Mai, C.; Thanh Diep, T.; Le, T.T.T.; Nguyen, V. Advances in colloidal dispersions: A review. J. Dispers. Sci. Technol. 2019, 1–16. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 27–40. [Google Scholar] [CrossRef]
- Cordani, M.; Somoza, Á. Targeting autophagy using metallic nanoparticles: A promising strategy for cancer treatment. Cell. Mol. Life Sci. 2019, 76, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Hoang Thi, T.T.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Csaba, N.; Garcia-Fuentes, M.; Alonso, M.J. The performance of nanocarriers for transmucosal drug delivery. Expert Opin. Drug Deliv. 2006, 3, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [Green Version]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Weissig, V.; Guzman-Villanueva, D. Nanopharmaceuticals (part 2): Products in the pipeline. Int. J. Nanomed. 2015, 10, 1245–1257. [Google Scholar] [CrossRef] [Green Version]
- Global Health Estimates 2016: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization (WHO): Geneva, Switzerland, 2018.
- Hillery, A.M.; Park, K. Drug Delivery—Fundamentals & Applications, 2nd ed.; CRC Press: New York, NY, USA, 2017. [Google Scholar]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- Borghardt, J.M.; Kloft, C.; Sharma, A. Inhaled therapy in respiratory disease: The complex interplay of pulmonary kinetic processes. Can. Respir. J. 2018, 2018, 2732017. [Google Scholar] [CrossRef] [Green Version]
- Mack, G.S. Pfizer dumps Exubera. Nat. Biotechnol. 2007, 25, 1331–1332. [Google Scholar] [CrossRef]
- Zaykov, A.N.; Mayer, J.P.; DiMarchi, R.D. Pursuit of a perfect insulin. Nat. Rev. Drug Discov. 2016, 15, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.J.; Grenha, A. Chapter 22—Technosphere®: An inhalation system for pulmonary delivery of biopharmaceuticals. In Mucosal Delivery of Biopharmaceuticals: Biology, Challenges and Strategies; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Jesus, S.; Schmutz, M.; Som, C.; Borchard, G.; Wick, P.; Borges, O. Hazard assessment of polymeric nanobiomaterials for drug delivery: What can we learn from literature so far. Front. Bioeng. Biotechnol. 2019, 7, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenha, A.; Seijo, B.; Remuñán-Lopez, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.P.; Vital, J.; Leiva, M.C.; Goncalves, L.M.D.; Taboada, P.; Remuñán-Lopez, C.; Vítor, J.; Almeida, A.J. Transfection of pulmonary cells by stable pDNA-polycationic hybrid nanostructured particles. Nanomedicine 2019, 14, 407–429. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, X.; Zhang, J.; Pan, S.; Yang, C.; Wei, Y.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. pH-responsive hybrid nanoparticle with enhanced dissociation characteristic for siRNA delivery. Int. J. Nanomed. 2018, 13, 6885–6902. [Google Scholar] [CrossRef] [Green Version]
- Abd Elwakil, M.M.; Mabrouk, M.T.; Helmy, M.W.; Abdelfattah, E.-Z.A.; Khiste, S.K.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable lactoferrin-chondroitin nanocomposites for combined delivery of doxorubicin and ellagic acid to lung carcinoma. Nanomedicine 2018, 13, 2015–2035. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Gaspar, M.M.; Eleutério, C.V.; Grenha, A.; Blanco, M.; Goncalves, L.M.D.; Taboada, P.; Almeida, A.J.; Remuñán-Lopez, C. Microencapsulated solid lipid nanoparticles as a hybrid platform for pulmonary antibiotic delivery. Mol. Pharm. 2017, 14, 2977–2990. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Pacheco, R.M. (Ed.) Tratado de Tecnología Farmacéutica, Volumen III: Formas de Dosificación; Editorial Síntesis, S.A.: Madrid, Spain, 2017; p. 458. [Google Scholar]
