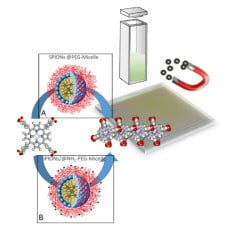
Influence of Magnetic Micelles on Assembly and Deposition of Porphyrin J-Aggregates
Abstract
:1. Introduction
2. Materials and Methods
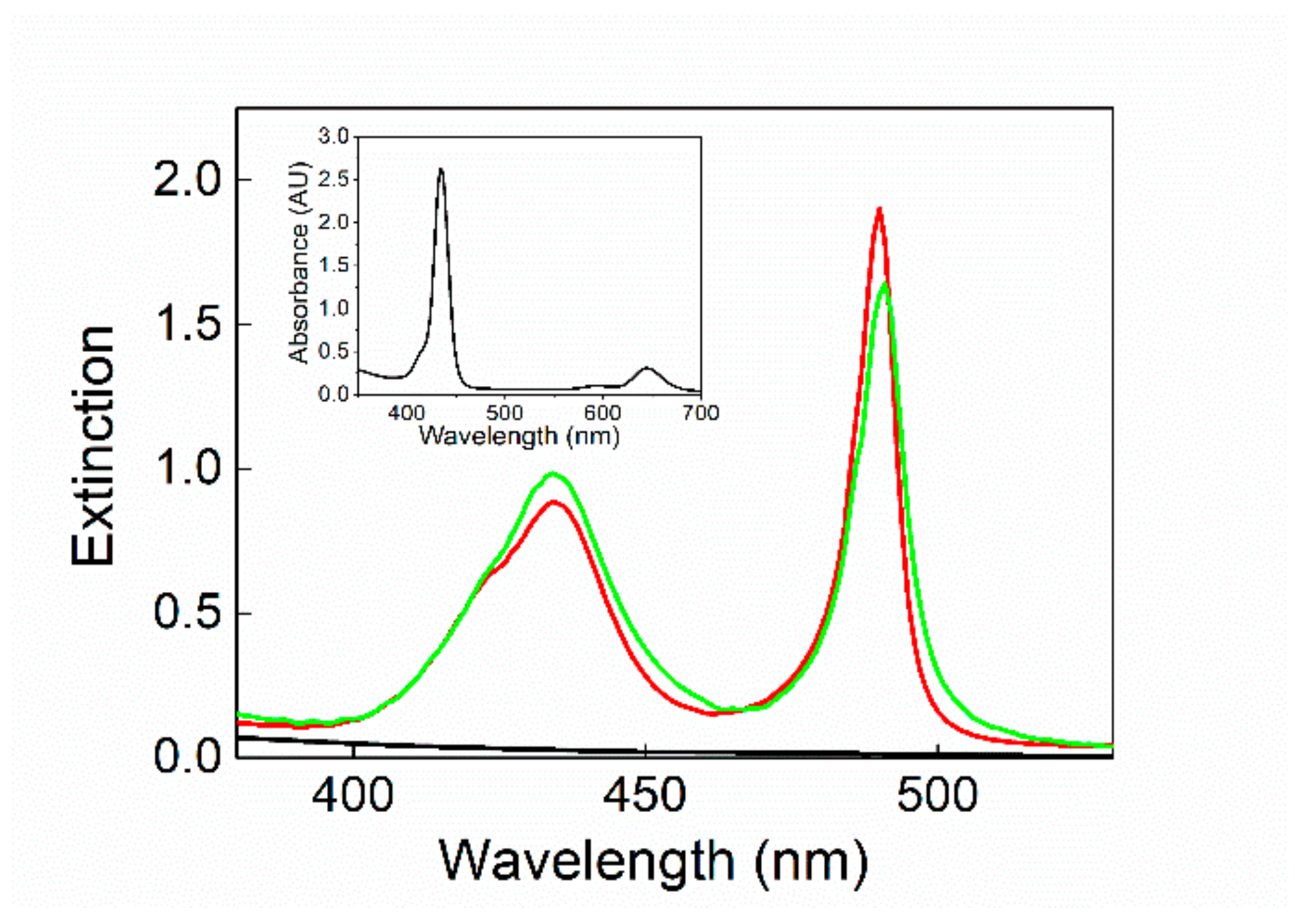
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cornia, A.; Seneor, P. The molecular way. Nat. Mater. 2017, 16, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.L.; Dai, Y.; Ma, Y.D.; Li, X.R.; Wei, W.; Huang, B.B. Two-dimensional metalloporphyrin monolayers with intriguing electronic and spintronic properties. J. Mater. Chem. C 2015, 3, 6901–6907. [Google Scholar] [CrossRef]
- Le Roy, J.J.; Cremers, J.; Thomlinson, I.A.; Slota, M.; Myers, W.K.; Horton, P.H.; Coles, S.J.; Anderson, H.L.; Bogani, L. Tailored homo- and hetero-lanthanide porphyrin dimers: A synthetic strategy for integrating multiple spintronic functionalities into a single molecule. Chem. Sci. 2018, 9, 8474–8481. [Google Scholar] [CrossRef]
- Cho, W.J.; Cho, Y.; Min, S.K.; Kim, W.Y.; Kim, K.S. Chromium Porphyrin Arrays As Spintronic Devices. J. Am. Chem. Soc. 2011, 133, 9364–9369. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, K.Q. Spin filtering, magnetic and electronic switching behaviors in manganese porphyrin-based spintronic devices. J. Mater. Chem. C 2013, 1, 4014–4019. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, K.Q. A nearly perfect spin filter and a spin logic gate based on a porphyrin/graphene hybrid material. Phys. Chem. Chem. Phys. 2018, 20, 3997–4004. [Google Scholar] [CrossRef]
- Wende, H.; Bernien, M.; Luo, J.; Sorg, C.; Ponpandian, N.; Kurde, J.; Miguel, J.; Piantek, M.; Xu, X.; Eckhold, P.; et al. Substrate-induced magnetic ordering and switching of iron porphyrin molecules. Nat. Mater. 2007, 6, 516–520. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, K.Q. Magnetic configuration dependence of magnetoresistance in a Fe-porphyrin-like carbon nanotube spintronic device. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Naaman, R.; Fontanesi, C.; Waldeck, D.H. Chirality and its role in the electronic properties of peptides: Spin filtering and spin polarization. Curr. Opin. Electrochem. 2019, 14, 138–142. [Google Scholar] [CrossRef]
- Abendroth, J.M.; Stemer, D.M.; Bloom, B.P.; Roy, P.; Naaman, R.; Waldeck, D.H.; Weiss, P.S.; Mondal, P.C. Spin Selectivity in Photoinduced Charge-Transfer Mediated by Chiral Molecules. ACS Nano 2019, 13, 4928–4946. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Capua, E.; Kesharwani, M.K.; Martin, J.M.L.; Sitbon, E.; Waldeck, D.H.; Naaman, R. Chirality-induced spin polarization places symmetry constraints on biomolecular interactions. Proc. Natl. Acad. Sci. USA 2017, 114, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaeli, K.; Kantor-Uriel, N.; Naaman, R.; Waldeck, D.H. The electron’s spin and molecular chirality—how are they related and how do they affect life processes? Chem. Soc. Rev. 2016, 45, 6478–6487. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.C.; Fontanesi, C.; Waldeck, D.H.; Naaman, R. Field and Chirality Effects on Electrochemical Charge Transfer Rates: Spin Dependent Electrochemistry. ACS Nano 2015, 9, 3377–3384. [Google Scholar] [CrossRef] [Green Version]