- Grenha, A.; Remuñán-López, C.; Carvalho, E.L.S.; Seijo, B. Microspheres containing lipid/chitosan nanoparticles complexes for pulmonary delivery of therapeutic proteins. Eur. J. Pharm. Biopharm. 2008, 69, 83–93. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Grenha, A.; Carrion-Recio, D.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control. Release 2012, 157, 383–390. [Google Scholar] [CrossRef]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remuñán-Lopez, C.; Forbes, B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Liu, Y.; Ke, L.; Fang, Y.; Yang, R.; Cui, F. Epsilon-poly-L-lysine guided improving pulmonary delivery of supramolecular self-assembled insulin nanospheres. Int. J. Biol. Macromol. 2015, 72, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gong, T.; Fu, H.; Wang, C.; Wang, X.; Chen, Q.; Zhang, Q.; He, Q.; Zhang, Z. Solid lipid nanoparticles for pulmonary delivery of insulin. Int. J. Pharm. 2008, 356, 333–344. [Google Scholar] [CrossRef]
- Buttini, F.; Colombo, G.; Kwok, P.C.L.; Wui, W.T. Chapter 6—Aerodynamic assessment for inhalation products: Fundamentals and current pharmacopoeial methods. In Inhalation Drug Delivery: Techniques and Products; John Wiley & Sons: Chichester, UK, 2013; pp. 91–119. [Google Scholar] [CrossRef]
- Sinsuebpol, C.; Chatchawalsaisin, J.; Kulvanich, P. Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery. Drug Des. Dev. Ther. 2013, 7, 861–873. [Google Scholar] [CrossRef] [Green Version]
- Kaye, R.S.; Purewal, T.S.; Alpar, H.O. Simultaneously manufactured nano-in-micro (SIMANIM) particles for dry-powder modified-release delivery of antibodies. J. Pharm. Sci. 2009, 98, 4055–4068. [Google Scholar] [CrossRef]
- De Backer, L.; Cerrada, A.; Pérez-Gil, J.; De Smedt, S.C.; Raemdonck, K. Bio-inspired materials in drug delivery: Exploring the role of pulmonary surfactant in siRNA inhalation therapy. J. Control. Release 2015, 220, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Lipka, J.; Semmler-Behnke, M.; Wenk, A.; Burkhardt, J.; Aigner, A.; Kreyling, W. Biokinetic studies of non-complexed siRNA versus nano-sized PEI F25-LMW/siRNA polyplexes following intratracheal instillation into mice. Int. J. Pharm. 2016, 500, 227–235. [Google Scholar] [CrossRef]
- Hu, Q.; Bai, X.; Hu, G.; Zuo, Y.Y. Unveiling the molecular structure of pulmonary surfactant corona on nanoparticles. ACS Nano 2017, 11, 6832–6842. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Jiao, B.; Shi, X.; Valle, R.P.; Fan, Q.; Zuo, Y.Y. Physicochemical properties of nanoparticles regulate translocation across pulmonary surfactant monolayer and formation of lipoprotein corona. ACS Nano 2013, 7, 10525–10533. [Google Scholar] [CrossRef]
- Nguyen, J.; Reul, R.; Betz, T.; Dayyoub, E.; Schmehl, T.; Gessler, T.; Bakowsky, U.; Seeger, W.; Kissel, T. Nanocomposites of lung surfactant and biodegradable cationic nanoparticles improve transfection efficiency to lung cells. J. Control. Release 2009, 140, 47–54. [Google Scholar] [CrossRef]
- Agnoletti, M.; Bohr, A.; Thanki, K.; Wan, F.; Zeng, X.; Boetker, J.P.; Yang, M.; Foged, C. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur. J. Pharm. Biopharm. 2017, 120, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.M.; Rejman, J.; Guan, S.; Rosenecker, J. Nebulisation of IVT mRNA complexes for intrapulmonary administration. PLoS ONE 2015, 10, e0137504. [Google Scholar] [CrossRef] [PubMed]