- Naaman, R.; Waldeck, D.H. Spintronics and Chirality: Spin Selectivity in Electron Transport Through Chiral Molecules. Annu. Rev. Phys. Chem. 2015, 66, 263–281. [Google Scholar] [CrossRef]
- Ray, S.G.; Daube, S.S.; Leitus, G.; Vager, Z.; Naaman, R. Chirality-induced spin-selective properties of self-assembled monolayers of DNA on gold. Phys. Rev. Lett. 2006, 96. [Google Scholar] [CrossRef]
- Xie, Z.; Markus, T.Z.; Cohen, S.R.; Vager, Z.; Gutierrez, R.; Naaman, R. Spin Specific Electron Conduction through DNA Oligomers. Nano Lett. 2011, 11, 4652–4655. [Google Scholar] [CrossRef]
- Di Nuzzo, D.; Kulkarni, C.; Zhao, B.; Smolinsky, E.; Tassinar, F.; Meskers, S.C.J.; Naaman, R.; Meijer, E.W.; Friend, R.H. High Circular Polarization of Electroluminescence Achieved via Self-Assembly of a Light-Emitting Chiral Conjugated Polymer into Multidomain Cholesteric Films. Acs Nano 2017, 11, 12713–12722. [Google Scholar] [CrossRef] [Green Version]
- Kettner, M.; Goehler, B.; Zacharias, H.; Mishra, D.; Kiran, V.; Naaman, R.; Fontanesi, C.; Waldeck, D.H.; Sek, S.; Pawlowski, J.; et al. Spin Filtering in Electron Transport Through Chiral Oligopeptides. J. Phys. Chem. C 2015, 119, 14542–14547. [Google Scholar] [CrossRef]
- Mondal, P.C.; Kantor-Uriel, N.; Mathew, S.P.; Tassinari, F.; Fontanesi, C.; Naaman, R. Chiral Conductive Polymers as Spin Filters. Adv. Mater. 2015, 27, 1924–1927. [Google Scholar] [CrossRef]
- Koplovitz, G.; Leitus, G.; Ghosh, S.; Bloom, B.P.; Yochelis, S.; Rotem, D.; Vischio, F.; Striccoli, M.; Fanizza, E.; Naaman, R.; et al. Single Domain 10 nm Ferromagnetism Imprinted on Superparamagnetic Nanoparticles Using Chiral Molecules. Small 2019, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiran, V.; Mathew, S.P.; Cohen, S.R.; Delgado, I.H.; Lacour, J.; Naaman, R. Helicenes-A New Class of Organic Spin Filter. Adv. Mater. 2016, 28, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Ben Dor, O.; Morali, N.; Yochelis, S.; Baczewski, L.T.; Paltiel, Y. Local Light-Induced Magnetization Using Nanodots and Chiral Molecules. Nano Lett. 2014, 14, 6042–6049. [Google Scholar] [CrossRef] [PubMed]
- Ben Dor, O.; Yochelis, S.; Mathew, S.P.; Naaman, R.; Paltiel, Y. A chiral-based magnetic memory device without a permanent magnet. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Verbiest, T.; Elshocht, S.V.; Kauranen, M.; Hellemans, L.; Snauwaert, J.; Nuckolls, C.; Katz, T.J.; Persoons, A. Strong Enhancement of Nonlinear Optical Properties Through Supramolecular Chirality. Science 1998, 282, 913–915. [Google Scholar] [CrossRef]
- Kim, C.-J.; Sánchez Castillo, A.; Ziegler, Z.; Ogawa, Y.; Noguez, C.; Park, J. Chiral atomically thin films. Nat. Nanotechnol. 2016, 11, 520–524. [Google Scholar] [CrossRef]
- Leclere, P.; Surin, M.; Lazzaroni, R.; Kilbinger, A.F.M.; Henze, O.; Jonkheijm, P.; Biscarini, F.; Cavallini, M.; Feast, W.J.; Meijer, E.W.; et al. Surface-controlled self-assembly of chiral sexithiophenes. J. Mater. Chem. 2004, 14, 1959–1963. [Google Scholar] [CrossRef]
- Tahara, K.; Yamaga, H.; Ghijsens, E.; Inukai, K.; Adisoejoso, J.; Blunt, M.O.; De Feyter, S.; Tobe, Y. Control and induction of surface-confined homochiral porous molecular networks. Nat. Chem. 2011, 3, 714–719. [Google Scholar] [CrossRef]
- Jack, C.; Karimullah, A.S.; Tullius, R.; Khorashad, L.K.; Rodier, M.; Fitzpatrick, B.; Barron, L.D.; Gadegaard, N.; Lapthorn, A.J.; Rotello, V.M.; et al. Spatial control of chemical processes on nanostructures through nano-localized water heating. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Bystrenova, E.; Facchini, M.; Cavallini, M.; Cacace, M.G.; Biscarini, F. Multiple length-scale patterning of DNA by stamp-assisted deposition. Angew. Chem. Int. Ed. 2006, 45, 4779–4782. [Google Scholar] [CrossRef]
- van Hameren, R.; Schön, P.; van Buul, A.M.; Hoogboom, J.; Lazarenko, S.V.; Gerritsen, J.W.; Engelkamp, H.; Christianen, P.C.M.; Heus, H.A.; Maan, J.C.; et al. Macroscopic Hierarchical Surface Patterning of Porphyrin Trimers via Self-Assembly and Dewetting. Science 2006, 314, 1433–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castriciano, M.A.; Gentili, D.; Romeo, A.; Cavallini, M.; Scolaro, L.M. Spatial control of chirality in supramolecular aggregates. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, J.M.; Berriman, J.A.; Kübel, C.; El-Hachemi, Z.; Naubron, J.-V.; Balaban, T.S. Electron Cryo-Microscopy of TPPS4⋅2HCl Tubes Reveals a Helical Organisation Explaining the Origin of their Chirality. ChemPhysChem 2013, 14, 3209–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, A.; Castriciano, M.A.; Occhiuto, I.; Zagami, R.; Pasternack, R.F.; Scolaro, L.M. Kinetic Control of Chirality in Porphyrin J-Aggregates. J. Am. Chem. Soc. 2014, 136, 40–43. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Donato, M.G.; Villari, V.; Micali, N.; Romeo, A.; Scolaro, L.M. Surfactant-like behavior of short-chain alcohols in porphyrin aggregation. J. Phys. Chem. B 2009, 113, 11173–11178. [Google Scholar] [CrossRef]
- Rubires, R.; Farrera, J.A.; Ribo, J.M. Stirring effects on the spontaneous formation of chirality in the homoassociation of diprotonated meso-tetraphenylsulfonato porphyrins. Chem. A Eur. J. 2001, 7, 436–446. [Google Scholar] [CrossRef]