- Kunda, N.K.; Alfagih, I.M.; Miyaji, E.N.; Figueiredo, D.B.; Goncalves, V.M.; Ferreira, D.M.; Dennison, S.R.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Pulmonary dry powder vaccine of pneumococcal antigen loaded nanoparticles. Int. J. Pharm. 2015, 495, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, V.; Chichester, J.A.; Ebensen, T.; Schwarz, K.; Hartman, C.E.; Shoji, Y.; Guzman, C.A.; Yusibov, V.; Sewald, K.; Braun, A. A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung. Vaccine 2014, 32, 3216–3222. [Google Scholar] [CrossRef]
- Scherließ, R.; Etschmann, C. DPI formulations for high dose applications—Challenges and opportunities. Int. J. Pharm. 2018, 548, 49–53. [Google Scholar] [CrossRef]
- Scherließ, R. Future of nanomedicines for treating respiratory diseases. Expert Opin. Drug Deliv. 2019, 16, 59–68. [Google Scholar] [CrossRef]
- Brinkac, L.; Voorhies, A.; Gomez, A.; Nelson, K.E. The threat of antimicrobial resistance on the human microbiome. Microb. Ecol. 2017, 74, 1001–1008. [Google Scholar] [CrossRef]
- Woods, A.; Rahman, K.M. Antimicrobial molecules in the lung: Formulation challenges and future directions for innovation. Future Med. Chem. 2018, 10, 575–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyt, C.E.; Hékimian, G.; Bréchot, N.; Chastre, J. Aerosol therapy for pneumonia in the intensive care unit. Clin. Chest Med. 2018, 39, 823–836. [Google Scholar] [CrossRef]
- Alves, A.D.; Cavaco, J.S.; Guerreiro, F.; Lourenco, J.P.; Rosa da Costa, A.M.; Grenha, A. Inhalable antitubercular therapy mediated by locust bean gum microparticles. Molecules 2016, 21, 702. [Google Scholar] [CrossRef] [Green Version]
- Global Tuberculosis Report 2018; World Health Organization (WHO): Geneva, Switzerland, 2018.
- Guerreiro, F.; Pontes, J.F.; Rosa da Costa, A.M.; Grenha, A. Spray-drying of konjac glucomannan to produce microparticles for an application as antitubercular drug carriers. Powder Technol. 2019, 342, 246–252. [Google Scholar] [CrossRef]
- Maretti, E.; Costantino, L.; Buttini, F.; Rustichelli, C.; Leo, E.; Truzzi, E.; Iannuccelli, V. Newly synthesized surfactants for surface mannosylation of respirable SLN assemblies to target macrophages in tuberculosis therapy. Drug Deliv. Transl. Res. 2018, 9, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Auger, M.J.; Ross, J.A. Chapter 1—The biology of the macrophage. In The Macrophage: The Natural Immune System, 1st ed.; Lewis, C.E., McGee, J.O.D., Eds.; Oxford University Press: New York, NY, USA, 1992. [Google Scholar]
- Maretti, E.; Costantino, L.; Rustichelli, C.; Leo, E.; Croce, M.A.; Buttini, F.; Truzzi, E.; Iannuccelli, V. Surface engineering of Solid Lipid Nanoparticle assemblies by methyl α-d-mannopyranoside for the active targeting to macrophages in anti-tuberculosis inhalation therapy. Int. J. Pharm. 2017, 528, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Melis, V.; Manca, M.L.; Bullita, E.; Tamburini, E.; Castangia, I.; Cardia, M.C.; Valenti, D.; Fadda, A.M.; Peris, J.E.; Manconi, M. Inhalable polymer-glycerosomes as safe and effective carriers for rifampicin delivery to the lungs. Colloids Surf. B Biointerfaces 2016, 143, 301–308. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [Green Version]
- Kwon, M.Y.; Wang, C.; Galarraga, J.H.; Pure, E.; Han, L.; Burdick, J.A. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials 2019, 222, 119451. [Google Scholar] [CrossRef]
- East, L.; Isacke, C.M. The mannose receptor family. Biochim. Biophys. Acta (BBA) 2002, 1572, 364–386. [Google Scholar] [CrossRef]
- Shah, K.; Chan, L.W.; Wong, T.W. Critical physicochemical and biological attributes of nanoemulsions for pulmonary delivery of rifampicin by nebulization technique in tuberculosis treatment. Drug Deliv. 2017, 24, 1631–1647. [Google Scholar] [CrossRef] [Green Version]