- Zagami, R.; Castriciano, M.A.; Romeo, A.; Trapani, M.; Pedicini, R.; Monsù Scolaro, L. Tuning supramolecular chirality in nano and mesoscopic porphyrin J-aggregates. Dye. Pigment. 2017, 142, 255–261. [Google Scholar] [CrossRef]
- Zagami, R.; Castriciano, M.A.; Romeo, A.; Scolaro, L.M. Spectroscopic investigations on chiral J-aggregates induced by tartaric acid in alcoholic solution. J. Porphyr. Phthalocyanines 2017, 21, 327–333. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Zagami, R.; Micali, N.; Scolaro, L.M. Kinetic effects of tartaric acid on the growth of chiral J-aggregates of tetrakis(4-sulfonatophenyl)porphyrin. Chem. Commun. 2012, 48, 4872–4874. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; De Luca, G.; Villari, V.; Scolaro, L.M.; Micali, N. Scaling the chirality in porphyrin J-nanoaggregates. J. Am. Chem. Soc. 2011, 133, 765–767. [Google Scholar] [CrossRef]
- Micali, N.; Villari, V.; Castriciano, M.A.; Romeo, A.; Scolaro, L.M. From fractal to nanorod porphyrin J-aggregates. concentration-induced tuning of the aggregate size. J. Phys. Chem. B 2006, 110, 8289–8295. [Google Scholar] [CrossRef] [PubMed]
- El-Hachemi, Z.; Escudero, C.; Acosta-Reyes, F.; Casas, M.T.; Altoe, V.; Aloni, S.; Oncins, G.; Sorrenti, A.; Crusats, J.; Campos, J.L.; et al. Structure vs. properties—Chirality, optics and shapes—In amphiphilic porphyrin J-aggregates. J. Mater. Chem. C 2013, 1, 3337–3346. [Google Scholar] [CrossRef] [Green Version]
- Zagami, R.; Romeo, A.; Castriciano, M.A.; Monsù Scolaro, L. Inverse Kinetic and Equilibrium Isotope Effects on Self-Assembly and Supramolecular Chirality of Porphyrin J-Aggregates. Chem. A Eur. J. 2017, 23, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Castriciano, M.A.; Zagami, R.; Pollicino, G.; Monsù Scolaro, L.; Pasternack, R.F. Effect of zinc cations on the kinetics of supramolecular assembly and the chirality of porphyrin J-aggregates. Chem. Sci. 2017, 8, 961–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Occhiuto, I.G.; Zagami, R.; Trapani, M.; Bolzonello, L.; Romeo, A.; Castriciano, M.A.; Collini, E.; Monsù Scolaro, L. The role of counter-anions in the kinetics and chirality of porphyrin J-aggregates. Chem. Commun. 2016, 52, 11520–11523. [Google Scholar] [CrossRef] [PubMed]
- Trapani, M.; Castriciano, M.A.; Romeo, A.; De Luca, G.; Machado, N.; Howes, B.D.; Smulevich, G.; Scolaro, L.M. Nanohybrid Assemblies of Porphyrin and Au-10 Cluster Nanoparticles. Nanomaterials 2019, 9, 1026. [Google Scholar] [CrossRef] [Green Version]
- Dressel, C.; Reppe, T.; Prehm, M.; Brautzsch, M.; Tschierske, C. Chiral self-sorting and amplification in isotropic liquids of achiral molecules. Nat. Chem. 2014, 6, 971–977. [Google Scholar] [CrossRef]
- Micali, N.; Engelkamp, H.; van Rhee, P.G.; Christianen, P.C.M.; Scolaro, L.M.; Maan, J.C. Selection of supramolecular chirality by application of rotational and magnetic forces. Nat. Chem. 2012, 4, 201–207. [Google Scholar] [CrossRef]