- Pourshahab, P.S.; Gilani, K.; Moazeni, E.; Eslahi, H.; Fazeli, M.R.; Jamalifar, H. Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid. J. Microencapsul. 2011, 28, 605–613. [Google Scholar] [CrossRef]
- Rawal, T.; Patel, S.; Butani, S. Chitosan nanoparticles as a promising approach for pulmonary delivery of bedaquiline. Eur. J. Pharm. Sci. 2018, 124, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Rodrigues, S.; Rosa da Costa, A.M.; Faleiro, L.; Buttini, F.; Grenha, A. Inhalable chitosan microparticles for simultaneous delivery of isoniazid and rifabutin in lung tuberculosis treatment. Drug Dev. Ind. Pharm. 2019, 45, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liao, W.; Wang, W.; Zhou, J.; Tan, W.; Xiang, W.; Zhang, J.; Guo, L.; Chen, T.; Ma, D.; et al. Genipin-crosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydr. Polym. 2018, 197, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ong, V.; Mei, V.; Cao, L.; Lee, K.; Chung, E.J. Nanomedicine for cystic fibrosis. SLAS Technol. 2019, 24, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, J.; Altay, A.; Zdimal, V.; Maskova, L.; Sonvico, F.; Quarta, E.; Rossi, A.; Buttini, F.; Colombo, G. Dry powder inhaler of colistimethate sodium for lung infections in cystic fibrosis: Optimization of powder construction. Drug Dev. Ind. Pharm. 2019, 1664–1673. [Google Scholar] [CrossRef]
- Akdag Cayli, Y.; Sahin, S.; Buttini, F.; Balducci, A.G.; Montanari, S.; Vural, I.; Oner, L. Dry powders for the inhalation of ciprofloxacin or levofloxacin combined with a mucolytic agent for cystic fibrosis patients. Drug Dev. Ind. Pharm. 2017, 43, 1378–1389. [Google Scholar] [CrossRef]
- Moreno-Sastre, M.; Pastor, M.; Esquisabel, A.; Sans, E.; Viñas, M.; Fleischer, A.; Palomino, E.; Bachiller, D.; Pedraz, J.L. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2016, 498, 263–273. [Google Scholar] [CrossRef]
- Schneider, C.S.; Xu, Q.; Boylan, N.J.; Chisholm, J.; Tang, B.C.; Schuster, B.S.; Henning, A.; Ensign, L.M.; Lee, E.; Adstamongkonkul, P.; et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv. 2017, 3, e1601556. [Google Scholar] [CrossRef] [Green Version]
- d’Angelo, I.; Casciaro, B.; Miro, A.; Quaglia, F.; Mangoni, M.L.; Ungaro, F. Overcoming barriers in Pseudomonas aeruginosa lung infections: Engineered nanoparticles for local delivery of a cationic antimicrobial peptide. Colloids Surf. B Biointerfaces 2015, 135, 717–725. [Google Scholar] [CrossRef]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; di Villa Bianca, R.D.E.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Du, J.; El-Sherbiny, I.M.; Smyth, H.D. Swellable ciprofloxacin-loaded nano-in-micro hydrogel particles for local lung drug delivery. AAPS PharmSciTech 2014, 15, 1535–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Liu, J.; Liu, C.; Yang, A.; Qiao, J. Quorum sensing for population-level control of bacteria and potential therapeutic applications. Cell. Mol. Life Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Husari, A.; Maurer, C.K.; Lu, C.; de Rossi, C.; Steinbach, A.; Hartmann, R.W.; Lehr, C.M.; Schneider, M. Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J. Control. Release 2014, 192, 131–140. [Google Scholar] [CrossRef]
- Cheng, T.Y.D.; Cramb, S.M.; Baade, P.D.; Youlden, D.R.; Nwogu, C.; Reid, M.E. The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics. J. Thorac. Oncol. 2016, 11, 1653–1671. [Google Scholar] [CrossRef] [Green Version]
- Fact Sheet on Lung Cancer; Global Cancer Observatory: Cancer Today, International Agency for Research on Cancer, World Health Organization (WHO): Lyon, France, 2019.
- Wu, L.; Leng, D.; Cun, D.; Foged, C.; Yang, M. Advances in combination therapy of lung cancer: Rationales, delivery technologies and dosage regimens. J. Control. Release 2017, 260, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Ghadiri, M.; Leong, C.R.; Young, P.M.; Traini, D. The potential to treat lung cancer via inhalation of repurposed drugs. Adv. Drug Deliv. Rev. 2018, 133, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Ohkoshi, T.; Hirota, K.; Sonavane, G.S.; Nakajima, T.; Terada, H.; Komuro, M.; Kitazato, K.; Makino, K. Preparation and properties of inhalable nanocomposite particles for treatment of lung cancer. Colloids Surf. B Biointerfaces 2009, 71, 177–182. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta 2015, 1856, 189–210. [Google Scholar] [CrossRef] [Green Version]
- Rosière, R.; Hureaux, J.; Levet, V.; Amighi, K.; Wauthoz, N. La chimiothérapie inhalée—Partie 1: Concept et challenges technologiques actuels. Rev. Des Mal. Respir. 2018, 35, 357–377. [Google Scholar] [CrossRef]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Mottaghitalab, F.; Farokhi, M.; Fatahi, Y.; Atyabi, F.; Dinarvand, R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. J. Control. Release 2019, 295, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gadoue, S.M.; Toomeh, D. Radio-sensitization efficacy of gold nanoparticles in inhalational nanomedicine and the adverse effect of nano-detachment due to coating inactivation. Phys. Med. 2019, 60, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold nanoparticles in cancer treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Kumar, R.; Korideck, H.; Ngwa, W.; Berbeco, R.I.; Makrigiorgos, G.M.; Sridhar, S. Third generation gold nanoplatform optimized for radiation therapy. Transl. Cancer Res. 2013, 2. [Google Scholar] [CrossRef]
- Di Pietro, P.; Zaccaro, L.; Comegna, D.; Del Gatto, A.; Saviano, M.; Snyders, R.; Cossement, D.; Satriano, C.; Rizzarelli, E. Silver nanoparticles functionalized with a fluorescent cyclic RGD peptide: A versatile integrin targeting platform for cells and bacteria. RSC Adv. 2016, 6, 112381–112392. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Ucakar, B.; Joudiou, N.; Riva, R.; Jérôme, C.; Gallez, B.; Danhier, F.; Préat, V. Paclitaxel-loaded multifunctional nanoparticles for the targeted treatment of glioblastoma. J. Drug Target. 2019, 27, 614–623. [Google Scholar] [CrossRef]
- Herter-Sprie, G.S.; Korideck, H.; Christensen, C.L.; Herter, J.M.; Rhee, K.; Berbeco, R.I.; Bennett, D.G.; Akbay, E.A.; Kozono, D.; Mak, R.H.; et al. Image-guided radiotherapy platform using single nodule conditional lung cancer mouse models. Nat. Commun. 2014, 5, 5870. [Google Scholar] [CrossRef] [Green Version]
- Ngwa, W.; Kumar, R.; Moreau, M.; Dabney, R.; Herman, A. Nanoparticle drones to target lung cancer with radiosensitizers and cannabinoids. Front. Oncol. 2017, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Hamzawy, M.A.; Abo-Youssef, A.M.; Salem, H.F.; Mohammed, S.A. Antitumor activity of intratracheal inhalation of temozolomide (TMZ) loaded into gold nanoparticles and/or liposomes against urethane-induced lung cancer in BALB/c mice. Drug Deliv. 2017, 24, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Kabary, D.M.; Helmy, M.W.; Abdelfattah, E.Z.A.; Fang, J.Y.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable multi-compartmental phospholipid enveloped lipid core nanocomposites for localized mTOR inhibitor/herbal combined therapy of lung carcinoma. Eur. J. Pharm. Biopharm. 2018, 130, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumour therapy by inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Karra, N.; Nassar, T.; Laenger, F.; Benita, S.; Borlak, J. Safety and proof-of-concept efficacy of inhaled drug loaded nano- and immunonanoparticles in a c-Raf transgenic lung cancer model. Curr. Cancer Drug Targets 2012, 13, 11–29. [Google Scholar] [CrossRef]