- Abdulrahman, N.A.; Fan, Z.; Tonooka, T.; Kelly, S.M.; Gadegaard, N.; Hendry, E.; Govorov, A.O.; Kadodwala, M. Induced Chirality through Electromagnetic Coupling between Chiral Molecular Layers and Plasmonic Nanostructures. Nano Lett. 2012, 12, 977–983. [Google Scholar] [CrossRef]
- Lei, S.; Surin, M.; Tahara, K.; Adisoejoso, J.; Lazzaroni, R.; Tobe, Y.; Feyter, S.D. Programmable Hierarchical Three-Component 2D Assembly at a Liquid−Solid Interface: Recognition, Selection, and Transformation. Nano Lett. 2008, 8, 2541–2546. [Google Scholar] [CrossRef]
- Gaeta, M.; Oliveri, I.P.; Fragala, M.E.; Failla, S.; D’Urso, A.; Di Bella, S.; Purrello, R. Chirality of self-assembled achiral porphyrins induced by chiral Zn(ii) Schiff-base complexes and maintained after spontaneous dissociation of the templates: A new case of chiral memory. Chem. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkas, N.; Palchik, O.; Brukental, I.; Nowik, I.; Gofer, Y. A Mesoporous Iron−Titanium Oxide Composite Prepared Sonochemically. J. Phys. Chem. B 2003, 107, 8772–8778. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Dulinska-Litewka, J.; Lazarczyk, A.; Halubiec, P.; Szafranski, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide NanoparticlesCurrent and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knuechel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Cui, H. Functional nanoparticles for magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 814–841. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Wahajuddin, S.A.; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Thandu, M.; Rapozzi, V.; Xodo, L.; Albericio, F.; Comuzzi, C.; Cavalli, S. “Clicking” Porphyrins to Magnetic Nanoparticles for Photodynamic Therapy. Chempluschem 2014, 79, 90–98. [Google Scholar] [CrossRef]
- Akbar, A.; Riaz, S.; Bashir, M.; Naseem, S. Effect of Fe3+/Fe2+ Ratio on Superparamagnetic Behavior of Spin Coated Iron Oxide Thin Films. IEEE Trans. Magn. 2014, 50, 4. [Google Scholar] [CrossRef]
- Depalo, N.; Carrieri, P.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Bertinetti, L.; Innocenti, C.; Sangregorio, C.; Curri, M.L. Biofunctionalization of Anisotropic Nanocrystalline Semiconductor–Magnetic Heterostructures. Langmuir 2011, 27, 6962–6970. [Google Scholar] [CrossRef] [PubMed]
- Valente, G.; Depalo, N.; de Paola, I.; Iacobazzi, R.M.; Denora, N.; Laquintana, V.; Comparelli, R.; Altamura, E.; Latronico, T.; Altomare, M.; et al. Integrin-targeting with peptide-bioconjugated semiconductor-magnetic nanocrystalline heterostructures. Nano Res. 2016, 9, 644–662. [Google Scholar] [CrossRef]