- Nan, Y. Lung carcinoma therapy using epidermal growth factor receptortargeted lipid polymeric nanoparticles coloaded with cisplatin and doxorubicin. Oncol. Rep. 2019, 42, 2087–2096. [Google Scholar] [CrossRef]
- Pancewicz-Wojtkiewicz, J. Epidermal growth factor receptor and notch signaling in non-small-cell lung cancer. Cancer Med. 2016, 5, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Pitts, A.E.; Corey, D.R. Inhibition of human telomerase by 2’-O-methyl-RNA. Proc. Natl. Acad. Sci. USA 1998, 95, 11549–11554. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Murdter, T.E.; Philippi, C.; Loretz, B.; Schaefer, U.F.; Lehr, C.M.; Schwab, M.; Ammon-Treiber, S. Pulmonary delivery and tissue distribution of aerosolized antisense 2’-O-Methyl RNA containing nanoplexes in the isolated perfused and ventilated rat lung. Eur. J. Pharm. Biopharm. 2012, 81, 478–485. [Google Scholar] [CrossRef]
- Choi, S.H.; Byeon, H.J.; Choi, J.S.; Thao, L.; Kim, I.; Lee, E.S.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer. J. Control. Release 2015, 197, 199–207. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hassanzadeh, F.; Mardani, A.; Rostami, M. Feasibility of haloperidol-anchored albumin nanoparticles loaded with doxorubicin as dry powder inhaler for pulmonary delivery. Pharm. Dev. Technol. 2015, 20, 183–196. [Google Scholar] [CrossRef]
- Lakkadwala, S.; Nguyen, S.; Nesamony, J.; Narang, A.S.; Boddu, S.H. Chapter 7—Smart Polymers in Drug Delivery. In Excipient Applications in Formulation Design and Drug Delivery; Narang, A.S., Boddu, S.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, Y.; Guo, Z.; Chen, J.; Lin, L.; Wu, J.; Tian, H.; Chen, X. Pulmonary delivery by exploiting doxorubicin and cisplatin co-loaded nanoparticles for metastatic lung cancer therapy. J. Control. Release 2019, 295, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q. Co-spray dried mannitol/poly(amidoamine)-doxorubicin dry-powder inhaler formulations for lung adenocarcinoma: Morphology, in vitro evaluation, and aerodynamic performance. AAPS PharmSciTech 2018, 19, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Humia, B.V.; Punjabi, A.R.; Padilha, F.F.; da Rocha, S.R.P. The interaction of dendrimer-doxorubicin conjugates with a model pulmonary epithelium and their cosolvent-free, pseudo-solution formulations in pressurized metered-dose inhalers. Eur. J. Pharm. Sci. 2017, 109, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; McLeod, V.M.; Ryan, G.M.; Kelly, B.D.; Haynes, J.M.; Williamson, M.; Thienthong, N.; Owen, D.J.; Porter, C.J.H. Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J. Control. Release 2014, 183, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.U.; Kuriakose, A.; Iyer, R.; Hernandez, E.; Gandee, L.; Zhang, S.; Takahashi, M.; Zhang, Z.; Saha, D.; Nguyen, K.T. Dual-drug containing core-shell nanoparticles for lung cancer therapy. Sci. Rep. 2017, 7, 13249. [Google Scholar] [CrossRef] [Green Version]
- Smulders, S.; Ketkar-Atre, A.; Luyts, K.; Vriens, H.; Nobre, S.D.S.; Rivard, C.; Van Landuyt, K.; Baken, S.; Smolders, E.; Golanski, L.; et al. Body distribution of SiO2-Fe3O4 core-shell nanoparticles after intravenous injection and intratracheal instillation. Nanotoxicology 2016, 10, 567–574. [Google Scholar] [CrossRef]