- Depalo, N.; Iacobazzi, R.M.; Valente, G.; Arduino, I.; Villa, S.; Canepa, F.; Laquintana, V.; Fanizza, E.; Striccoli, M.; Cutrignelli, A.; et al. Sorafenib delivery nanoplatform based on superparamagnetic iron oxide nanoparticles magnetically targets hepatocellular carcinoma. Nano Res. 2017, 10, 2431–2448. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Collings, P.J. Resonance Light-Scattering—A New Technique for Studying Chromophore Aggregation. Science 1995, 269, 935–939. [Google Scholar] [CrossRef]
- Akins, D.L.; Zhu, H.R.; Guo, C. Absorption and raman-scattering by aggregated meso-tetrakis(p-sulfonatophenyl)porphine. J. Phys. Chem. 1994, 98, 3612–3618. [Google Scholar] [CrossRef]
- Ribo, J.M.; Crusats, J.; Farrera, J.A.; Valero, M.L. Aggregation in water solutions of tetrasodium diprotonated meso-tetrakis(4-sulfonatophenyl)porphyrin. J. Chem. Soc. Chem. Commun. 1994, 681–682. [Google Scholar] [CrossRef]
- Gandini, S.C.M.; Gelamo, E.L.; Itri, R.; Tabak, M. Small angle X-ray scattering study of meso-tetrakis (4-sulfonatophenyl) porphyrin in aqueous solution: A self-aggregation model. Biophys. J. 2003, 85, 1259–1268. [Google Scholar] [CrossRef] [Green Version]
- Schwab, A.D.; Smith, D.E.; Rich, C.S.; Young, E.R.; Smith, W.F.; de Paula, J.C. Porphyrin nanorods. J. Phys. Chem. B 2003, 107, 11339–11345. [Google Scholar] [CrossRef]
- Scolaro, L.M.; Romeo, A.; Castriciano, M.A.; Micali, N. Unusual optical properties of porphyrin fractal J-aggregates. Chem. Commun. 2005, 24, 3018–3020. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Castriciano, M.A.; Scolaro, L.M. Spectroscopic and kinetic investigations on porphyrin J-aggregates induced by polyamines. J. Porphyr. Phthalocyanines 2010, 14, 713–721. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Villari, V.; Micali, N.; Scolaro, L.M. Structural rearrangements in 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin J-aggregates under strongly acidic conditions. J. Phys. Chem. B 2003, 107, 8765–8771. [Google Scholar] [CrossRef]
- Depalo, N.; Fanizza, E.; Vischio, F.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Striccoli, M.; Giannelli, G.; Agostiano, A.; Curri, M.L.; et al. Imaging modification of colon carcinoma cells exposed to lipid based nanovectors for drug delivery: A scanning electron microscopy investigation. RSC Adv. 2019, 9, 21810–21825. [Google Scholar] [CrossRef] [Green Version]
- Pasternack, R.F.; Gibbs, E.J.; Collings, P.J.; de Paula, J.C.; Turzo, L.C.; Terracina, A. A nonconventional approach to supramolecular formation dynamics. The kinetics of assembly of DNA-bound porphyrins. J. Am. Chem. Soc. 1998, 120, 5873–5878. [Google Scholar] [CrossRef]
- Monsù Scolaro, L.; Castriciano, M.; Romeo, A.; Mazzaglia, A.; Mallamace, F.; Micali, N. Nucleation effects in the aggregation of water-soluble porphyrin aqueous solutions. Phys. A Stat. Mech. Its Appl. 2002, 304, 158–169. [Google Scholar] [CrossRef]