- Dufort, S.; Bianchi, A.; Henry, M.; Lux, F.; Le Duc, G.; Josserand, V.; Louis, C.; Perriat, P.; Cremillieux, Y.; Tillement, O.; et al. Nebulized gadolinium-based nanoparticles: A theranostic approach for lung tumor imaging and radiosensitization. Small 2015, 11, 215–221. [Google Scholar] [CrossRef]
- Bianchi, A.; Dufort, S.; Lux, F.; Fortin, P.Y.; Tassali, N.; Tillement, O.; Coll, J.L.; Cremillieux, Y. Targeting and in vivo imaging of non-small-cell lung cancer using nebulized multimodal contrast agents. Proc. Natl. Acad. Sci. USA 2014, 111, 9247–9252. [Google Scholar] [CrossRef] [Green Version]
- McBride, A.A.; Price, D.N.; Lamoureux, L.R.; Elmaoued, A.A.; Vargas, J.M.; Adolphi, N.L.; Muttil, P. Preparation and characterization of novel magnetic nano-in-microparticles for site-specific pulmonary drug delivery. Mol. Pharm. 2013, 10, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Mejias, J.C.; Roy, K. In-vitro and in-vivo characterization of a multi-stage enzyme-responsive nanoparticle-in-microgel pulmonary drug delivery system. J. Control. Release 2019, 316, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Duncan, R. Polymeric carriers: Preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv. Drug Deliv. Rev. 2009, 61, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Whittaker, M.; McIntosh, M.P.; Pouton, C.W.; Phipps, S.; Kaminskas, L.M. A comparison of the lung clearance kinetics of solid lipid nanoparticles and liposomes by following the 3H-labelled structural lipids after pulmonary delivery in rats. Eur. J. Pharm. Biopharm. 2018, 125, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef]


| Respiratory Disease | Main Limitations | Improvements from Lung Delivery | Proteins | Antibiotics | Anticancer Drugs |
|---|---|---|---|---|---|
| Asthma | Low therapeutic efficacy of delivered drugs; inefficient control of the disease; airway inflammation | n.a. * | x | ||
| COPD | Persistent inflammation; parenchymal lung tissue destruction; abnormalities of the small airways | n.a. * | x | x | |
| Pneumonia | Low amount of drug reaches infection site; antimicrobial resistance | Higher drug accumulation in infection site; co-localisation of drug and infectious agent | x | x | |
| Cystic fibrosis | Thick viscous mucus; recurrent lung infections; progressive impairment of lung airways | Mucus-penetrating carriers; increased lung drug retention; delivery of genetic material to restore CFTR function | x | x | |
| Tuberculosis | Reduced amount of drug reaches infection site; antimicrobial resistance; long therapeutic regimen | Co-localisation of drug and infectious agent; reduction in antibiotic resistance incidence; possibility of add-on therapy (along with oral); reduce treatment duration | x | ||
| Lung cancer | Non-specificity of drugs; difficulties to reach the affected tissues; severity of systemic adverse effects | Vectorisation to cancer cells; reduction in systemic adverse effects | x |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontes, J.F.; Grenha, A. Multifunctional Nanocarriers for Lung Drug Delivery. Nanomaterials 2020, 10, 183. https://doi.org/10.3390/nano10020183
Pontes JF, Grenha A. Multifunctional Nanocarriers for Lung Drug Delivery. Nanomaterials. 2020; 10(2):183. https://doi.org/10.3390/nano10020183
Chicago/Turabian StylePontes, Jorge F., and Ana Grenha. 2020. "Multifunctional Nanocarriers for Lung Drug Delivery" Nanomaterials 10, no. 2: 183. https://doi.org/10.3390/nano10020183
APA StylePontes, J. F., & Grenha, A. (2020). Multifunctional Nanocarriers for Lung Drug Delivery. Nanomaterials, 10(2), 183. https://doi.org/10.3390/nano10020183