- Paulo, P.M.R.; Costa, S.M.B. Non-covalent dendrimer–porphyrin interactions: The intermediacy of H-aggregates? Photochem. Photobiol. Sci. 2003, 2, 597–604. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Angelini, N.; Micali, N.; Longo, A.; Mazzaglia, A.; Scolaro, L.M. Structural features of meso-tetrakis(4-carboxyphenyl)porphyrin interacting with amino-terminated poly(propylene oxide). Macromolecules 2006, 39, 5489–5496. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Scolaro, L.M. Aggregation of meso-tetrakis(4-sulfonatophenyl)porphyrin on polyethyleneimine in aqueous solutions and on a glass surface. J. Porphyr. Phthalocyanines 2002, 6, 431–438. [Google Scholar] [CrossRef]
- Vlaming, S.M.; Augulis, R.; Stuart, M.C.A.; Knoester, J.; van Loosdrecht, P.H.M. Exciton Spectra and the Microscopic Structure of Self-Assembled Porphyrin Nanotubes. J. Phys. Chem. B 2009, 113, 2273–2283. [Google Scholar] [CrossRef] [Green Version]









| J-Aggregates | K | N | ||
|---|---|---|---|---|
| [SPIONs@PEG-micelle]/µM [a] | − | − | ||
| − | 1.8 × 10−2 ± 1 × 10−4 | 0.8 ± 0.05 | ||
| 15 | 2.7 × 10−2 ± 1 × 10−3 | 0.7 ± 0.02 | ||
| k0 | kc | m | n | |
| [SPIONs@PEG-micelle]/µM [b] | − | − | − | − |
| 0 | 2.7 × 10−4 ± 7 × 10−6 | 1.9 × 10−3 ± 8 × 10−6 | 3.0 ± 0.1 | 4.0 ± 0.1 |
| 15 | − | 1.6 × 10−2 ± 4 × 10−5 | 2.3 ± 0.1 | 1.3 ± 0.1 |
| [SPIONs@NH2-PEG-micelle]/µM[b] | − | − | − | − |
| 0 | 2.7 × 10−4 ± 7 × 10−6 | 1.9 × 10−3 ± 8 × 10−6 | 3.0 ± 0.1 | 4.0 ± 0.1 |
| 15 | 4.1 × 10−4 ± 2 × 10−7 | 1.7 × 10−3 ± 9 × 10−6 | 2.2 ± 0.1 | 3.6 ± 0.3 |
| 22 | 1.2 × 10−4 ± 5 × 10−6 | 2.6 × 10−3 ± 5 × 10−6 | 3.0 ± 0.3 | 4.3 ± 0.1 |
| 45 | 2.5 × 10−3 ± 7 × 10−7 | 9.1 × 10−3 ± 8 × 10−6 | 3.5 ± 0.2 | 3.0 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castriciano, M.A.; Trapani, M.; Romeo, A.; Depalo, N.; Rizzi, F.; Fanizza, E.; Patanè, S.; Monsù Scolaro, L. Influence of Magnetic Micelles on Assembly and Deposition of Porphyrin J-Aggregates. Nanomaterials 2020, 10, 187. https://doi.org/10.3390/nano10020187
Castriciano MA, Trapani M, Romeo A, Depalo N, Rizzi F, Fanizza E, Patanè S, Monsù Scolaro L. Influence of Magnetic Micelles on Assembly and Deposition of Porphyrin J-Aggregates. Nanomaterials. 2020; 10(2):187. https://doi.org/10.3390/nano10020187
Chicago/Turabian StyleCastriciano, Maria Angela, Mariachiara Trapani, Andrea Romeo, Nicoletta Depalo, Federica Rizzi, Elisabetta Fanizza, Salvatore Patanè, and Luigi Monsù Scolaro. 2020. "Influence of Magnetic Micelles on Assembly and Deposition of Porphyrin J-Aggregates" Nanomaterials 10, no. 2: 187. https://doi.org/10.3390/nano10020187
APA StyleCastriciano, M. A., Trapani, M., Romeo, A., Depalo, N., Rizzi, F., Fanizza, E., Patanè, S., & Monsù Scolaro, L. (2020). Influence of Magnetic Micelles on Assembly and Deposition of Porphyrin J-Aggregates. Nanomaterials, 10(2), 187. https://doi.org/10.3390/nano10020187